Abstract
Cell wall remodeling is important for plants to adapt to environmental stress. Under salt stress, cortical microtubules undergo a depolymerization-reassembly process to promote the biosynthesis of stress-adaptive cellulose, but the regulatory mechanisms underlying this process are still largely unknown. In this study, we reveal that FERONIA (FER), a potential cell wall sensor, interacts with COMPANION OF CELLULOSE SYNTHASE1 (CC1) and its closest homolog, CC2, two proteins that are required for cortical microtubule reassembly under salt stress. Biochemical data indicate that FER phosphorylates CC1 on multiple residues in its second and third hydrophobic microtubule-binding regions and that these phosphorylations modulate CC1 trafficking and affect the ability of CC1 to engage with microtubules. Furthermore, CC1 phosphorylation level is altered upon exposure to salt stress, which coincides with the changes of microtubule organization. Together, our study outlines an important intracellular mechanism that maintains microtubule arrays during salt exposure in plant cells.
A receptor-like kinase controls cell wall biosynthesis under salt stress by regulating microtubule organization.
INTRODUCTION
Environmental stress from high salinity severely impedes plant growth and development, affecting crop productivity. To grow in high-salt environments, plants need to adjust their physiology, metabolism, and morphology (1). The capacity of plants to endure salt stress heavily relies on cell wall remodeling (2). Plant cell walls are typically composed of cellulose, hemicelluloses, pectins, lignin, many glycoproteins, water and ions, with the most abundant molecule in the wall being water (3). Cell wall porosity, which allows exchange of cellular water and gas, is an important characteristic of plant cell wall structure and function (4). Remodeling of the cell wall matrix, including that of pectins, can influence cell wall porosity (5, 6). Salt stress–triggered cell wall remodeling leads to the changes of cell wall polymer abundance as well as cell wall organization and elasticity (7, 8), ultimately improving plant adaptation to salt stress. Cell wall changes need be constantly monitored by cell wall integrity sensors (8–10), which allows rapid cell wall remodeling without losing cell wall integrity. Now, the precise regulatory mechanisms of cell wall remodeling under salt stress are still largely unknown.
Catharanthus roseus receptor-like kinase 1–like (CrRLK1L) family proteins are considered potential cell wall sensors in plants (11). CrRLK1L subfamily proteins harbor two extracellular malectin-like domains that may bind to cell wall polymers and an intracellular kinase domain that transmits signals via the phosphorylation of intracellular substrates (12). In Arabidopsis, there are 17 CrRLK1L family members, among which FERONIA (FER) is the most extensively studied. A growing body of evidence supports that FER regulates both plant growth and development, such as root hair development, pavement cell morphogenesis, flowering, and fertilization as well as plant responses to biotic and abiotic stresses (13–18). As a cell wall sensor, FER directly binds to demethylesterified pectin via its external malectin domains, initiating internal cell wall repair pathways to deal with salt stress (8, 9, 19). In addition to FER, another two members of the CrRLK1L subfamily, THESEUS1 (THE1) and HERCULES1 (HERK1), are also required for the maintenance of cell wall integrity under salt stress by monitoring pectin status (8). Our previous study showed that FER functions together with the cell wall–localized leucine-rich repeat extensins 3/4/5 and rapid alkalinization factor 22/23 to regulate salt tolerance (16, 20). Moreover, FER may regulate other cell wall–related processes, such as microtubule organization. For instance, FER modulates cortical microtubule rearrangement by activating RHO-RELATED PROTEIN FROM PLANTS 6 (ROP6) signaling in response to mechanical stress (19, 21), and FER participates in the regulation of root-nutating growth by controlling microtubule organization and Ca2+ signaling (22). However, it has also been reported that microtubule response to mechanical stress is FER-independent as average orientation and anisotropy of cortical microtubule arrays after ablation were not affected in the fer-4 mutant and disruptions of both FER and microtubule assembly conferred additive effects on turgor-dependent cell bursting (23). Therefore, the relationship between FER and microtubule regulation needs to be further investigated. Identification of components that directly link FER to microtubules might aid to clarify such relationships.
In plant cells, cellulose biosynthesis is carried out by plasma membrane–localized cellulose synthase (CESA) complexes (CSCs) (24). Because of their catalytic activity, the CSCs move in the plasma membrane with the direction of the movement typically steered by cortical microtubules (25, 26). Hence, the layout of the cortical microtubules determines the direction of cellulose microfibril deposition, which subsequently controls cell morphology and ultimately the shape of whole plants. CSCs are connected to cortical microtubules via several proteins, including CELLULOSE SYNTHASE INTERACTING1 (CSI1) and COMPANION OF CELLULOSE SYNTHASE (CC) (27–29). Cortical microtubule organization and CSC behavior are dynamically regulated in response to salt stress. At the early stage of salt stress (~30 min to 2 hours after exposure), accompanying by a depolymerization of cortical microtubules, CSCs are internalized into small CESA compartments (smaCCs)/microtubule-associated CESA compartments (MASCs) in the cytosol (28). This process is regulated by several microtubule-associated proteins (MAPs), including atypical MAP kinase PROPYZAMIDE-HYPERSENSITIVE 1, microtubule stabilizing protein SPIRAL1, and MAP65 (30–34). The transient depolymerization of cortical microtubules is crucial for salt tolerance in plants. For instance, application of oryzalin, a microtubule depolymerizing agent, promotes seedling survival during salt stress, whereas the overstabilization of cortical microtubules with taxol leads to a reduced survival rate (35, 36). The two CC proteins, CC1 and CC2, facilitate the reassembly of cortical microtubules under salt stress and aid in the return of the CSCs to the plasma membrane to continue cellulose synthesis (28, 37). Simultaneous mutations of CC1 and CC2 genes result in reduced hypocotyl elongation under salt stress (28). Nuclear magnetic resonance analyses revealed that four conserved hydrophobic regions within the cytosolic N-terminal domain of the CC proteins bind to cortical microtubules (37). Nevertheless, upstream components that regulate the CC-mediated reconfiguration of cortical microtubules under salt stress remain unknown.
In this study, we found that FER interacts with and phosphorylates CC1 and that this phosphorylation is crucial for the regulation of CC1 trafficking and cortical microtubule organization under salt stress. We thus outline a molecular link between cell wall integrity and intracellular cell wall feedback pathway, which greatly advances our understanding of how plants maintain cell wall integrity under high salinity.
RESULTS
FER is identified as an interacting partner of CC1
To decipher upstream components that link cell wall integrity and CC-mediated cortical microtubule organization under salt stress, we conducted an immunoprecipitation–mass spectrometry (IP-MS) assay using functional GFP-CC1 transgenic plants (28) to search for CC1-interacting proteins. The IP-MS data revealed several cellulose biosynthesis–related components, such as CESA1, CESA2, CESA3, CESA5, CESA6, CESA9, and CSI1 (fig. S1A), highlighting the prominent role of CC1 in regulating cellulose biosynthesis. In addition, FER, a CrRLK1L family protein that acts as a putative cell wall sensor and regulates plant salt tolerance (9), was copurified with CC1 (Fig. 1A), suggesting a potential interaction between CC1 and FER. This interaction was further supported by IP-MS assay using functional FER-GFP transgenic plants, which showed that CC1 was copurified with FER-GFP (green fluorescent protein) (Fig. 1A). To validate the interaction between these two proteins, split luciferase (split-LUC) complementation assay in Nicotiana benthamiana and coimmunoprecipitation (Co-IP) assay in Arabidopsis (Fig. 1, B and C) were performed, which supported the interaction of CC1 with FER. Gene expression profiling based on The Arabidopsis Information Resource (TAIR) transcriptomics database showed that CC1 and FER are coexpressed across different tissues and developmental stages (fig. S1B). Collectively, these results suggested that FER and CC1 may function together in certain biological processes.
Fig. 1. CC1 interacts with FER.
(A) IP-MS assay showing the interaction of CC1 with FER. The peptides of CC1 and FER identified in GFP-CC1 IP-MS data (top) and FER-GFP IP-MS data (bottom) are presented. (B) split-LUC complementation assay showing the interaction of FER with CC1. Fluorescence was detected at 48 hours after infiltration of the indicated constructs into N. benthamiana. (C) Analysis of the interaction of FER with CC1 in planta using Co-IP assay. Proteins were extracted from 8-day-old seedlings, and Venus-CC1 proteins were immunoprecipitated using GFP-Trap beads. Immunoblottings were performed using anti-GFP and anti-FER antibodies. (D) Analysis of the interaction of FER with CC1 using BiFC assay. The combination of YN-CC1 and FLS2-YC was used as a negative control. Yellow fluorescent protein (YFP) fluorescence was detected at 48 hours after the infiltration of the indicated constructs into N. benthamiana leaves. Scale bars, 50 μm. (E) Colocalization of mScarlet-CC1 and FER-GFP at the plasma membrane in the etiolated seedling of dual-labeled transgenic plants. Scale bars, 10 μm. (F) Fluorescence intensity plot of mScarlet-CC1 and FER-GFP along the yellow lines as shown in (E).
To determine where the interaction of these two proteins occurs in a living plant cell, we performed a bimolecular fluorescence complementation (BiFC) assay. Overexpression of full-length FER, but not its kinase-dead variant (FERK565R), causes cell death and autofluorescence in N. benthamiana leaves, probably due to the constitutive kinase activity of FER (38). Therefore, FERK565R was used in our BiFC assay. When FERK565R-YC was cotransformed with YN-CC1 in N. benthamiana leaves, strong and clear fluorescence was detected at the plasma membrane (Fig. 1D), again corroborating the interaction between FER and CC1. We did not observe fluorescence when YN-CC1, and the well-known receptor-like kinase FLAGELLIN-SENSITIVE 2 (FLS2)–YC were coexpressed (Fig. 1D), indicating that CC1 interacts specifically with FER. Using stable dual-labeled mScarlet-CC1/FER-GFP transgenic Arabidopsis plants, we similarly found that FER and CC1 were colocalized at the plasma membrane (Fig. 1, E and F), but colocalization of FER and CC1 was also observed in dispersed cytosolic puncta (fig. S1, C and D), which are likely the trans-Golgi network (TGN) where CC1 and FER are known to reside (28, 39). CC2, a homolog of CC1, works redundantly with CC1 in the regulation of hypocotyl elongation under salt stress (28). Our BiFC assay indicated that FER also interacted with CC2 (fig. S1E), supporting that FER is a regulator of CC1/CC2 proteins.
fer-4 and cc1 cc2 mutants exhibit similar defects in hypocotyl elongation upon exposure to oryzalin, isoxaben, and salt stress
CC1 and CC2 play a key role in cortical microtubule organization and cellulose synthesis (28), and simultaneous mutations of these two genes result in shorter hypocotyls upon exposure to oryzalin, a microtubule depolymerizing agent, and isoxaben, a cellulose synthesis inhibitor (Fig. 2, A to D, and fig. S2A) (40, 41). By contrast, cc1 cc2 double mutant exhibited only slightly reduced hypocotyl growth under normal conditions (Fig. 2A and fig. S2A). Similar to the cc1 cc2 double mutant, the fer-4 mutant also displayed reduced hypocotyl elongation compared to the wild type (WT) when grown on a medium supplemented with oryzalin or isoxaben (Fig. 2, A to D, and fig. S2A). CC1/CC2 proteins are required for the reassembly of cortical microtubules under salt stress, and cc1 cc2 double mutant exhibits reduced hypocotyl elongation under salt stress (28). Notably, the fer-4 mutant displayed a comparable hypocotyl elongation inhibition phenotype as cc1 cc2 double mutant under salt stress (Fig. 2, E and F, and fig. S2B). Together, these results indicate that plants lacking FER and CC1/CC2 react similarly to microtubule depolymerization, cellulose synthesis inhibition, and salt stress.
Fig. 2. fer-4 and cc1 cc2 mutants exhibit a similar defect in hypocotyl elongation.
(A) Hypocotyl phenotype of seedlings grown on 1/2 MS media or 1/2 MS media supplemented with 300 nM oryzalin in the dark for 4 days. Scale bars, 2 mm. (B) Relative hypocotyl length of the seedlings shown in (A). Values were obtained by dividing the length of hypocotyls grown on oryzalin media with that on MS media. Values are the means ± SD (n > 33 seedlings). (C) Hypocotyl phenotype of seedlings grown on 1/2 MS media or 1/2 MS media supplemented with 2 nM isoxaben in the dark for 4 days. Scale bars, 2 mm. (D) Relative hypocotyl length of the seedlings shown in (C). Values are the means ± SD (n > 33 seedlings). (E) Hypocotyl phenotype of seedlings grown on 1/2 MS media or 1/2 MS media supplemented with 120 mM NaCl in the dark for 9 days. Scale bars, 2 mm. (F) Relative hypocotyl length of the seedlings shown in (E). Values are the means ± SD (n > 33 seedlings). The relative hypocotyl lengths of the representative seedlings shown in (A), (C), and (E) are marked by red asterisks in the histograms of (B), (D), and (F). Different letters in (B), (D), and (F) indicate statistically significant differences (P < 0.01, one-way ANOVA).
To further explore the genetic interaction between FER and CC1/CC2, cc1 cc2 double mutant was crossed with the fer-4 mutant to generate cc1 cc2 fer-4 triple mutant. Under normal conditions, cc1 cc2 fer-4 triple mutant displayed an obvious growth defect in hypocotyl elongation (Fig. 2A and fig. S2A), indicating that simultaneous mutations of FER and CC1/CC2 conferred an additive effect on hypocotyl elongation. However, when treated with oryzalin or isoxaben, the cc1 cc2 fer-4 triple mutant exhibited a similar ratio of hypocotyl growth reduction as the cc1 cc2 mutant (Fig. 2, B and D), indicating that FER and CC1/CC2 genetically function together to regulate microtubule polymerization and cellulose synthesis. Under salt stress, cc1 cc2 fer-4 triple mutant exhibited more severe hypocotyl elongation inhibition compared to the fer-4 and cc1 cc2 mutants (Fig. 2, E and F), suggesting that FER and CC1/CC2 may also be involved in independent pathways to respond to salt stress.
In addition to hypocotyl elongation, we analyzed hypocotyl width under different stress conditions. Under normal conditions, cc1 cc2 double mutant exhibited similar, whereas fer-4 and cc1 cc2 fer-4 mutants exhibited wider, hypocotyls as compared to the WT (fig. S3, A and B). After oryzalin treatment, hypocotyl enlargement was observed in all the genotypes (fig. S3, A and B). However, when comparing hypocotyl width under oryzalin conditions to that under control conditions, we found that the fer-4 mutant exhibited a similar ratio of hypocotyl swelling as the WT, whereas cc1 cc2 displayed wider hypocotyls (fig. S3C). Intriguingly, cc1 cc2 fer-4 triple mutant exhibited a slightly reduced ratio of hypocotyl swelling compared to the WT and fer-4 mutant after oryzalin treatment (fig. S3C), suggesting that the width increase observed in cc1cc2 mutant is FER-dependent. It is plausible that FER is required for the feedback regulation of hypocotyl width under oryzalin conditions in the absence of CC1/CC2. Isoxaben treatment led to similar hypocotyl width changes in WT and cc1 cc2 (fig. S3, A to C). However, fer-4 and cc1 cc2 fer-4 showed slimmer hypocotyl width than the WT (fig. S3, A to C). Under salt stress, cc1 cc2 displayed a similar hypocotyl width ratio as WT, whereas both fer-4 and cc1 cc2 fer-4 mutants displayed increased hypocotyl width ratio (fig. S3, D to F). These results indicated that FER, but not CC1/CC2, is involved in the regulation of hypocotyl swelling during isoxaben and NaCl treatment.
We next analyzed the cell aspect ratio (length/width) in hypocotyl cells, about 4 to 10 cells from apical hook, treated with oryzalin, isoxaben, and NaCl. Under normal conditions, the cell aspect ratio in the fer-4 and cc1 cc2 mutants was not affected compared to the WT (fig. S4, A and B). After oryzalin, isoxaben, or NaCl treatment, we observed a notable reduction in the length/width ratio of hypocotyl cells in the fer-4 and cc1 cc2 mutants compared to the WT (fig. S4, A to D). Notably, cc1 cc2 mutants exhibited a similar reduction as the fer-4 mutant after treatment with oryzalin and isoxaben, but they displayed a slightly reduced length/width ratio compared to the fer-4 mutant under salt stress (fig. S4, A to D). cc1 cc2 fer-4 triple mutant exhibited a similar or slightly reduced length/width ratio compared to the cc1 cc2 double mutant after oryzalin, isoxaben, and NaCl treatments (fig. S4, A to D). Collectively, these results suggested that FER and CC1/CC2 are involved in a common pathway to regulate cell aspect ratio in response to oryzalin, isoxaben, and salt stress.
FER phosphorylates six residues in the second and third hydrophobic regions of CC1
The data above showed that FER is physically and genetically linked to CC1. We next investigated how FER and CC1 may act together. Given that FER is a receptor-like kinase, we tested whether it can phosphorylate CC1. CC1 consists of an N-terminal cytosolic domain (1 to 120 amino acid, CC1N) and a C-terminal apoplastic domain linked via a transmembrane domain (121 to 342 amino acid, CC1C) (fig. S1F). In the CC1N, there are four hydrophobic regions that are proposed to bind to microtubules (fig. S1F) (37). Our BiFC assay indicated that FER interacts with the N-terminal but not the C-terminal domain of CC1 (fig. S1G), so CC1N was used for in vitro kinase assay. After incubation with the cytosolic domain of FER (FERCD), clear phosphorylation of CC1N was observed, and this phosphorylation was fully eliminated when treated with lambda protein phosphatase (λPPase) (Fig. 3A), indicating that FER was capable of phosphorylating CC1N in vitro. Notably, a clear band shift of CC1N on a Coomassie Brilliant Blue (CBB)–stained gel was detected after incubation with FERCD, and this band shift disappeared when λPPase was added to the reaction buffer (Fig. 3A), supporting the phosphorylation of CC1N by FERCD.
Fig. 3. FER phosphorylates CC1.
(A) In vitro kinase assay showing the phosphorylation of CC1 N terminus (CC1N) by the FERCD. Immunoblottings were performed using an anti-pSer antibody. The loading of recombinant CC1N and FERCD was detected by CBB staining. GST protein was used as a negative control. Red triangle in the CBB staining gel indicates the shifted band of CC1 after incubation with FERCD. (B) Analysis of CC1 phosphorylation in CC1pro::Venus-CC1/cc1 cc2 and CC1pro::Venus-CC1/cc1 cc2 fer-4 plants. Proteins were extracted from 8-day-old seedlings, and Venus-CC1 proteins were immunoprecipitated using GFP-Trap beads. Immunoblottings were performed using anti-pSer and anti-GFP antibodies. The sample treated with λPPase was used to indicate the phosphorylation band of CC1. (C) Diagram of phosphorylation sites in the N-terminal domain of CC1 identified by LC-MS/MS analysis. Dark blue boxes represent the four hydrophobic regions of CC1N. The phosphorylated Ser and Thr residues are pointed out by orange and blue lines, respectively. (D) In vitro kinase assays showing the phosphorylation of the WT and the mutated variants of CC1N by FERCD. Immunoblottings were performed using an anti-pSer antibody. The recombinant CC1N and FERCD were detected by CBB staining. 6A indicates the substitutions of S47, T48, S52, S74, S77, and S79 with Ala. (E) Analysis of the phosphorylation of CC1 in Arabidopsis. Venus-CC1WT and Venus-CC16A were immunoprecipitated from transgenic plants using GFP-Trap beads. Immunoblottings were performed using anti-pSer and anti-GFP antibodies.
To determine whether FER phosphorylates CC1 in vivo, we generated CC1pro::Venus-CC1/ cc1 cc2 fer-4 plants by crossing CC1pro::Venus-CC1/cc1 cc2 plant with the fer-4 mutant. We next immunoprecipitated Venus-CC1 from the transgenic plants before subjecting the samples to immunoblotting assays. The results showed that the protein abundance of the immunoprecipitated CC1 was comparable between the two samples. However, by using an anti-phosphoserine (anti-pSer) or anti-phosphothreonine (anti-pThr) antibody, we found that the phosphorylation level of CC1 was substantially reduced in the fer-4 mutant (Fig. 3B and fig. S5A), indicating that FER is needed for CC1 phosphorylation in planta.
To pinpoint the phosphorylation sites of CC1N by FER, we performed liquid chromatography–tandem MS (LC-MS/MS) analysis of CC1N after incubation with FERCD in a kinase reaction buffer. This assay revealed that six Ser or Thr residues located within or nearby the second and third hydrophobic regions of CC1 exhibited elevated phosphorylation levels after incubation with FERCD (Fig. 3C). To validate that these six residues, namely, Ser47, Thr48, Ser52, Ser74, Ser77, and Ser79, are phosphorylated by FER, we substituted them with Ala and used the mutated variant (CC1N6A) for in vitro kinase assay. The results showed that FERCD phosphorylated CC1N6A to a much lower extent compared to the CC1NWT (Fig. 3D). Protein sequence alignment indicated that, with the exception of S79, all the phosphorylated Ser/Thr residues are conserved in AtCC2 as well as CC1 homologs across different plant species (fig. S5, B and C), highlighting the importance of these phosphorylation sites.
To determine the phosphorylation of the six residues in planta, we generated CC1pro::Venus-CC16A transgenic plants in the cc1 cc2 mutant and selected three independent CC1pro::Venus-CC16A transgenic lines with comparable CC1 protein abundance to CC1pro::Venus-CC1WT (Fig. 3E). Immunoblotting assays of immunoprecipitated Venus-CC1WT and Venus-CC16A using an anti-pSer antibody showed that the phosphorylation level of CC16A was markedly reduced compared to the CC1WT (Fig. 3E), corroborating that the six residues are the phosphorylation sites of CC1 in vivo.
Neither CC16A nor CC16D rescues the phenotypes of cc1 cc2 double mutant under oryzalin and salt stress conditions
Considering that FER-mediated CC1 phosphorylation sites are located in two hydrophobic regions that have been identified as microtubule-binding domains (MBDs) (37), we hypothesized that CC1 phosphorylation is important for its biological functions. To assess this, we first analyzed the hypocotyl elongation and cell aspect ratio of CC1pro::Venus-CC1WT/cc1 cc2, CC1pro::Venus-CC16A/cc1 cc2, and CC1pro::Venus-CC16D/cc1 cc2 transgenic plants. The cc1 cc2 double mutant had shorter hypocotyls and reduced length/width ratio than the WT when grown on a medium supplemented with oryzalin, isoxaben, or NaCl, and these phenotypes were almost fully rescued by the Venus-CC1WT transgene (Fig. 4, A to F, and fig. S4, A to D). However, Venus-CC16A could not restore these phenotypes under oryzalin and salt stress conditions (Fig. 4, A, B, E, and F, and fig. S4, A to D), indicating the importance of CC1 phosphorylation in regulating plant tolerance to oryzalin and salt stress. Intriguingly, we found that Venus-CC16A could partially rescue the reduced hypocotyl growth and reduced length/width ratio of hypocotyl cells in the cc1 cc2 double mutant under isoxaben treatment (Fig. 4, C and D, and fig. S4, A and B). These results implied that the Venus-CC16A variant was still functional and that phosphorylations on the six residues primarily contribute to the regulation of microtubule assembly rather than CSC-mediated cellulose synthesis. Unexpectedly, phospho-mimic variant Venus-CC16D also failed to rescue the hypocotyl elongation and the cell aspect ratio of the cc1 cc2 double mutant under oryzalin and salt stress conditions (Fig. 4, A, B, E, and F, and fig. S4, A to D) but partially restored the hypocotyl elongation and the length/width ratio of hypocotyl cells under isoxaben treatment (Fig. 4, C and D, and fig. S4, A and B). Collectively, these results suggested that a certain phosphorylation status is required for CC1 to respond to oryzalin and salt stress.
Fig. 4. Both CC16A and CC16D fail to rescue the hypocotyl elongation phenotypes of the cc1 cc2 mutant under oryzalin and salt stress conditions.
(A) Hypocotyl phenotype of seedlings grown on 1/2 MS media or 1/2 MS media supplemented with 300 nM oryzalin in the dark for 4 days. 6A and 6D represent the substitutions of six Ser/Thr residues with Ala and Asp, respectively. Scale bars, 2 mm. (B) Relative hypocotyl length of the seedlings shown in (A). Values are the means ± SD (n > 36 seedlings). (C) Hypocotyl phenotype of seedlings grown on 1/2 MS media or 1/2 MS media supplemented with 2 nM isoxaben in the dark for 4 days. Scale bars, 2 mm. (D) Relative hypocotyl length of the seedlings shown in (C). Values are the means ± SD (n > 32 seedlings). (E) Hypocotyl phenotype of seedlings grown on 1/2 MS media or 1/2 MS media supplemented with 120 mM NaCl in the dark for 9 days. Scale bars, 2 mm. (F) Relative hypocotyl length of the seedlings shown in (E). Values are the means ± SD (n > 33 seedlings). The relative hypocotyl lengths of the representative seedlings shown in (A), (C), and (E) are marked by red asterisks in the histograms. Different letters in (B), (D), and (F) indicate statistically significant differences (P < 0.01, one-way ANOVA).
FER-mediated phosphorylation controls CC1 trafficking under salt stress
To further understand the biological significance of FER-mediated CC1 phosphorylation, we analyzed the subcellular localization of CC1WT, CC16A, and CC16D. Our observations showed that, under normal conditions, both CC16A and CC16D exhibited similar localization patterns as CC1WT, i.e., both the plasma membrane and cytosol (fig. S6A). Moreover, time-lapse imaging revealed that CC16A and CC16D moved along linear tracks at the plasma membrane as CC1WT under normal conditions (fig. S6B). Previous research indicates that CC1 is internalized upon salt exposure and is recycled to the plasma membrane after prolonged salt treatment (28), and we thus observed the trafficking of each CC1 variant after NaCl treatment. Following 2 hours of salt exposure, CC1WT was clearly internalized in both the WT and fer-4 mutant (Fig. 5, A and B). Quantification of the CC1 protein density at the plasma membrane showed that CC1WT was internalized at a slightly faster rate in the fer-4 mutant compared to the WT (Fig. 5B). After 30 hours of NaCl exposure, CC1WT abundance at the plasma membrane was recovered to the steady-state level in the WT (Fig. 5, A and B). However, in the fer-4 mutant, CC1WT was unable to return to the plasma membrane (Fig. 5, A and B), indicating that FER-mediated phosphorylation is crucial for CC1 to repopulate the plasma membrane during salt stress. Similar to the behavior of CC1WT in the fer-4 mutant, CC16A failed to return to the plasma membrane during prolonged exposure to salt stress (Fig. 5, A and B). We found that salt stress–triggered CC16D internalization was blocked (Fig. 5, A and B), suggesting that CC1 dephosphorylation is necessary for its internalization during salt stress. Moreover, the CC16D exhibited a “fuzzy” fluorescence pattern after long-term salt exposure (Fig. 5, A and B), indicating that precise regulation of CC1 phosphorylation is important for its proper trafficking under salt stress. These results could explain our phenotypic assays showing that neither CC16A nor CC16D rescued the hypocotyl growth of the cc1 cc2 mutant under salt stress.
Fig. 5. FER-mediated phosphorylation modulates CC1 trafficking and its binding to microtubules.
(A) Observation of CC1 localization in CC1pro::Venus-CC1/cc1 cc2, CC1pro::Venus-CC1/cc1 cc2 fer-4, CC1pro::Venus-CC16A/cc1 cc2, and CC1pro::Venus-CC16D/cc1 cc2 etiolated hypocotyls after NaCl treatment for 0, 2, 6, and 30 hours. Scale bars, 10 μm. h, hours. (B) Quantification of the CC1 protein density at the plasma membrane in different transgenic lines as shown in (A) after NaCl treatment. Data are shown as means ± SD of cells from three independent experiments. The exact numbers of cells examined in each sample are indicated in the parentheses. Different letters indicate statistically significant differences (P < 0.01, one-way ANOVA). The CC1 density of the representative cells shown in (A) is marked by red asterisks. (C) In vitro microtubule-binding assay. The recombinant GST-His-CC1NWT and GST-His-CC1N6E (substitutions of six phosphorylated Ser/Thr residues with Glu) proteins were incubated with microtubules (MTs) and then centrifuged at a high speed. The tubulin and CC1 proteins in the supernatant (S) and pellet (P) fractions were detected by CBB staining. (D) Comparison of CC1NWT and CC1N6E abundance in the pellets after incubation with or without microtubules. Values are the means ± SD of three biological replicates. **P < 0.01, two-sided Student’s t test. (E) In vitro microtubule-bundling assay. Recombinant GST-His-CC1NWT and GST-His-CC1N6E proteins were incubated with microtubules and then centrifuged at a low speed. The tubulin and CC1 proteins in the supernatant (S) and pellet (P) fractions were detected by CBB staining. (F) Quantification of tubulins in the pellets after incubation with or without CC1 proteins. Values are the means ± SD of three biological replicates. Different letters indicate statistically significant differences (P < 0.01, one-way ANOVA). (G) Rhodamine-labeled microtubule imaging assay showing the bundling of microtubules after incubation with WT CC1N and CC1N6E. Scale bars, 10 μm.
FER-mediated CC1 phosphorylation affects microtubule binding and bundling
As the FER-mediated phosphorylation of CC1 might affect its interaction with microtubules, we next examined CC1’s microtubule-binding characteristics in vitro. To this end, microtubule-binding spin-down and microtubule-bundling assays were conducted. Microtubule-binding spin-down assays revealed that the microtubule-binding ability of CC1N6E, a phospho-mimic variant of CC1N, was substantially lower than CC1NWT (Fig. 5, C and D), indicating that CC1 phosphorylation at the six residues reduces the affinity of CC1 to microtubules. This is probably caused by an increase in negative charges in CC1N6E that may repel microtubule surfaces that are also negatively charged. In contrast to CC1N6E, the CC1N6A engaged with microtubules in a similar affinity as CC1NWT (fig. S7), most likely because the recombinant CC1NWT proteins purified from Escherichia coli exist in a dephosphorylated form due to the absence of its kinase.
For the microtubule-bundling assay, prepolymerized and stabilized microtubules were incubated with each CC1 variant and then centrifuged at a low speed. Without incubation with CC1NWT, most single microtubules were retained in the supernatant, while a certain amount of aggregated microtubules were precipitated. However, the abundance of microtubules in the pellet increased after addition of CC1NWT (Fig. 5, E and F), indicating that CC1NWT promotes microtubule aggregation. By contrast, probably due to the reduced affinity to microtubules, CC1N6E did not induce microtubule aggregation (Fig. 5, E and F). The difference between CC1NWT and CC1N6E in promoting microtubule aggregation was confirmed via rhodamine-labeled microtubule imaging (Fig. 5G). These results indicated that CC1 phosphorylation affects its ability to engage with and bundle microtubules.
fer-4 and cc1 cc2 mutants exhibit a similar defect in cortical microtubule regulation under salt stress
cc1 cc2 double mutant seedlings display abnormal cortical microtubule arrangements under salt stress, i.e., rapid cortical microtubule dissociation and defects in reassembly (28), suggesting that CCs are critical for the reorganization of cortical microtubules under salt stress. To investigate whether FER has a similar role as CCs in regulating cortical microtubule organization under salt stress, we generated PDF1pro::mCherry-MBD/WT, PDF1pro::mCherry-MBD/cc1 cc2, and PDF1pro::mCherry-MBD/fer-4 plants. MBD is derived from mammalian MAP4 that has been widely used to trace microtubule behavior in plants (21). In this assay, we chose to observe cells in both the upper (about 3 to 8 cells from apical hook) and middle (about 9 to 15 cells from apical hook) regions of etiolated hypocotyls (Fig. 6A). Without salt stress, we did not observe any obvious differences in cortical microtubule organization among WT, fer-4, and cc1 cc2 mutant cells (Fig. 6, B to E). Following 2 hours of salt exposure, the cortical microtubule array was depolymerized in the middle region cells of the WT hypocotyls (Fig. 6, B and C). However, this change was more prominent in both the cc1 cc2 and fer-4 mutants, with obvious microtubule fragmentation and reduced microtubule density (Fig. 6, B and C). Prolonged salt exposure (72 hours) led to a substantial loss in microtubule array density in the middle part of hypocotyls in all the three genotypes (Fig. 6, B and C). By contrast, in the cells of the upper region of hypocotyls, 2 hours of NaCl treatment only slightly perturbed cortical microtubule arrays in the WT, cc1 cc2, and fer-4 plants (Fig. 6, D and E). After 72 hours of NaCl treatment, cortical microtubule arrays were well organized in the WT, but the arrays were sparser in both mutants (Fig. 6, D and E). These results indicated that both FER and CC1/CC2 function in the regulation of cortical microtubule behavior/organization during salt stress.
Fig. 6. FER-mediated CC1 phosphorylation is required for cortical microtubule organization under salt stress.
(A) Cartoon depicting the hypocotyl of Arabidopsis seedling grown in the dark. For cortical microtubule imaging, the cells in the upper and middle regions of hypocotyls were observed. (B) Representative cortical microtubule networks in the middle region of etiolated hypocotyls after NaCl treatment for 0, 2, and 72 hours, respectively. Scale bars, 10 μm. (C) Quantification of the density of cortical microtubule networks shown in (B). (D) Representative cortical microtubule networks in the upper region of etiolated hypocotyls after NaCl treatment for 0, 2, and 72 hours, respectively. Scale bars, 10 μm. (E) Quantification of the density of cortical microtubule networks shown in (D). (F) Representative cortical microtubule networks in the upper region of hypocotyls after NaCl treatment for 0, 2, and 72 hours. Scale bars, 10 μm. (G) Quantification of the density of cortical microtubule networks shown in (F). The cortical microtubule density of the representative cells shown in (B), (D), and (F) are marked by red asterisks in the histograms of (C), (E), and (G). Data in (C), (E), and (G) are shown as means ± SD of cells from three independent experiments, and the exact numbers of cells examined in each sample are indicated in the parentheses. Different letters indicate statistically significant differences (P < 0.01, one-way ANOVA). (H) Analysis of CC1 phosphorylation under salt stress. Venus-CC1 proteins were immunoprecipitated from 8-day-old seedlings after NaCl treatment for the indicated time points, and immunoblottings were performed using anti-pSer and anti-GFP antibodies. (I) Analysis of CC1 phosphorylation in the WT and fer-4 mutant after NaCl treatment for 0, 2, 8, and 28 hours. The sample treated with λPPase was used as a negative control.
To underscore the relevance of CC1 phosphorylation facilitated by FER in controlling cortical microtubule organization under salt stress, PDF1pro::mCherry-MBD was introduced also into CC1pro::Venus-CC1WT/cc1 cc2, CC1pro::Venus-CC16A/cc1 cc2, and CC1pro::Venus-CC16D/cc1 cc2 plants. Similar to the observation above, microtubules were disorganized in all three lines after 72 hours of salt treatment in cells of the middle region of hypocotyls (fig. S8). By contrast, in cells of the upper region of hypocotyls, cortical microtubules in the Venus-CC1WT/cc1 cc2 transgenic plants showed significant recovery following a 72-hour NaCl exposure (Fig. 6, F and G). However, we did not observe a similar recovery in both Venus-CC16A/cc1 cc2 and Venus-CC16D/cc1 cc2 transgenic plants (Fig. 6, F and G), indicating that both CC1 phosphorylation and dephosphorylation are important for the organization of cortical microtubules under salt stress.
CC1 phosphorylation is dynamically regulated under salt stress
Our data indicated that CC1 trafficking and cortical microtubules are tightly regulated under salt stress, and it is likely that these processes require dynamic changes of CC1 phosphorylation. To assess how CC1 phosphorylation changes during salt exposure, we enriched Venus-CC1 at different intervals following NaCl treatment and probed it with an anti-pSer antibody. After 2 hours of salt exposure, the phosphorylation level of CC1 was substantially decreased compared with the control, i.e., steady-state level. However, after 8 hours of treatment, the CC1 phosphorylation level was partially recovered and was fully restored to the steady-state level after 28 hours treatment (Fig. 6H), indicating a dynamic change of CC1 phosphorylation during salt stress exposure. In the fer-4 mutant, the phosphorylation level of CC1 was much lower than the WT under normal conditions, and it was further reduced after 2 hours of NaCl treatment (Fig. 6I). Notably, in contrast to the WT, the phosphorylation level of CC1 in the fer-4 mutant could not be restored to the steady-state level after a 28-hour period of salt stress (Fig. 6I), indicating that FER is essential for the phosphorylation of CC1 during prolonged salt stress. These findings indicated that FER participates in the dynamic regulation of CC1 phosphorylation under salt stress, which may ultimately contribute to the temporal regulation of CC1 behavior and cortical microtubule organization in response to salt stress.
DISCUSSION
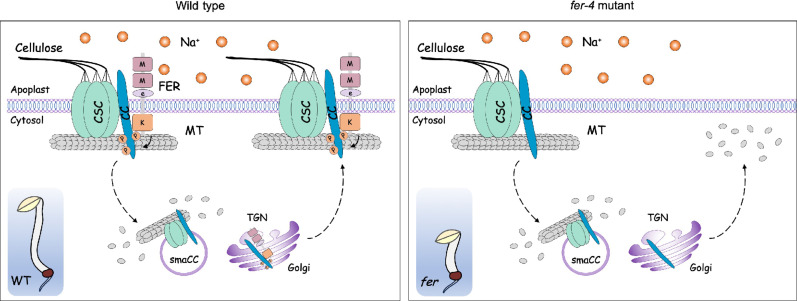
Cell wall integrity is an important factor that not only governs plant growth and development but also equips plants with the ability to withstand environmental stresses (10, 42). Under harsh conditions, such as high salinity, drought, and temperature stress, the cell wall needs to be extensively remodeled, which is critical for improving plant adaptation to unfavorable conditions (10, 43). Nevertheless, cell wall remodeling relies on the precise monitoring of cell wall status to avoid the loss of cell wall integrity. Now, molecular mechanisms underlying the regulation of cell wall integrity under salt stress remain largely unknown. In this study, we found that the putative cell wall sensor FER controls CC-mediated cortical microtubule organization during salt stress through direct phosphorylation (Fig. 7), which uncovers a molecular mechanism of cell wall integrity regulation under salt stress.
Fig. 7. Proposed model for the regulation of cortical microtubule via FER-CC module under salt stress.
FER controls cortical microtubule organization under salt stress through the regulation of CC1 phosphorylation. Under normal conditions, FER-mediated CC1 phosphorylation may facilitate the movement of CSCs along cortical microtubules and thus promotes cellulose biosynthesis. Upon exposure to salt stress, dephosphorylation of CC1 triggers its internalization with CSCs into smaCCs and TGN/Golgi, and meanwhile cortical microtubules are depolymerized. At the growth recovery stage after salt stress treatment, recycled or newly synthesized CC1 proteins are rephosphorylated by FER, allowing CC1 to return back to the plasma membrane and reassemble cortical microtubules. In the fer-4 mutant, the phosphorylation of CC1 cannot be dynamically regulated, and thus CC1 trafficking to the plasma membrane is disrupted under salt stress, leading to defects in microtubule reorganization and reduced hypocotyl growth under salt stress.
When exposed to salt stress, plants typically exhibit three stages of responses, i.e., an initial salt stress sensing and signal transduction phase, a growth stop and quiescence phase, and a growth recovery phase (44). These different stages are closely linked to changes in cortical microtubule organization and cellulose biosynthesis. During the growth stop and quiescence phase, CSCs and CC1/CC2 are internalized along with the depolymerization of cortical microtubules (28). Depolymerization of cortical microtubules is important for plant salt tolerance (30, 31, 33, 35). For instance, disruption of MAP65-1, an MAP that promotes the depolymerization of cortical microtubules, results in increased sensitivity to salt stress (33). Our data showed that the salt-induced depolymerization of cortical microtubules was not affected in fer-4 and cc1 cc2 mutants, implying that their hypersensitivity to salt stress is not due to a defect in microtubule depolymerization. During the growth recovery stage, cortical microtubules reassemble into new “stress-tolerant” arrays, and CSCs return to the plasma membrane to resume cellulose synthesis (28). Here, a precise level of CC1 phosphorylation is needed for correct CC1 trafficking dynamics as both CC16A and CC16D displayed defects and failed to reassemble cortical microtubules at the recovery stage. It is plausible that FER adjusts the CC1 phosphorylation to assure coordinated trafficking and microtubule array behavior. FER also controls recycling of other plasma membrane–localized proteins, such as brassinosteroid (BR) receptor BR INSENSITIVE1 (BRI1), FLS2, and PIN-FORMED (PIN) auxin transporter (22, 39, 45), suggesting a general role of FER in modulating protein trafficking. Given that FER and CC1 are also colocalized in the TGN, it is still unclear whether FER phosphorylates CC1 in the TGN or at the plasma membrane to regulate CC1 function.
Clathrin-mediated endocytosis (CME) is responsible for the internalization of many plasma membrane–localized proteins (46), such as PIN2 and BRI1 (47, 48). BRI1 directly interacts with adaptor protein complex 2 (AP-2), a key complex of the CME pathway, and a mutation in the recognition motif of AP-2 results in BRI1 endocytosis defects (49). Another example of phosphorylation-based internalization is natural resistance-associated macrophage protein 1 (NRAMP1), an important metal transporter (50). It is plausible that the phosphorylation status of CC1 might control its internalization via CME. Instead of AP-2, the CSC and therefore also CC1 are mainly endocytosed by the TPLATE complex (51). Perhaps the phosphorylation level of CC1 here represents a way for the TPLATE complex to regulate the CSC at the plasma membrane.
Four hydrophobic regions of CC1 are proposed to underpin the interactions between CC1 and microtubules (37). Here, we found that the phosphorylation of six Ser/Thr residues in the second and third hydrophobic regions of CC1 protein prevents its binding to microtubules, further supporting the role of the hydrophobic regions of CC1 binding to microtubules. During cellulose synthesis, CC1 and CC2 associate with the CESAs in the CSC and move along cortical microtubules to support direct cell expansion. Our data imply that, under nonstressed conditions, or during the growth recovery stage after salt treatment, CC1 exists in a phosphorylated state, which should reduce its interaction with cortical microtubules. We hypothesize that a low affinity between CC1 and microtubules may support a smooth movement of CSCs along microtubules, a connection that is then largely facilitated by CSI1 (29). Upon salt stress, the CC1, and perhaps CC2, becomes dephosphorylated to increase their interactions with the microtubules, presumably to aid in the bundling and stabilization of the microtubules to build a new stress-adopted array. The phosphorylation-dephosphorylation of CC1 and CC2 may therefore need to be coordinated. The identification of phosphatases that work in parallel to FER to control this modification would be important to fully elucidate this mechanism.
Our data uncovered that cc1 cc2 and fer-4 mutations conferred additive effects on hypocotyl elongation under normal conditions and high salinity. It is here possible that the biological functions of CC1 and CC2 are not completely lost in the fer-4 mutant. This hypothesis is supported by our data showing that residual CC1 phosphorylation was detected in the fer-4 mutant. Perhaps other CrRLK1L subfamily proteins, such as THE1 and HERK1 that also participate in regulating salt tolerance (8), may also contribute to the phosphorylation of CC1/CC2. Another possibility is that FER might also regulate cell wall biosynthesis in a CC1/CC2-independent manner. In our FER-GFP IP-MS data, several other CSC-associated proteins, including CESA3 and CSI1, were immunoprecipitated together with FER-GFP (fig. S1A). Notably, a previous large-scale phosphoproteomics study revealed that alterations in the phosphorylation levels of CC1, CESA1, CESA3, and CSI1 are significantly reduced in the fer-4 mutant (52), indicating that FER might phosphorylate other CSC-related components to regulate cellulose synthesis. A recent study reported that FER associates with CSI1 to negatively regulate immunity in roots (53). These results imply that FER may play a broader role in regulating cellulose synthesis. Further investigation is needed to validate any physical interactions of FER with CESAs and CSI1 as well as possible phosphorylation of these proteins by FER. This could constitute a more intricate regulation of cell wall synthesis via a major cell wall integrity sensor. In addition, we cannot rule out that CC1/CC2 may regulate salt tolerance independently of FER regulation, and this notion needs to be further investigated.
As the front line of environmental communication, cell wall elements are crucial in helping plants cope with various environmental stresses, not only by strengthening the cell wall but also by transducing cell wall signals to activate intracellular stress responses. While this is a complex process, FER appears to have an important role that allows plants to efficiently control trade-off between plant growth and stress responses and thereby avoid excessive inhibition of growth and overactivation of stress responses. With the discovery of an FER-controlled cortical microtubule disassembly-reassembly process via CC1/CC2, we outline that one such mechanism is important for plant salt tolerance.
MATERIALS AND METHODS
Plant materials and growth conditions
All plants were in the Columbia-0 (Col-0) ecotype in this study. fer-4 and cc1 cc2 mutants and FERpro::FER-GFP transgenic plants were described in previous studies (16, 28). cc1 cc2 was crossed with fer-4 to generate cc1 cc2 fer-4 mutants. CC1pro::Venus-CC1/cc1 cc2, CC1pro::Venus-CC16A/cc1 cc2, and CC1pro::Venus-CC16D/cc1 cc2 transgenic plants were generated by Agrobacterium-mediated transformation. CC1pro::Venus-CC1/cc1 cc2 fer-4 plants were generated via the crossing of CC1pro::Venus-CC1/cc1 cc2 with the fer-4 mutant.
Seeds were sterilized and sown on 1/2 Murashige and Skoog (MS) media containing 0.8% agar and 2.35 mM MES (Sigma-Aldrich, V900336) at pH 5.7 and stratified for at least 2 days at 4°C. For hypocotyl length measurement, seedlings were grown on 1/2 MS media and 1/2 MS media supplemented with 300 nM oryzalin or 2 nM isoxaben for 4 days in the dark, and hypocotyl length was quantified using ImageJ. For hypocotyl width measurement, images were captured using an M205 FA fluorescence stereomicroscope (Leica), and hypocotyl width was quantified using ImageJ. For cell aspect ratio measurement, hypocotyls were stained for 30 min using a propidium iodide solution (25 mg/ml), and images were captured using a TCS SMD confocal laser scanning microscope (Leica). Cell length and width were measured using ImageJ (https://imagej.net/, RRID:SCR_003070). To analyze hypocotyl growth under salt stress, seedlings were grown on 1/2 MS media and 1/2 MS media supplemented with 120 mM NaCl for 9 days in the dark, and hypocotyl length, hypocotyl width, and cell aspect ratio were quantified using ImageJ.
Plasmid construction
To construct the CC1pro::Venus-CC1 plasmid, the native CC1 promoter (4899 bp upstream of the start codon of CC1), full-length CC1 sequence, and the native CC1 terminator (444 bp downstream of the stop codon of CC1) were amplified from Col-0 genomic DNA and cloned into GreenGate entry vectors and then assembled with N-Venus tag, D-dummy, and hygromycin resistance cassettes following the instructions as previously reported (54). CC1pro::Venus-CC16A and CC1pro::Venus-CC16D constructs were generated by polymerase chain reaction–based point mutation. The CC1pro::mScarlet-CC1 plasmid was constructed in a similar way as CC1pro::Venus-CC1, except that the mScarlet sequence was cloned into the pGGB000 entry vector and used as an N-terminal tag.
For BiFC assay, the full-length CC1 (1 to 342 amino acid), the N terminus (1 to 120 amino acid), and the C terminus (121 to 342 amino acid) of CC1, CC2, and FERK565R were amplified and cloned into BiFC vectors using the Gateway cloning technology (Thermo Fisher Scientific). For split-LUC assay, the CDS of FER was amplified and cloned into the pCambia1300-nLUC vector using the Gateway cloning technology, and the CDS of CC1 was amplified from cDNAs and cloned into the pCambia1300-cLUC vector using the ClonExpress II One Step Cloning Kit (Vazyme, C112).
To generate the PDF1pro::mCherry-MBD plasmid, the PDF1 promoter (1456 bp upstream of the start codon of PDF1) was amplified from the PDF1pro::mCitrine-MBD line (23), and the MBD (55) was amplified and cloned into GreenGate entry vectors (54). The destination vector was assembled with the PDF1 promoter, N-mCherry-linker, MBD domain, D-dummy, UBQ10 terminator, and kanamycin (for the cc1 cc2 mutant background) or basta resistance (for the Col-0 and fer-4 mutant). The PDF1pro::mCherry-MBD plasmid (kanamycin resistance) was also transformed into CC1pro::Venus-CC1/cc1 cc2, CC1pro::Venus-CC16A/cc1 cc2, and CC1pro::Venus-CC16D/cc1 cc2 plants to generate MBD marker lines. All GreenGate assemblies in this study were done using the Golden Gate Assembly kit (NEB, E1602S). Primers used for plasmid construction are listed in table S1.
Protein extraction and IP-MS analysis
Plant materials were ground to fine powder with a mortar and pestle in liquid nitrogen. A protein extraction buffer [50 mM Mops (pH 7.0), 2 mM EDTA (pH 7.0), and one tablet of cOmplete EDTA-free protease inhibitor cocktail (Roche, 04693132001) per 50 ml of extraction buffer] was added to ground samples at a ratio of 3:1 (v/v) and mixed well by vortexing. The homogenized mixture was centrifuged at 10,000g for 15 min at 4°C to remove the debris, and the obtained supernatant was centrifuged at 100,000g for 1 hour at 4°C to precipitate the microsomal fraction. The microsomal pellet was resuspended with 500 μl of a buffer [10 mM tris-HCl (pH 7.5), 150 mM NaCl, 0.5 mM EDTA, and 0.5% NP-40]. After immunoprecipitation with GFP-Trap Agarose beads (ChromoTek, gtma-20, RRID:AB_2631358), the samples were subjected to on-bead trypsin digestion according to the manufacturer’s instructions. The obtained peptides were desalted by using MicroSpin C18 (SEM SS18V) columns (The Nest Group). The desalted samples were dried in a speed-vacuum centrifuge for 2 hours and then dissolved with 10 μl of a buffer containing 2% acetonitrile and 0.05% trifluoroacetic acid, and 6-μl samples were loaded for LC-MS/MS analyses. All samples were analyzed on the Ultimate 3000 RSLCnano system (Thermo Fisher Scientific) coupled with the Q Exactive Plus Orbitrap mass spectrometer (Thermo Fisher Scientific) using the shotgun method. The data analysis was performed with Proteome Discoverer 2.3 (https://thermofisher.com/order/catalog/product/IQLAAEGABSFAKJMAUH, RRID:SCR_014477).
Immunoblotting and Co-IP assays
Eight-day-old seedlings were frozen and ground in liquid nitrogen. Proteins were extracted using a lysis buffer [50 mM tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1x cOmplete protease inhibitor cocktail]. The extraction solution was centrifuged at 14,000g and 4°C for 10 min, and the supernatant was boiled with a 1x SDS loading buffer. Proteins were separated by SDS–polyacrylamide gel electrophoresis (PAGE) using a 7.5% (w/v) acrylamide gel. Immunoblottings were performed using anti-GFP (1:3,000, Sigma-Aldrich, 11814460001, RRID:AB_390913) and anti-ACTIN (1:5,000, ABclonal, AC009, RRID:AB_2771701) antibodies, and goat anti-mouse horseradish peroxidase (HRP) conjugate (1:10,000, Bio-Rad, 1721011, RRID:AB_2617113) was used as a secondary antibody.
For Co-IP assays, total proteins were extracted from 8-day-old CC1pro::Venus-CC1/cc1 cc2 seedlings using the same lysis buffer described above. After centrifugation, the supernatant was incubated with anti-GFP magarose beads (Smart-Lifesciences, SM038001) at 4°C for 4 hours. The beads were washed twice with a wash buffer [50 mM tris-HCl (pH 7.5) and 150 mM NaCl], once with 1x phosphate-buffered saline (PBS), and boiled with a 1x SDS loading buffer for 10 min. Immunoblottings were performed using anti-GFP and anti-FER (1:2,000, our lab) antibodies. A goat anti-mouse HRP conjugate or goat anti-rabbit HRP conjugate (1:10,000, Bio-Rad, 1721019, RRID:AB_11125143) was used as secondary antibodies. The anti-FER antibody was produced by ABclonal Technology (Wuhan, China) using the antigen of the 28 to 300 amino acid of the FER protein fused with glutathione S-transferase (GST), and the polyclonal antibody was produced in rabbit.
BiFC and split-LUC assays
Agrobacteria carrying the indicated plasmids were cultured overnight at 28°C in a liquid LB medium, and then agrobacterial cells were spun down and resuspended in an infiltration buffer [10 mM MgCl2, 10 mM MES (pH 5.7), and 200 μM acetosyringone] with a final concentration of OD600 (optical density at 600 nm) = 0.5. After incubation at room temperature for 3 hours in the dark, agrobacterial cultures were infiltrated into N. benthamiana leaves, and fluorescence was detected after infiltration for 48 hours. For BiFC assay, the leaves were detached and imaged using a TCS SMD FLCS confocal laser scanning microscope (Leica). For split-LUC assay, luciferin was injected into N. benthamiana leaves and fluorescence was detected using a NightShade LB985 (Berthold).
Recombinant protein expression and in vitro kinase assay
Recombinant His-FERCD was purified as described previously (17). For recombinant GST-CC1N (the N terminus of CC1, 1 to 120 amino acid) purification, the CC1N protein was fused to GST by cloning into the expression vector pGEX4T-1, and the constructed plasmid was transformed into strain BL21 (WEDI, EC1001). After induction overnight at 16°C with 1 mM isopropyl-β-d-thiogalactopyranoside, the cell culture was centrifuged and the pellet was resuspended in a lysis buffer [50 mM Hepes, 300 mM NaCl, and 1 mM tris(2-carboxyethyl)phosphine (TCEP) at pH 7.5], and then the strains were lysed using a high-pressure homogenizer. The mixture was centrifuged at 14,000g and 4°C for 40 min, and then the supernatant was incubated at 4°C for 1 hour with a Glutathione Sepharose 4B (GE HealthCare Life Sciences, 17-0756-05). After incubation, the recombinant protein was eluted with a lysis buffer containing 10 mM l-glutathione reduced (Sigma-Aldrich, V900456) at 4°C.
For in vitro kinase assay, the recombinant GST-CC1NWT or GST-CC1N6A was incubated with His-FERCD in a 25-μl kinase buffer [50 mM Hepes (pH 7.5), 1 mM MgCl2, 1 mM DTT, and 1 mM adenosine triphosphate] at room temperature for 30 min. The sample treated with 2 μl of λPPase (400,000 units/ml) (New England Biolabs, P0753S), 2.5 μl of a 10x NEBuffer Pack for Protein MetalloPhosphatases (PMP) buffer, and 2.5 μl of 10 mM MnCl2 was used as a control. After incubation, the samples were boiled with a 1x SDS loading buffer for 10 min. Proteins were separated by SDS-PAGE using a 10% (w/v) acrylamide gel. Immunoblottings were performed using an anti-pSer antibody (1:125, Abcam, ab9332, RRID:AB_307184). The loading of recombinant CC1N and FERCD was detected by CBB staining.
In vivo phosphorylation assay
Seeds were sown on a 1/2 MS medium containing 0.8% agar, 1% sucrose, and 2.35 mM MES at pH 5.7 and grown under long-day conditions for 8 days. Proteins were extracted using a lysis buffer [50 mM tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1 mM DTT, 1 mM PMSF, 1x cOmplete protease inhibitor cocktail, and 1x phosphatase inhibitor cocktail set II (Millipore, 524625)]. The extraction was centrifuged twice at 14,000g and 4°C for 10 min, and then the supernatant was incubated with anti-GFP magarose beads at 4°C for 4 hours. The beads were washed four times with a washing buffer [50 mM tris-HCl (pH 7.5), 150 mM NaCl, and 0.1% Triton X-100] and twice with 1x PBS and then resuspended in 25 μl of 1x PBS. As a control, a sample was incubated in a solution containing 2 μl of λPPase, 2.5 μl of a 10x PMP buffer, and 2.5 μl of 10 mM MnCl2. After incubation, the samples were boiled with a 1x SDS sample buffer for 10 min. Proteins were separated by SDS-PAGE using a 7.5% (w/v) acrylamide gel. Immunoblottings were performed using anti-pSer, anti-pThr (1:1000, Cell Signaling Technology, 9386, RRID:AB_331239), and anti-GFP antibodies.
Sequence analysis of CC1
The amino acid sequence of Arabidopsis thaliana CC1 was used to conduct Basic Local Alignment Search Tool (BLAST) analysis in the NCBI (National Center for Biotechnology Information) database (https://blast.ncbi.nlm.nih.gov/, RRID:SCR_006472) to search for CC1 homologs in representative plant species, including Arabidopsis lyrata, Populus trichocarpa, Glycine max, Solanum tuberosum, Oryza sativa, Zea mays, Sorghum bicolor, Amborella trichopoda, and Marchantia polymorpha. The protein sequence alignment was done by using Clustal Omega (https://ebi.ac.uk/Tools/msa/clustalo/, RRID:SCR_001591), and GeneDoc 2.7 (https://softpedia.com/get/Science-CAD/GeneDoc.shtml) was used to visualize the alignment result. The aligned sequences were used to generate sequence logos using WEBLOGO (https://weblogo.berkeley.edu/, RRID:SCR_010236).
Live cell imaging and imaging analysis
The in planta live cell imaging was conducted according to the protocol described previously (56). Three-day-old etiolated hypocotyls were used for imaging. For salt treatment, hypocotyls were transferred to an MS medium supplemented with 200 mM NaCl and then wrapped with aluminum foil and grown in the dark for the indicated time. Before imaging, samples were mounted in Milli-Q water and covered with 0.8% agarose pad (Bioline). All live cell imaging was performed with a 3i Marianas CSU-W1 SoRa spinning disk confocal microscope and 100× numerical aperture 1.49 Apo total internal reflection fluorescence oil-immersion objective (Nikon). The mCherry- and Venus-labeled lines were excited at 561 and 515 nm, respectively. Images were recorded using a 3i SlideBook (https://intelligent-imaging.com/slidebook.php, RRID:SCR_014423).
To analyze the movement trajectories of the CC1 variant, time-lapse images were taken with 10-s intervals for 10 min. The background was subtracted using the “Subtract Background” tool (rolling ball radius, 30 to 50 pixels), and the “StackReg” plugin (57) was used to correct focal drift in Fiji (https://imagej.net/software/fiji/, RRID:SCR_002285) (58). The average intensity projections of time-lapse images were generated to visualize movement trajectories.
To monitor the CC1 behavior under salt stress, images of the Venus-tagged CC1 variants were acquired at the plasma membrane/cortex focal plane. To quantify the plasma membrane CC1 density, the “ThunderSTORM” plugin (59) was first used to extract individual foci of CC1 with default settings in Fiji (58). Then, the Golgi/TGN-localized CC1 and smaCCs were excluded, i.e., only foci with the following parameters were considered as plasma membrane–localized CC1: a nonzero offset, a localization error (uncertainty) between 5 and 60 nm, a sigma of 50 to 500 nm, and an intensity below 500,000 (integrated value) (60). The density was calculated by dividing the resulting puncta count by the area of region of interest (puncta/μm2). For each biological replicate and each time point, ~20 cells from four to five etiolated hypocotyls were quantified. Three independent biological replicates were conducted.
To monitor microtubule networks under salt stress, Z-stack imaging (0.3-μm spacing) was acquired along the upper and middle regions of hypocotyls. The background was subtracted using the Subtract Background tool (rolling ball radius, 50 pixels) in Fiji. The average intensity projection of Z-stack images was generated in Fiji for visualization and quantification of microtubule networks. The quantification of the microtubule density was performed as described in previous publications (61–63). A 10-μm line was drawn vertically to the orientation of most microtubules in the cell, and the number of microtubules across this line was quantified. Three lines were drawn for each cell, and the average value was calculated and considered as numbers of microtubules per micrometer. For each biological replicate and each time point, ~10 cells from three etiolated hypocotyls were imaged and quantified. Three biological replicates were conducted for microtubule imaging under salt stress.
To check the in planta colocalization of FER and CC1, the 3-day-old etiolated hypocotyls of dual-labeled mScarlet-CC1/FER-GFP transgenic plants were analyzed. GFP and mScarlet signals were excited at 488 and 561 nm, respectively. Line scan analysis was performed using the “Plot Profile” tool in Fiji to reflect the colocalization of mScarlet-CC1 and FER-GFP.
Quantification and statistical analysis
Statistical analyses were carried out by Student’s t test (two-sided) for two groups and one-way analysis of variance (ANOVA) followed by Duncan’s analysis for multiple groups. All graphs were generated by GraphPad Prism 10 (http://graphpad.com/, RRID:SCR_002798).
Acknowledgments
We would like to thank O. Hamant for providing PDF1pro:mCitrine-MBD seeds. We would like to thank the Center for Advanced Bioimaging (CAB) Denmark for the microscopy support.
Funding: X.L. was supported by the China Postdoctoral Science Foundation (grant no. 2023M743492), Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (grant no. GZC20241718), the Shanghai “Super Postdoctoral” Incentive Program (grant no. 2032643), and the National Natural Science Foundation of China (grant no. 32400242). S.P. was funded by a Villum, two Novo Nordisk, and Danish National Research Foundation grants (25915, 19OC0056076, 20OC0060564, and DNRF155, respectively). C.Z. was supported by the National Key Research and Development Program of China (grant no. 2023YFF1002100), the National Natural Science Foundation of China (grant nos. 32070295 and 32270283), the Science and Technology Commission of Shanghai Municipality (grant no. 22ZR1469600), the Key Laboratory of Plant Design, and the National Key Laboratory of Plant Molecular Genetics.
Author contributions: S.P. and C.Z. conceived and designed the experiments. X.L., L.W., L.L., Y.Li, M.O., M.S., Y.Liu, Y.Z., and M.R. performed experiments. X.L. and L.L. performed phenotypic analyses. X.L. performed biochemical assays. L.W. performed in vitro microtubule assays and cellular imaging analyses. X.L. and L.W. performed proteomics analyses. X.L., L.W., S.P., and C.Z. analyzed data and wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The MS proteomics data are available via ProteomeXchange (http://proteomexchange.org, RRID:SCR_004055) with identifiers PXD047667, PXD047595, and PXD047608. The Arabidopsis reference genome (TAIR10) database (https://arabidopsis.org/) was used in this study.
Supplementary Materials
This PDF file includes:
Figs. S1 to S8
Table S1
REFERENCES AND NOTES
- 1.Zhang H., Zhu J., Gong Z., Zhu J.-K., Abiotic stress responses in plants. Nat. Rev. Genet. 23, 104–119 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Liu J., Zhang W., Long S., Zhao C., Maintenance of cell wall integrity under high salinity. Int. J. Mol. Sci. 22, 3260 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosgrove D. J., Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6, 850–861 (2005). [DOI] [PubMed] [Google Scholar]
- 4.O’Driscoll D., Read S. M., Steer M. W., Determination of cell-wall porosity by microscopy: Walls of cultured cells and pollen tubes. Acta Bot. Neerl. 42, 237–244 (1993). [Google Scholar]
- 5.Wu H.-C., Bulgakov V. P., Jinn T.-L., Pectin methylesterases: Cell wall remodeling proteins are required for plant response to heat stress. Front. Plant Sci. 9, 1612 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okawa R., Hayashi Y., Yamashita Y., Matsubayashi Y., Ogawa-Ohnishi M., Arabinogalactan protein polysaccharide chains are required for normal biogenesis of plasmodesmata. Plant J. 113, 493–503 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Wang Z., Wang M., Yang C., Zhao L., Qin G., Peng L., Zheng Q., Nie W., Song C.-P., Shi H., Zhu J.-K., Zhao C., SWO1 modulates cell wall integrity under salt stress by interacting with importin ɑ in Arabidopsis. Stress Biol. 1, 9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gigli-Bisceglia N., van Zelm E., Huo W., Lamers J., Testerink C., Arabidopsis root responses to salinity depend on pectin modification and cell wall sensing. Development 149, dev200363 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng W., Kita D., Peaucelle A., Cartwright H. N., Doan V., Duan Q., Liu M.-C., Maman J., Steinhorst L., Schmitz-Thom I., Yvon R., Kudla J., Wu H.-M., Cheung A. Y., Dinneny J. R., The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 28, 666–675.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colin L., Ruhnow F., Zhu J.-K., Zhao C., Zhao Y., Persson S., The cell biology of primary cell walls during salt stress. Plant Cell 35, 201–217 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaahtera L., Schulz J., Hamann T., Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 5, 924–932 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Franck C. M., Westermann J., Boisson-Dernier A., Plant malectin-like receptor kinases: From cell wall integrity to immunity and beyond. Annu. Rev. Plant Biol. 69, 301–328 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Li C., Yeh F.-L., Cheung A. Y., Duan Q., Kita D., Liu M.-C., Maman J., Luu E. J., Wu B. W., Gates L., Jalal M., Kwong A., Carpenter H., Wu H.-M., Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife 4, e06587 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan Q., Liu M.-J. J., Kita D., Jordan S. S., Yeh F.-L. J., Yvon R., Carpenter H., Federico A. N., Garcia-Valencia L. E., Eyles S. J., Wang C.-S., Wu H.-M., Cheung A. Y., FERONIA controls pectin- and nitric oxide-mediated male-female interaction. Nature 579, 561–566 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Stegmann M., Monaghan J., Smakowska-Luzan E., Rovenich H., Lehner A., Holton N., Belkhadir Y., Zipfel C., The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355, 287–289 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Zhao C., Zayed O., Yu Z., Jiang W., Zhu P., Hsu C. C., Zhang L., Tao W. A., Lozano-Duran R., Zhu J. K., Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 115, 13123–13128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X., Jiang W., Li Y., Nie H., Cui L., Li R., Tan L., Peng L., Li C., Luo J., Li M., Wang H., Yang J., Zhou B., Wang P., Liu H., Zhu J.-K., Zhao C., FERONIA coordinates plant growth and salt tolerance via the phosphorylation of phyB. Nat. Plants 9, 645–660 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Zhu S., Fu Q., Xu F., Zheng H., Yu F., New paradigms in cell adaptation: Decades of discoveries on the CrRLK1L receptor kinase signalling network. New Phytol. 232, 1168–1183 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Lin W., Tang W., Pan X., Huang A., Gao X., Anderson C. T., Yang Z., Arabidopsis pavement cell morphogenesis requires FERONIA binding to pectin for activation of ROP GTPase signaling. Curr. Biol. 32, 497–507.e4 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Zhao C., Jiang W., Zayed O., Liu X., Tang K., Nie W., Li Y., Xie S., Li Y., Long T., Liu L., Zhu Y., Zhao Y., Zhu J.-K., The LRXs-RALFs-FER module controls plant growth and salt stress responses by modulating multiple plant hormones. Natl. Sci. Rev. 8, nwaa149 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang W., Lin W., Zhou X., Guo J., Dang X., Li B., Lin D., Yang Z., Mechano-transduction via the pectin-FERONIA complex activates ROP6 GTPase signaling in Arabidopsis pavement cell morphogenesis. Curr. Biol. 32, 508–517.e3 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Li E., Wang G., Zhang Y.-L., Kong Z., Li S., FERONIA mediates root nutating growth. Plant J. 104, 1105–1116 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Malivert A., Erguvan O., Chevallier A., Dehem A., Friaud R., Liu M., Martin M., Peyraud T., Hamant O., Verger S., FERONIA and microtubules independently contribute to mechanical integrity in the Arabidopsis shoot. PLOS Biol. 19, e3001454 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFarlane H. E., Döring A., Persson S., The cell biology of cellulose synthesis. Annu. Rev. Plant Biol. 65, 69–94 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Paredez A. R., Somerville C. R., Ehrhardt D. W., Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312, 1491–1495 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Anderson C. T., Kieber J. J., Dynamic construction, perception, and remodeling of plant cell walls. Annu. Rev. Plant Biol. 71, 39–69 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Gu Y., Kaplinsky N., Bringmann M., Cobb A., Carroll A., Sampathkumar A., Baskin T. I., Persson S., Somerville C. R., Identification of a cellulose synthase-associated protein required for cellulose biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 107, 12866–12871 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endler A., Kesten C., Schneider R., Zhang Y., Ivakov A., Froehlich A., Funke N., Persson S., A mechanism for sustained cellulose synthesis during salt stress. Cell 162, 1353–1364 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Pedersen G. B., Blaschek L., Frandsen K. E. H., Noack L. C., Persson S., Cellulose synthesis in land plants. Mol. Plant 16, 206–231 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Fujita S., Pytela J., Hotta T., Kato T., Hamada T., Akamatsu R., Ishida Y., Kutsuna N., Hasezawa S., Nomura Y., Nakagami H., Hashimoto T., An atypical tubulin kinase mediates stress-induced microtubule depolymerization in Arabidopsis. Curr. Biol. 23, 1969–1978 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Galva C., Kirik V., Lindeboom J. J., Kaloriti D., Rancour D. M., Hussey P. J., Bednarek S. Y., Ehrhardt D. W., Sedbrook J. C., The microtubule plus-end tracking proteins SPR1 and EB1b interact to maintain polar cell elongation and directional organ growth in Arabidopsis. Plant Cell 26, 4409–4425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurepa J., Wang S., Smalle J., The role of 26S proteasome-dependent proteolysis in the formation and restructuring of microtubule networks. Plant Signal. Behav. 7, 1289–1295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou S., Chen Q., Li X., Li Y., MAP65-1 is required for the depolymerization and reorganization of cortical microtubules in the response to salt stress in Arabidopsis. Plant Sci. 264, 112–121 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q., Lin F., Mao T., Nie J., Yan M., Yuan M., Zhang W., Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis. Plant Cell 24, 4555–4576 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C., Li J., Yuan M., Salt tolerance requires cortical microtubule reorganization in Arabidopsis. Plant Cell Physiol. 48, 1534–1547 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Yang P., Jin J., Zhang J., Wang D., Bai X., Xie W., Hu T., Zhao X., Mao T., Qin T., MDP25 mediates the fine-tuning of microtubule organization in response to salt stress. J. Integr. Plant Biol. 64, 1181–1195 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Kesten C., Wallmann A., Schneider R., McFarlane H. E., Diehl A., Khan G. A., van Rossum B.-J., Lampugnani E. R., Szymanski W. G., Cremer N., Schmieder P., Ford K. L., Seiter F., Heazlewood J. L., Sanchez-Rodriguez C., Oschkinat H., Persson S., The companion of cellulose synthase 1 confers salt tolerance through a Tau-like mechanism in plants. Nat. Commun. 10, 857 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo H., Nolan T. M., Song G., Liu S., Xie Z., Chen J., Schnable P. S., Walley J. W., Yin Y., FERONIA receptor kinase contributes to plant immunity by suppressing jasmonic acid signaling in Arabidopsis thaliana. Curr. Biol. 28, 3316–3324.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Yu M., Li R., Cui Y., Chen W., Li B., Zhang X., Bu Y., Cao Y., Xing J., Jewaria P. K., Li X., Bhalerao R. P., Yu F., Lin J., The RALF1-FERONIA interaction modulates endocytosis to mediate control of root growth in Arabidopsis. Development 147, dev189902 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Morejohn L. C., Bureau T. E., Mole-Bajer J., Bajer A. S., Fosket D. E., Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 172, 252–264 (1987). [DOI] [PubMed] [Google Scholar]
- 41.Persson S., Wei H. R., Milne J., Page G. P., Somerville C. R., Identification of genes required for cellulose synthesis by regression analysis of public microarray data sets. Proc. Natl. Acad. Sci. U.S.A. 102, 8633–8638 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voxeur A., Höfte H., Cell wall integrity signaling in plants: “To grow or not to grow that’s the question”. Glycobiology 26, 950–960 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Yu B., Zheng W., Xing L., Zhu J.-K., Persson S., Zhao Y., Root twisting drives halotropism via stress-induced microtubule reorientation. Dev. Cell 57, 2412–2425.e6 (2022). [DOI] [PubMed] [Google Scholar]
- 44.van Zelm E., Zhang Y., Testerink C., Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 71, 403–433 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Liu M.-C. J., Yeh F.-L. J., Yvon R., Simpson K., Jordan S., Chambers J., Wu H.-M., Cheung A. Y., Extracellular pectin-RALF phase separation mediates FERONIA global signaling function. Cell 187, 312–330.E22 (2024). [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y., Persson S., Hirst J., Robinson M. S., van Damme D., Sánchez-Rodríguez C., Change your TPLATE, change your fate: Plant CME and beyond. Trends Plant Sci. 20, 41–48 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Kim S. Y., Xu Z.-Y., Song K., Kim D. H., Kang H., Reichardt I., Sohn E. J., Friml J., Juergens G., Hwang I., Adaptor protein complex 2–mediated endocytosis is crucial for male reproductive organ development in Arabidopsis. Plant Cell 25, 2970–2985 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Rubbo S., Irani N. G., Kim S. Y., Xu Z.-Y., Gadeyne A., Dejonghe W., Vanhoutte I., Persiau G., Eeckhout D., Simon S., Song K., Kleine-Vehn J., Friml J., De Jaeger G., Van Damme D., Hwang I., Russinova E., The clathrin adaptor complex AP-2 mediates endocytosis of BRASSINOSTEROID INSENSITIVE1 in Arabidopsis. Plant Cell 25, 2986–2997 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu D., Kumar R., Claus L. A. N., Johnson A. J., Siao W., Vanhoutte I., Wang P., Bender K. W., Yperman K., Martins S., Zhao X., Vert G., Van Damme D., Friml J., Russinova E., Endocytosis of BRASSINOSTEROID INSENSITIVE1 is partly driven by a canonical Tyr-based motif. Plant Cell 32, 3598–3612 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castaings L., Alcon C., Kosuth T., Correia D., Curie C., Manganese triggers phosphorylation-mediated endocytosis of the Arabidopsis metal transporter NRAMP1. Plant J. 106, 1328–1337 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Sánchez-Rodríguez C., Shi Y., Kesten C., Zhang D., Sancho-Andrés G., Ivakov A., Lampugnani E. R., Sklodowski K., Fujimoto M., Nakano A., Bacic A., Wallace I. S., Ueda T., Van Damme D., Zhou Y., Persson S., The cellulose synthases are cargo of the TPLATE adaptor complex. Mol. Plant 11, 346–349 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Wang P., Clark N. M., Nolan T. M., Song G., Bartz P. M., Liao C.-Y., Montes-Serey C., Katz E., Polko J. K., Kieber J. J., Kliebenstein D. J., Bassham D. C., Walley J. W., Yin Y., Guo H., Integrated omics reveal novel functions and underlying mechanisms of the receptor kinase FERONIA in Arabidopsis thaliana. Plant Cell 34, 2594–2614 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu G., Zhang L., Xue H., Chen Y., Liu X., del Pozo J. C., Zhao C., Lozano-Duran R., Macho A. P., Cell wall-mediated root development is targeted by a soil-borne bacterial pathogen to promote infection. Cell Rep. 43, 114179 (2024). [DOI] [PubMed] [Google Scholar]
- 54.Lampropoulos A., Sutikovic Z., Wenzl C., Maegele I., Lohmann J. U., Forner J., GreenGate—A novel, versatile, and efficient cloning system for plant transgenesis. PLOS ONE 8, e83043 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marc J., Granger C. L., Brincat J., Fisher D. D., Kao T.-H., McCubbin A. G., Cyr R. J., A GFP–MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell 10, 1927–1939 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McFarlane H. E., Mutwil-Anderwald D., Verbančič J., Picard K. L., Gookin T. E., Froehlich A., Chakravorty D., Trindade L. M., Alonso J. M., Assmann S. M., Persson S., A G protein-coupled receptor-like module regulates cellulose synthase secretion from the endomembrane system in Arabidopsis. Dev. Cell 56, 1484–1497.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thevenaz P., Ruttimann U. E., Unser M., A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A., Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ovesný M., Křížek P., Borkovec J., Švindrych Z., Hagen G. M., ThunderSTORM: A comprehensive ImageJ plug-in for PALM and STORM data analysis and super-resolution imaging. Bioinformatics 30, 2389–2390 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider R., Ehrhardt D. W., Meyerowitz E. M., Sampathkumar A., Tethering of cellulose synthase to microtubules dampens mechano-induced cytoskeletal organization in Arabidopsis pavement cells. Nat. Plants 8, 1064–1073 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C., Lu H., Li W., Yuan M., Fu Y., A ROP2-RIC1 pathway fine-tunes microtubule reorganization for salt tolerance in Arabidopsis. Plant Cell Environ. 40, 1127–1142 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Li J., Wang X., Qin T., Zhang Y., Liu X., Sun J., Zhou Y., Zhu L., Zhang Z., Yuan M., Mao T., MDP25, a novel calcium regulatory protein, mediates hypocotyl cell elongation by destabilizing cortical microtubules in Arabidopsis. Plant Cell 23, 4411–4427 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X., Qin T., Ma Q., Sun J., Liu Z., Yuan M., Mao T., Light-regulated hypocotyl elongation involves proteasome-dependent degradation of the microtubule regulatory protein WDL3 in Arabidopsis. Plant Cell 25, 1740–1755 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S8
Table S1