Abstract
Lead (Pb), a dense, soft, blue-gray metal, is widely used in metallurgy, cables, storage batteries, pigments, and other industrial applications. Pb has been shown to cause degenerative changes in the nervous system. Necroptosis, a form of non-apoptotic programmed cell death modality, is closely associated with neurodegenerative diseases. Whether the TNF-R1-RIPK1/RIPK3 pathway is involved in the neurodegeneration induced by Pb has yet to be determined. Here, we explored the role of the TNF-R1-RIPK1/RIPK3 signaling pathway in the Pb-induced necroptosis by using HT-22 cells, primary mouse hippocampal neurons, and C57BL/6 mice models, demonstrating that Pb exposure elevated lead levels in murine whole blood and hippocampal tissue in a dose-response relationship. Protein expression levels of PARP, c-PARP, RIPK1, p-RIPK1, RIPK3, MLKL, and p-MLKL in the hippocampal tissues were elevated, while the protein expression of caspase-8 was decreased. Furthermore, Pb exposure reduced the survival rates in HT-22 cells and primary mouse hippocampal neurons, while increasing the protein expressions of RIPK1 and p-MLKL. Collectively, these novel findings suggest that the TNF-R1/RIPK1/RIPK3 signaling pathway is associated with Pb-induced neurotoxicity in hippocampal neurons in mice.
Keywords: Lead, Mice, Hippocampus, TNF-R1-RIPK1/RIPK3, Necroptosis
1. Introduction
Lead (Pb), a class 2B carcinogen, is harmful to human health. Humans are exposed to Pb through diverse routes, for example, inhalation of contaminated air, exposure to Pb-containing dust and soil, and consumption of contaminated food and water [1, 2]. Long-term Pb exposure in humans increase the risk of cardiovascular and kidney disease[3, 4]. Pb can cause genotoxicity and hepatotoxicity in zebrafish at environmentally relevant concentrations (5 ppm)[5], and Pb (800μM) exposure has been shown to induce transgenerational developmental neurotoxicity in Drosophila Melanogaster[6], The adverse effects of Pb extend to multiple systems [7, 8], with the nervous system being highly susceptible to its toxic effects [9, 10].
Neurotoxicity induced by Pb is often characterized by memory and learning deficits, attention deficits and autism, posing a potential risk factor for neurodegenerative diseases[11–13]. In vivo investigations have demonstrated that perinatal intake of drinking water containing 0.1% Pb could triggered neuronal cell inflammation reactions in rats’ forebrain cortex, hippocampus and cerebellum[14] and impaired spatial learning and memory[15]. Epidemiological investigations have revealed that Pb exposure in the fetal and neonatal periods decreased glycogen levels and metabolic rate in neurons and astrocytes, reducing glucose utilization and impairing brain energy metabolism[16, 17]. Notably, the developing brain is extremely susceptible to Pb toxicity as it can readily cross the placenta and blood-brain barriers. In turn, Pb can alter neurodevelopmental pathways with long-lasting effects on motor skills, emotional well-being, social interactions and cognitive functions extending into adulthood[18]. The rising levels of environmental Pb have drawn attention and contemporary concern to its neurotoxic effects[19].
Numerous studies have delved into the intricate mechanisms of Pb neurotoxicity. Pb neurotoxicity was invoked to be mediated via GSK-3β and CDK5-dependent hyperphosphorylation of Tau protein, resulting in disruption of cytoskeletal stability[20]. It has been shown that Pb diminished the density of hippocampal dendritic spines by downregulating SNX6 and Homer1 expression, resulting in the impairments in learning and memory of rats[21]. Moreover, Pb can impact the cholinergic, dopaminergic and glucose metabolism systems, thereby disrupting normal neurotransmission [22]. Programmed cell death (PCD) plays a significant role in the neurotoxicity induced by Pb[23]. For example, in vivo studies have shown that Pb increased apoptosis in the cerebral cortex, hippocampus and cerebellum of rats, thereby exerting its neurotoxic effects[24]. Furthermore, Pb can cause a blockage of autophagic flow in PC12 cells, in turn, causing cell death[25]. Additionally, chronic low-level Pb exposure promoted ferroptosis in mice hippocampal neurons via activating microglia cells [26]. Overall, the mechanism of Pb neurotoxicity is multifactorial, including oxidative stress[19], apoptosis[27], autophagy dysregulation[28], epigenetic changes[11, 29], activation of the immune system by microglial and astrocytes[30], and altered calcium-dependent processes[31]. Despite these insights, the precise mechanism of Pb neurotoxicity remains incompletely understood.
Necroptosis is a mode of PCD, marked by cell membrane destruction, organelle swelling and release of inflammatory factors[32]. Under pathological conditions, TNF-α triggers the activation of its receptor, TNF-R1. Inhibition of the intracellular apoptosis factor caspase-8 triggers complex I formation, activating the phosphorylation of receptor and Receptor-Interacting Protein 1 (RIPK1). Subsequently, RIPK1 phosphorylates and activates Receptor-Interacting Protein 3 (RIPK3)[33], which in turn phosphorylates. Next, Mixed Lineage Kinase Domain-Like (MLKL) is phosphorylated and activated. Activated MLKL transfer aggregates to the plasma membrane, leading to plasma membrane damage[34, 35]. This, in turn, leads to cell rupture, with spillage of cellular contents into the extracellular space, ultimately leading to cell necroptosis[36–39]. Recent studies have highlighted the significant contribution of necroptosis to the pathogenesis and progression of neurodegenerative disease[36, 40, 41]. Additionally, it has been found that Pb causes necroptosis in lymphocytes and renal cells[42, 43]. Whether Pb leads to necroptosis in neurons and the mechanism of necrosis has yet to be determined. Herein, the present study aims to integrate in vitro and in vivo studies to address the contribution of necroptosis in Pb-induced neurodegeneration.
2. Materials and Methods
2.1. Cell Culture
HT22 cells were generously provided by Professor Zou Yunfeng’s research group at the School of Public Health, Guangxi Medical University. The cells were cultured in DMEM (Gibco, USA) containing 10% FBS (BI, ISR) and 1% penicillin/streptomycin(Solarbio, Beijing, China), and cultured under standardized conditions of 5% CO2 at 37°C[44]. Daily observation of cell morphology and growth were performed, with medium changes on alternate days. Once the cell density reached about 80–90%, the cells could be passaged or seed-plated for further treatment with Pb. At the end of each experiment, the total cellular protein was extracted for protein expression analysis.
2.2. MTT Colorimetric Assay
Cell viability was determined with the MTT(3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide) method. Briefly, HT-22 cell (5×103) was inoculated into a 96-well plate. The cells were then divided into 0, 1, 2.5, 5, 10, 25, 50, 100, 200, and 500 μmol/L Pb(Ac)2 groups, with each group having 6 duplicate wells. Following 24 hours of Pb exposure, the used medium was disposed, and fresh medium including 10 μL of 5mg/ml MTT solution (Beyotime, China) was added and cultured for 4 hours under standardized conditions of 5% CO2 at 370°C. At the end of the incubation, the medium was discarded. Subsequently, DMSO was added and the cells were cultured for 10 min. After incubation, The absorbance (OD value) of the cells in each well was measured at 490 nm using an enzyme meter (Thermo, USA). Cell viability was expressed as a percentage of untreated control cells[45].
2.3. Primary Hippocampal Neuron Culture
C57BL/6 mice (18 days pregnant) were anesthetized with 1.2% Avertin, and fetal mice were extracted via laparotomy. Subsequently, fetal mice were decapitated and transferred to dissection medium (DM) fluid (10×1L Hanks Balanced Salts, 0.35g NaHCO3, 10 mL HEPES, 6 g glucose, 100 μL gentamycin, 3 g BSA, 1.44 g MgSO4, NaOH adjusts pH to 7.3). Following that, the fetal mice’s brains were dissected out and the hippocampus was dissociated, and transferred to fresh DM fluid. Then, the hippocampus was cut and digested with papain (Worthington, USA) in a 37 °C incubator for 30 min with shaking every 10 min. Next, the plating medium was added to terminate the digestion. The collected neuronal cells were resuspended with plating medium and separated using a 40 μm cell filter. Subsequently, the cells were plated in poly-L-lysine-coated experimental plates, and then cultured cell incubator with 5% CO2 at 37 °C. After four hours, the plating medium was switched to the B27 neuronal culture medium, followed by half-exchange every 2 days [46]. The purity of the hippocampal neuronal cells was characterized by immunofluorescence after culture to day 7.
2.4. Immunofluorescence Analysis
Primary mouse hippocampal neuronal cells were inoculated onto glass coverslips in 6-well plates and cultured at 37 °C to 50%−80% density. The cells were then fixed with 4% paraformaldehyde for 10 min and washed three times with PBS to remove the fixative. Subsequently, the cells were blocked with TBSTx containing 1% BSA for 60 minutes at room temperature. Following this, the cells were incubated with Rabbit anti-neurofilament-L (1:100, Cell Signaling Technology, #2837) and Mouse anti-GFAP (1:100, Cell Signaling Technology, #3670) at 4 °C overnight. The cells were then rinsed three times with PBS and incubated with goat anti-rabbit IgG (1:1000, Alexa Fluor® 594 Conjugate, Cell Signaling Technology, #8889) and goat anti-mouse IgG (1:1000, Alexa Fluor® 488 Conjugate, Cell Signaling Technology, #4408) for 1 hour at room temperature. After another round of three rinses with PBS, the cells were stained with DAPI (10 μg/mL) for 15 minutes at room temperature. Finally, fluorescence images were captured under the EVOS Fluorescence Microimaging(Thermo, USA), and quantized using Image J software.
2.5. Flow CytoMetry
The identified primary mouse hippocampal neurons were cultured until day 7 and then divided into control, low (L)-Pb, medium (M)-Pb, and high (H)- Pb groups. Each group was treated with a solution containing 0, 12.5, 50, and 200 μmol/L Pb (Ac)2 for 24 hours. After finishing the incubation, the supernatant was collected, cells were flushed with 1×PBS. Subsequently, each cells vial was digested by adding 1 mL of 0.25% trypsin and placed in the incubator for 3 min. The digestion was terminated by adding medium, and the liquid was collected and centrifuged. At the end of centrifugation, the supernatant was discarded and resuspended in PBS containing PI working solution (5 μg/mL). Flow cytometry (Beckman, USA) was used for detection, with the experiments repeated thrice.
2.6. Western Blotting
After Pb exposure, the total protein was extracted from primary hippocampal neuron cells, HT22 cells, and mice hippocampus using RIPA lysis buffer (CWBIO, China) containing protease and phosphatase inhibitors. After completion of lysis, low temperature and high-speed centrifugation and collection of the supernatant to measure the protein content by the BCA assay. Refer to previous articles for specific steps in protein immunoblotting[47]. Briefly, 20 μg of standard protein samples were subjected to gel electrophoresis, polyvinylidene difluoride membrane transfer, containment, primary antibody(1:1000) incubation at 4 °C overnight. The antibodies used in this study were as follows: Rabbit anti-TNF-R1(Cell Signaling Technology,#13377), RIPK1(Cell Signaling Technology, #3493), Phospho-RIP (Ser166) (Cell Signaling Technology, #31122), RIPK3(Cell Signaling Technology, #95702), MLKL(Cell Signaling Technology, #37705), Phospho-MLKL (Ser345) (Cell Signaling Technology, #37333), PARP(Cell Signaling Technology, #9532), Caspase-8 (Cell Signaling Technology, #4790), GAPDH (Cell Signaling Technology, #2118), Cleaved PARP (Cell Signaling Technology, #9544). Subsequently, the membranes were incubated with goat anti-rabbit IgG (1:500, Cell Signaling Technology, #7074) for 1 h at room temperature. Finally, membranes were detected using a supersensitive chemiluminescence detection kit (Beyotime, China), then quantified with Image J software for further comparative analysis.
2.7. Animals
Eight-week-old SPF-grade male adult C57BL/6 mice were purchased from the Experimental Animal Center of Guangxi Medical University Animal Center [SCXK (Gui) 2014-0002]. All experimental procedures in mice followed animal ethical guidelines and complied with animal ethics requirements. All mice were cage-reared in SPF-grade chambers at a suitable temperature (24 ± 1 °C), humidity of 55±10%, and 12 hours of light. After 7 days of acclimatization, the mice were randomly divided into 4 groups (n = 10): control, low (L)-Pb, medium (M)-Pb, and high (H)- Pb groups. The mice in L, M, H- Pb groups received oral gavage (i.g.) with 12.5, 25, and 50 mg/kg Pb acetate (Sigma, USA) once a day, 7 days per week for 8 weeks, while mice in the control group received an equivalent volume of double-distilled water via i.g. After behavioral testing, the mice were anesthetized intraperitoneally with 1.2% Avertin. Next, blood was collected from the abdominal aorta, and the animals were sacrificed. Subsequently, the brain tissue was dissected out on dry ice, and the hippocampus tissue was collected and stored at −80 °C. Additionally, the heart, liver, spleen, lungs, kidneys and testicles of the mice were dissected, weighed, and the organ coefficients were calculated (organ coefficient (%) = organ weight/fasting body weight × 100%). Finally, the organs were separately collected into the freezing tube and maintained in a − 80 °C refrigerator for future experiments.
2.8. Behavioral Testing
The Morris water maze test was used to evaluate the mice’s spatial learning and memory abilities. The mice were petted for 1–2 minutes daily for one week before the experiment, in addition, the mice were moved to the water maze experimental room and acclimatized for 3 hours per day to help them adapt to the experimental environment. In addition, the light conditions in the acclimatization phase were kept the same as in the formal testing phase. The test program comprised 5 days of orientation navigation training, with an exploratory trial on the 6th day[48]. During the testing period, mice were immersed in water from fixed positions in each quadrant, and oriented to the pool wall with white patterns of different geometric shapes, which could provide references for the mice to establish spatial learning and memory. During the training period, if the mice failed to locate the hidden platform for more than 60 s, its latency was automatically registered as 60 seconds. Next, the tester guided the mice to climb onto the platform and allowed them to stay there for 15 seconds. After training, the mice were removed, dried, and placed the mice under the heater for 5 min, and returned to the cages. After orientation navigation training, exploratory testing was conducted by removing the hidden platform. The escape latency and the times all mice crossed the platform were recorded.
2.9. Pb Concentration Detection
Refer to previous research[47] for specific steps. Blood (1 mL) or hippocampus tissues (10 mg) were placed in a microwave disintegrator for disintegration. The digested samples were transferred in a 110~150°C electrothermal acidifier to evaporate the acid. After about 0.1 mL of sample remained, the sample was cooled to room temperature. Then wash the digestion tank with double-distilled water and volume the sample to 10 ml. Pb concentration was analyzed using Nexion300DICP-MS (PerkinElmer5771, USA).
2.10. Statistical Analysis
Data were expressed as Mean ± standard deviation (), and statistically analyzed using SPSS 23.0. For the water maze experiment, repeated measures analysis of variance was applied, while one-way analysis of variance (ANOVA) was used for other data, with LSD tests for multiple comparisons[49]. The Welch test was utilized if the data did not satisfy the homogeneity of variance requirement, and the Games-Howell test was employed for subsequent multiple comparisons. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Effects of Pb exposure on cell viability and morphology of HT-22 cells and primary mouse hippocampal neurons
After 24 hours of Pb treatment, HT22 cell viability decreased at Pb concentration >2.5 μmol/L (Fig 1A, P<0.05 or 0.01). Compared with the Control group, cell survival rates of primary mouse hippocampal neurons in the M-Pb and H-Pb groups were reduced by 6.69% and 23.18% (Fig 1B, P<0.01 or 0.001). Immunofluorescence showed that the purity of the primary mouse hippocampal neurons exceeded 90% (Fig 1C). Morphological analyses showed cell body shrinkage, and synaptic rupture in the L-Pb group. Cell body shrinkage, fewer protrusions, extensive synaptic damage, and cell death were observed in both the M-Pb and H-Pb groups, with the most obvious damage observed at the highest Pb exposure concentrations (Fig 1D).
Fig 1.
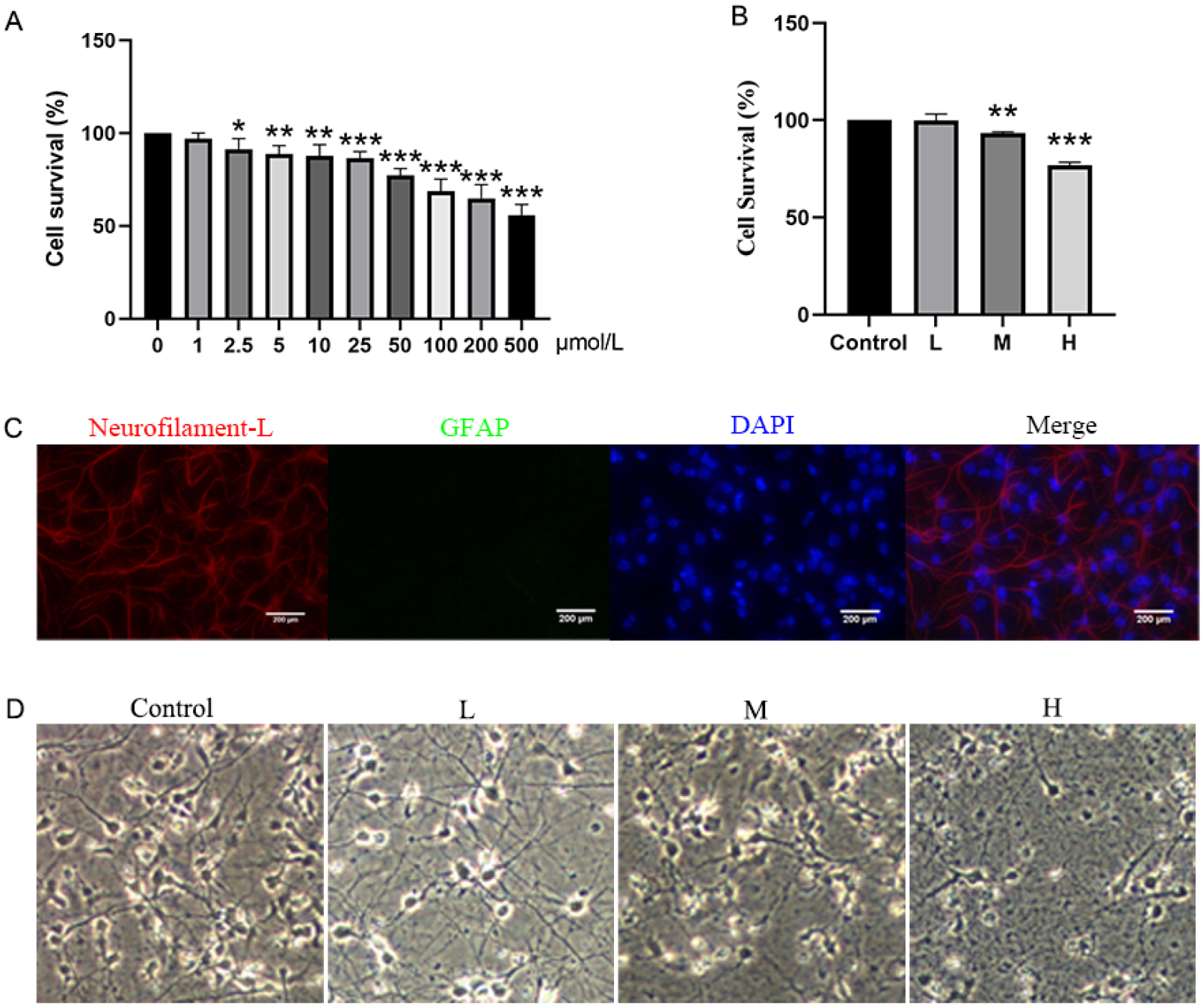
Effects of Pb exposure on cell viability and morphology of HT-22 cells and primary mouse hippocampal neurons
Note: Effect of Pb exposure on the survival rate of HT-22 cells (A) and primary mouse hippocampal neurons (B);(C) Identification of primary mouse hippocampal neurons, Neurofilament-L(red), GFAP(green), DAPI(blue), Scale bar = 200 μm; (D) Effects of Pb exposure on the morphology of primary mouse hippocampal neurons. L, M, and H groups were treated with 12.5, 50, and 200 μmol/L Pb(Ac)2 for 24 h; Values are presented as mean±SD (n=3). *P<0.05, **P<0.01 and ***P<0.001: compared to the Control group.
3.2. Effects of Pb exposure on TNF-R1-RIPK1/RIPK3 signaling pathway of HT-22 cells
RIPK1, p-RIPK1, and MLKL were elevated in HT-22 cells in the M and H-Pb groups compared to the Control(Fig 2B–C and 2H, P<0.05 or 0.01). Moreover, RIPK3 and p-MLKL levels in HT-22 cells were increased in all tested concentrations of Pb treatments after 24-hour exposures (Fig 2D and 2I, P<0.05 or 0.01). TNF-R1 protein expression in HT-22 cells in the H-Pb group was increased (Fig 2E, P<0.01). Additionally, PARP and c-PARP protein levels were elevated in the H-Pb group. (Fig 2F–G, P< 0.01 or 0.001).
Fig 2.
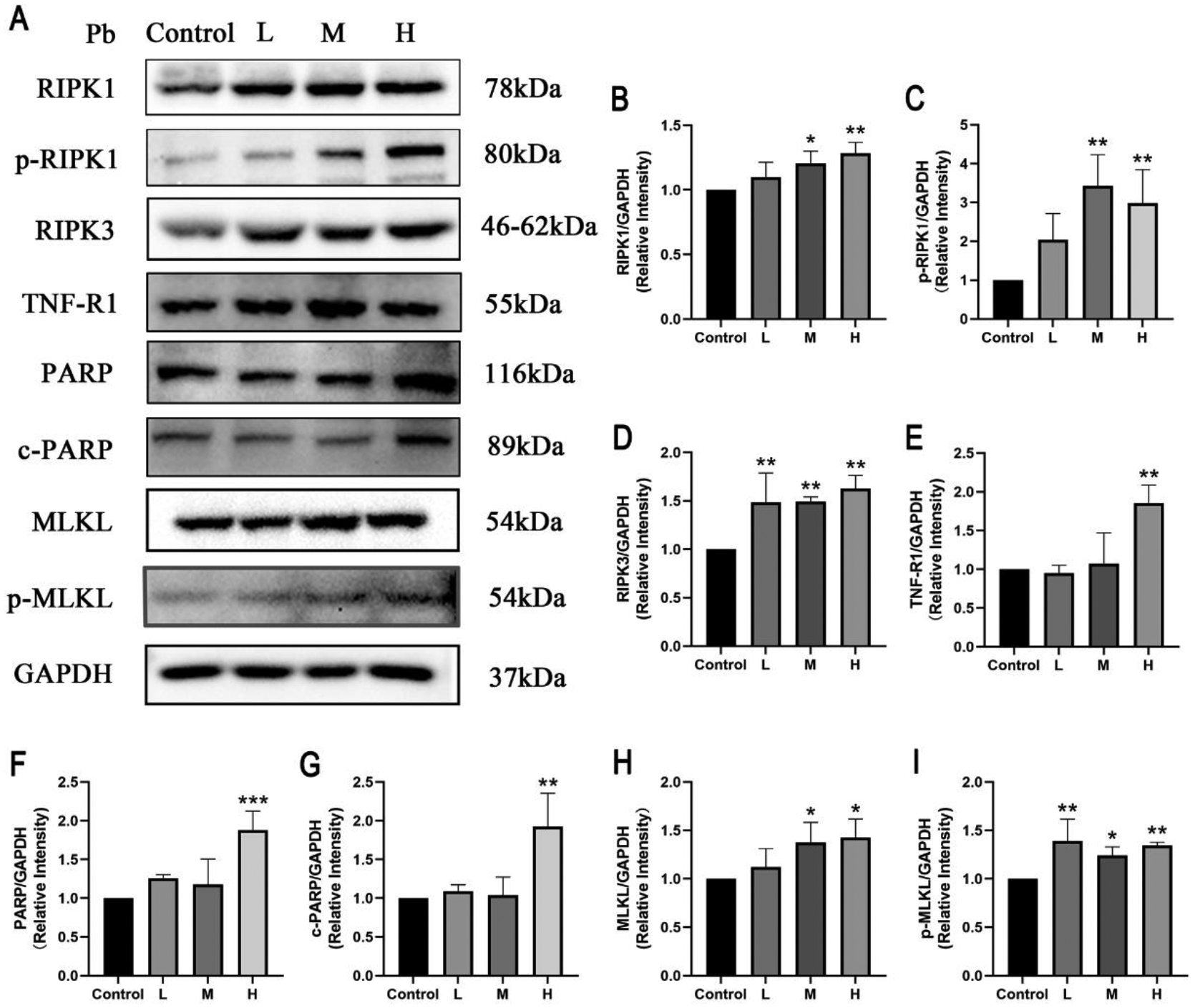
Effects of Pb exposure on TNF-R1-RIPK1/RIPK3 signaling pathway of HT-22 cells
Note: Representative protein bands (A) and the protein expression (B-I) in the HT-22 cells. The relative quantity of all proteins was normalized to GAPDH. L, M, and H groups were treated with, 12.5, 50, and 200 μmol/L Pb(Ac)2 for 24 h; Values are presented as mean±SD (n=3). *P<0.05 and **P<0.01: compared to the Control group.
3.3. Effects of Pb exposure on TNF-R1-RIPK1/RIPK3 signaling pathway of primary hippocampal neurons
Compared with the Control group, PARP and c-PARP protein expression in primary hippocampal neurons in all tested concentrations of Pb treated groups was increased(Fig 3F–G, P<0.05, 0.01 or 0.001), and RIPK1, p-RIPK1 protein expression in primary hippocampal neurons of the M-Pb and H-Pb groups was increased (Fig 3B–C, P<0.01). Concomitantly, the expression of MLKL protein was higher in primary hippocampal neurons in the M and H-Pb groups, and the phosphorylation of MLKL was higher in the H-Pb group, compared with the Control (Fig 3H–I, P<0.05 or 0.01). However, Pb failed to alter RIPK3 and TNF-R1 protein expression levels in mice hippocampal neurons (Fig 3D–E).
Fig 3.
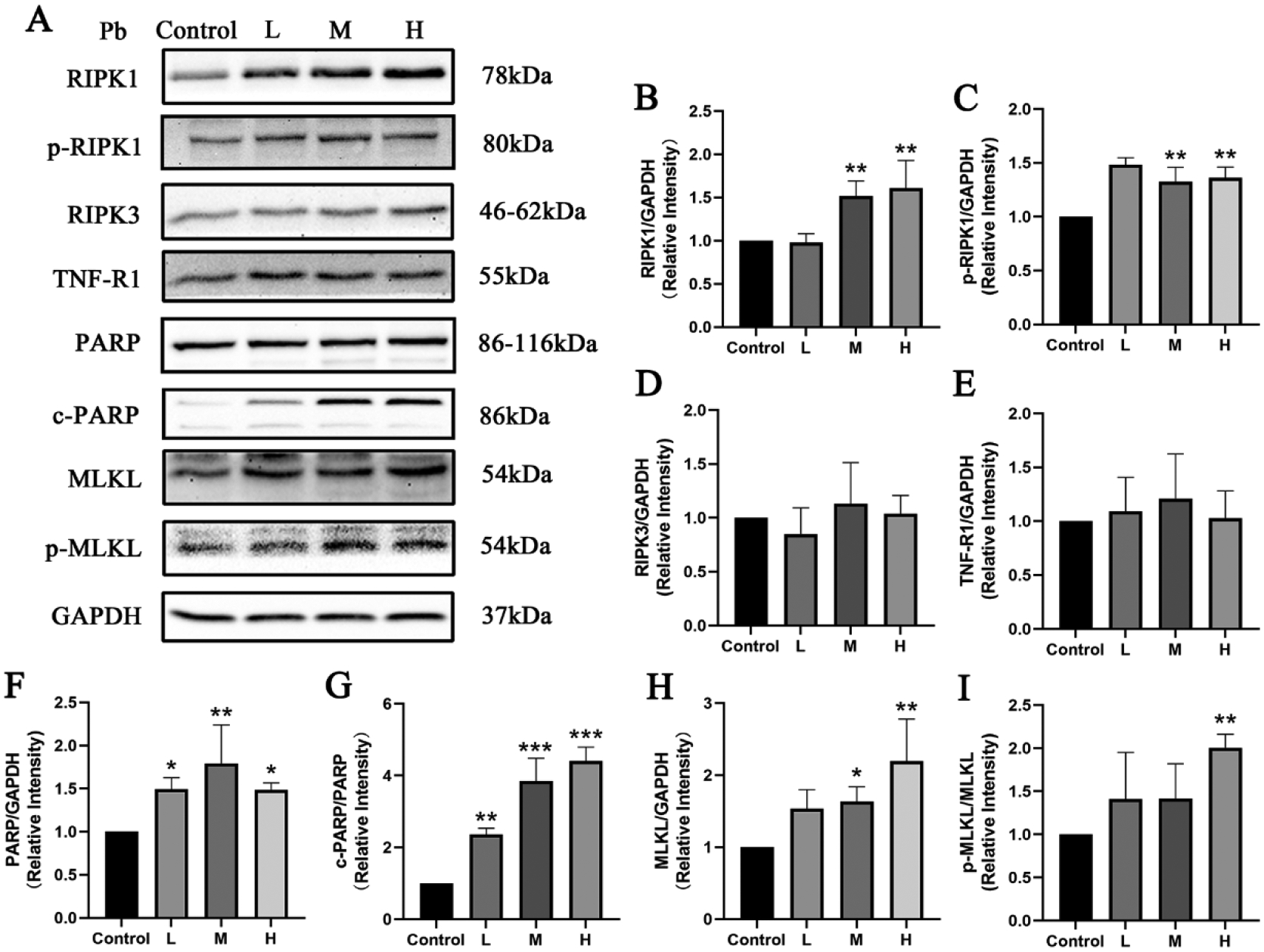
Effects of Pb exposure on TNF-R1-RIPK1/RIPK3 signaling pathway of the primary mouse hippocampal neurons
Note: Representative protein bands (A) and protein expressions (B-I) in the primary mouse hippocampal neurons. The relative quantity of all proteins was normalized to GAPDH. L, M, and H groups were treated with, 12.5, 50, and 200 μmol/L Pb(Ac)2 for 24 h; Values are presented as mean± SD (n=3). *P<0.05, **P<0.01 and ***P<0.001: compared to the Control group.
3.4. Effects of Pb exposure on the body weight of mice
The body weight of all mice was increased as exposure time increased (Fig 4). Compared to the Control group, body weights in H-Pb mice were significantly decreased at the 1st, 4th, 6th, 7th and 8th weeks (Fig 4, P<0.05).
Fig 4.
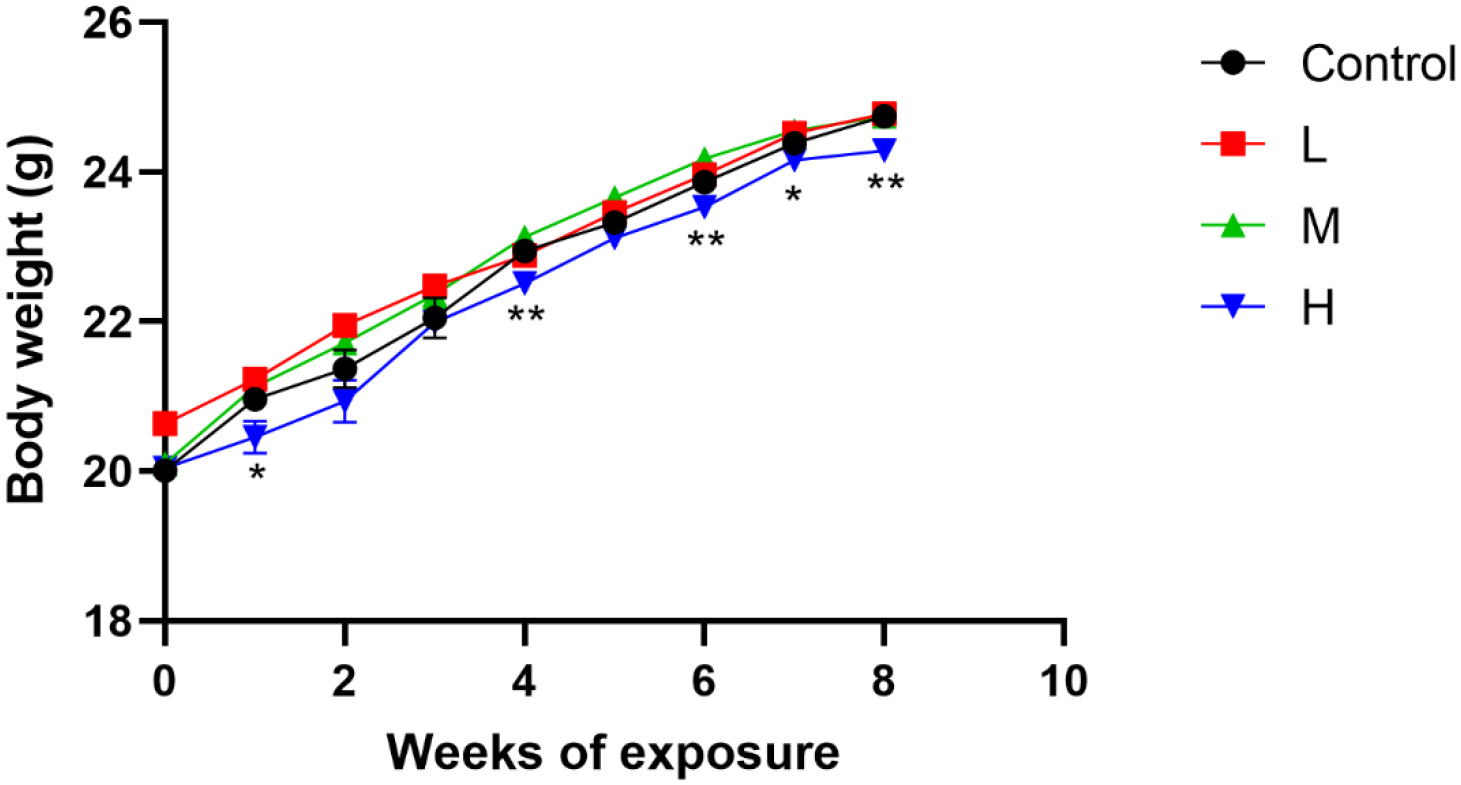
Effects of Pb exposure on the body weight of mice (n=10)
Note: L, M, and H groups received oral gavage (i.g.) with 12.5, 25, and 50 mg/kg Pb acetate; The data are presented as mean±SEM. *P<0.05 and **P<0.01: compared to the Control group.
3.5. Effects of Pb exposure on the spatial learning and memory abilities of mice
The escape latency and swimming distance were longer in the H-Pb group on days 2 to 5 and the M-Pb group on the 4th day compared with the Control group (Fig 5A and B, P<0.05 or 0.01). Compared to the Control group, the number of crossing the platform in the M-Pb and H-Pb groups were decreased (Fig 5C, P<0.01).
Fig 5.
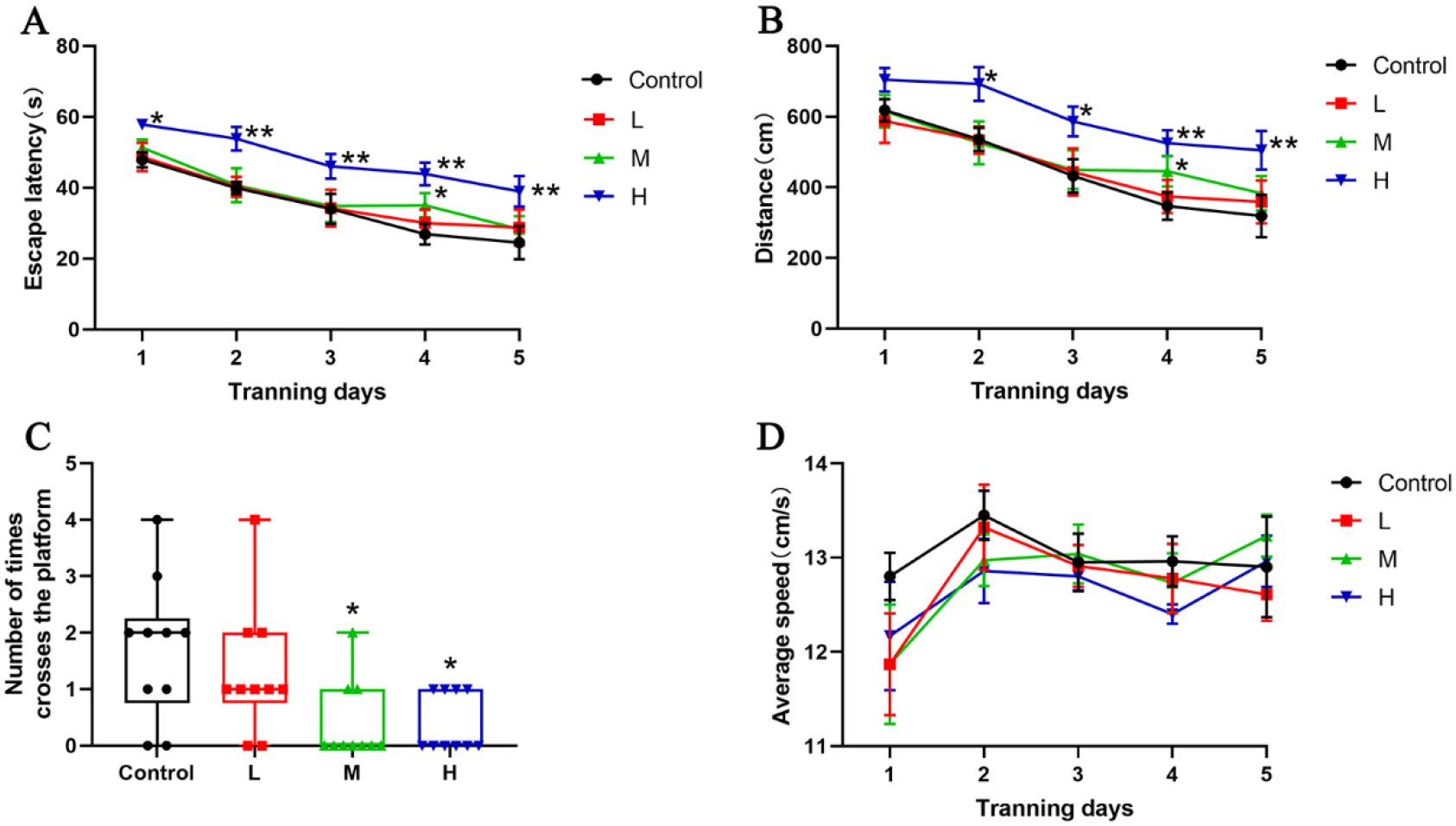
Effects of Pb exposure on mice spatial learning and memory abilities
Note: (A) The escape latency, (B) the swimming distance, (C)the number of crossing the platform, and (D) the average swimming speed of mice. L, M, and H groups received oral gavage (i.g.) with 12.5, 25, and 50 mg/kg Pb acetate; Values are presented as mean±SEM (n=10). *P<0.05 and **P<0.01: compared to the Control group.
3.6. Effects of Pb exposure on the organ coefficient of mice
Heart coefficients of the L-Pb and M-Pb groups were higher than the Control group (P<0.05). The H-Pb group lungs coefficients were higher than the Control group(P<0.05), and liver coefficients of the M-Pb group were higher than in the Control (P<0.05); However, Pb exposure did not change in spleen, kidney and testis coefficients (P>0.05). (Table 1)
Table 1.
Effects of Pb exposure on the organ coefficient of mice (n=10, )
| Group (mg/kg) | Heart | Lung | Liver | Spleen | Kidney | Testicles |
|---|---|---|---|---|---|---|
| Control | 0.62±0.03 | 0.64±0.03 | 4.43±0.07 | 0.27±0.02 | 1.13±0.04 | 0.85±0.03 |
| L-Pb | 0.75±0.05* | 0.68±0.02 | 4.49±0.07 | 0.25±0.02 | 1. 15±0.03 | 0.77±0.03 |
| M-Pb | 0.79±0.05* | 0.70±0.04 | 4.76±0.09* | 0.28±0.02 | 1.17±0.03 | 0.91±0.05 |
| H-Pb | 0.66±0.03 | 0.77±0.03* | 4.29±0.13 | 0.24±0.01 | 1.22±0.03 | 0.88±0.03 |
Note: L, M, H- Pb groups received oral gavage (i.g.) with 12.5, 25, and 50 mg/kg Pb acetate; Data are presented as mean±SED(n=10).
P<0.05: compared to the Control group.
3.7. Effects of Pb exposure on Pb concentration in whole blood and hippocampus of mice
Pb exposure increased Pb concentration in both the whole blood and hippocampus of mice in a dose-dependent relationship compared to the Control (Fig 6, rblood=0.898, rhippocampus=0.787).
Fig 6.
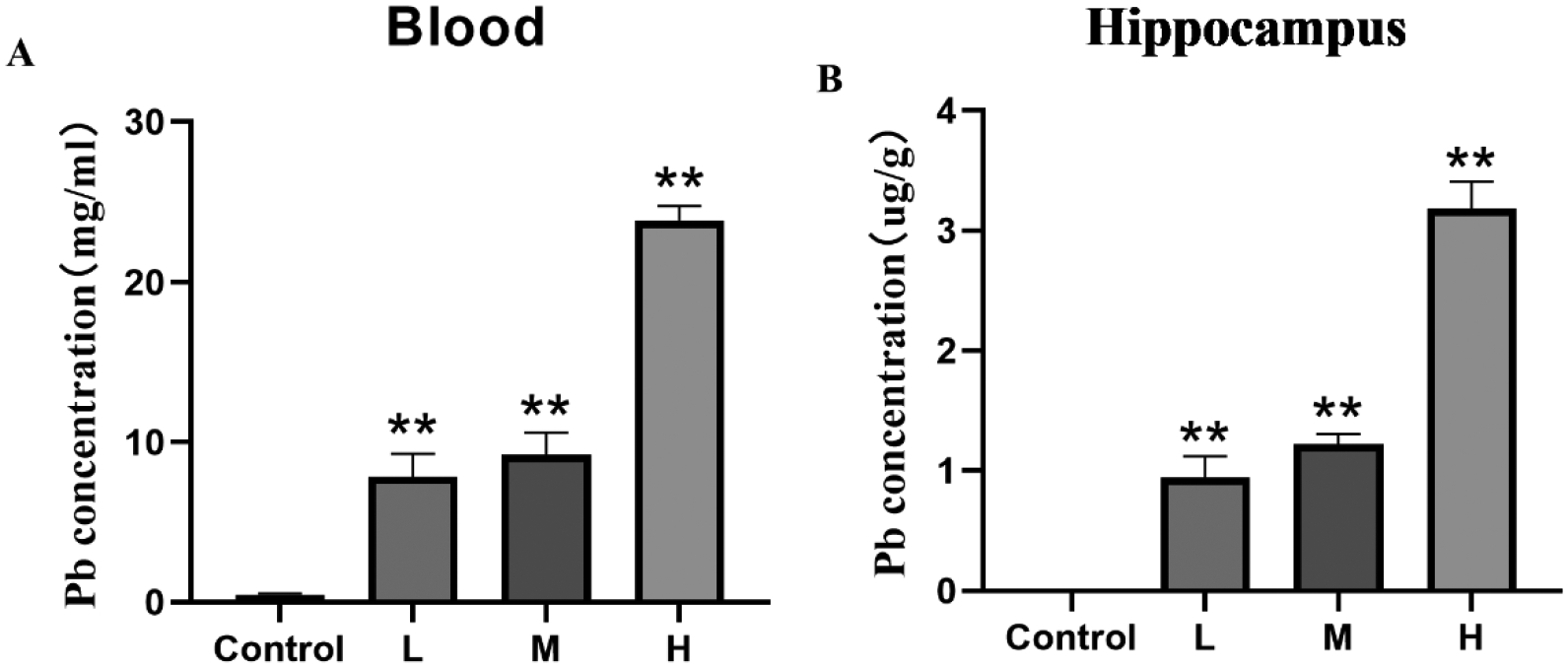
Effects of Pb exposure on Pb concentration in the whole blood and hippocampus of mice
Note: Pb concentration in the whole blood (A) and hippocampus (B) of mice. L, M, and H groups received oral gavage (i.g.) with 12.5, 25, and 50 mg/kg Pb acetate; Data are presented as mean±SEM (n=8).**P<0.01: compared to the Control group.
3.8. Effects of Pb exposure on the TNF-R1-RIPK1/RIPK3 signaling pathway in hippocampus of mice
The protein expression of TNF-R1 in hippocampus of mice of the H-Pb group was significantly increased compared to the Control group (Fig 7E, P<0.05). Furthermore, Pb increased PARP protein expressions in the hippocampus of L, M and H-Pb groups, and c-PARP protein expression in the M-Pb and H-Pb groups (Fig 7F–G, P<0.05 or 0.01). In addition, all tested concentrations of Pb significantly reduced caspase-8 protein expression in murine hippocampus (Fig 7J, P<0.05 or 0.01). The protein expressions of RIPK1 and p-RIPK1 in the hippocampus of M-Pb and H-Pb groups were elevated compared to the Control group (Fig 7B–C, P<0.05 or 0.01). Likewise, the L, M and H-Pb group of RIPK3 protein expression was higher than in the Control group (Fig 7D, P<0.05, 0.01 or 0.001). Additionally, MLKL and p-MLKL protein expression were increased in the M-Pb and H-Pb groups compared to the Control group (Fig 7H–I, P<0.05 or 0.01).
Fig 7.
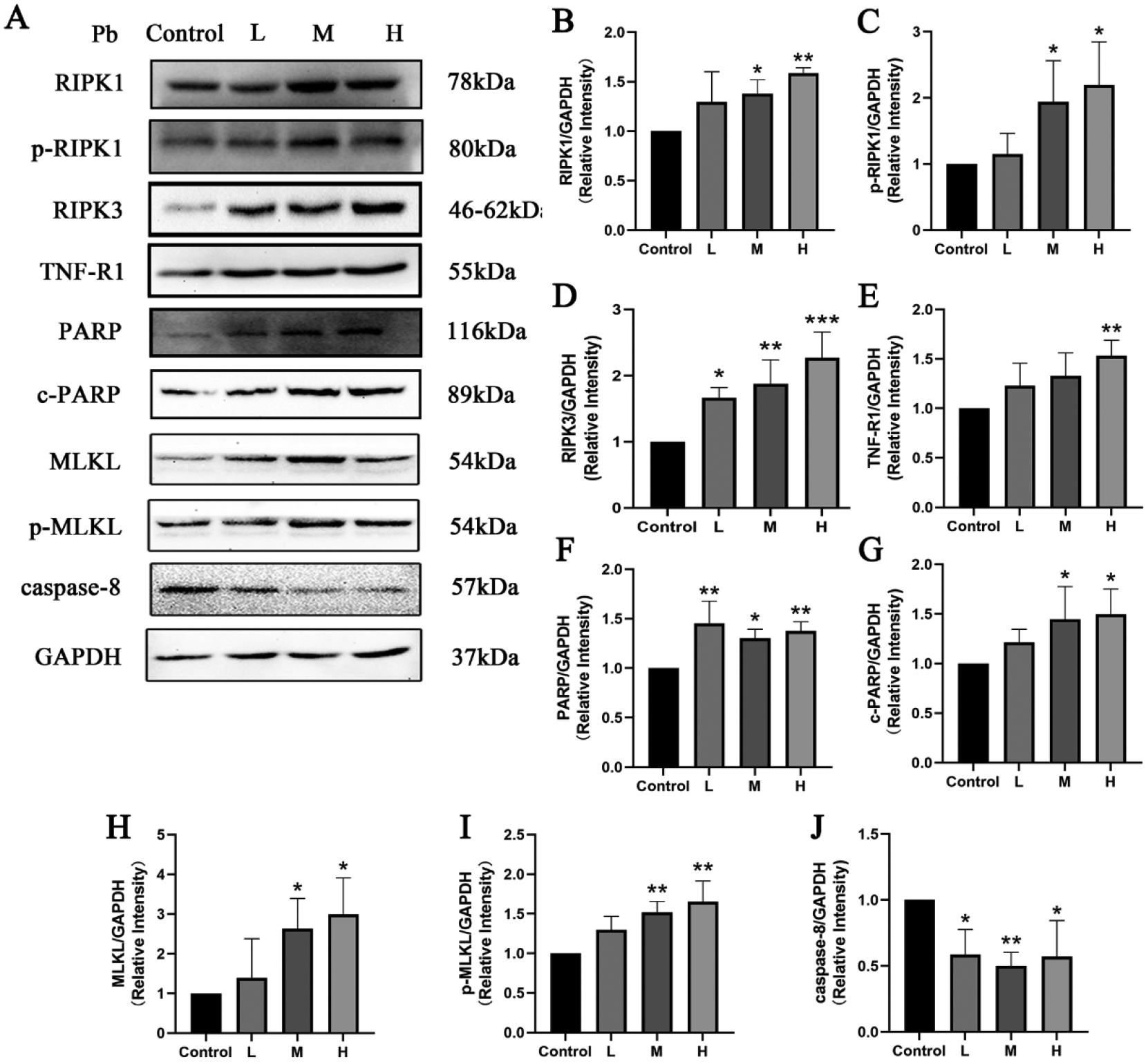
Effects of Pb exposure on the TNF-R1-RIPK1/RIPK3 signaling pathway in the hippocampus of mice
Note: Representative protein bands (A) and protein expressions (B-J) in the hippocampus of mice. The relative quantity of all proteins was normalized to GAPDH. L, M, and H groups received oral gavage (i.g.) with 12.5, 25, and 50 mg/kg Pb acetate; Data are presented as mean±SD (n=3). *P<0.05 and **P<0.01: compared to the Control group.
4. Discussion
Pb is a known health risk to humans, and its exposure in non-occupational populations occurs primarily through gastrointestinal intake[50]. Various studies have shown that Pb exposure caused gastrointestinal irritation and decreased appetite, leading to reduced food consumption, growth retardation and mild weight loss[51, 52]. The present study revealed that administering 50 mg/kg of Pb via oral gavage for 8 weeks decreased body weight in mice. Pb can cause damage to several organs of the body, and the results of the present study showed that Pb altered the heart and lung coefficient in mice, which is consistent with previous studies[8, 53]. Blood Pb levels are often used to reflect the degree of Pb poisoning in an organism[54], and the ICP-MS results showed an increase in both blood and hippocampal Pb levels in mice, which were positively correlated with the dose of Pb exposure. There are large variations in patterns or sources of Pb-exposure and its severity of outcomes among countries[55] Humans can be exposed to higher concentrations of Pb through multiple routes of intake, including air, soil, water, buildings, and household products, and Pb exposure in animals often exceeds the approximate exposure in humans[56].
Epidemiologic investigations have demonstrated that low-level Pb exposure is a risk factor for IQ drops [57, 58], cardiovascular disease[59], and hearing loss[60]. According to the World Health Organization, there is no safe blood lead concentration[61]. Various studies showed that Pb could induce cognitive impairment[62, 63], as well as AD-related neuropathological changes[64, 65], and nerve cell damage is the basis of its pathology. Numerous studies have indicated that exposure to Pb reduced the cellular viability in various cell lines, such as SH-SY5Y[66], PC12[67], and HT-22 cells[68]. Pb at 20 μg/d showed significant cytotoxic effects on embryonic hippocampal cells in Wistar rats, resulting in decreased cell viability[69]. Exposure of human iPSC-derived neural cells to low doses of Pb resulted in differentiated neurons exhibiting altered calcium homeostasis and synaptic plasticity, as well as elevated markers of AD pathogenesis[70]. The present study showed that Pb caused neurodegeneration in primary hippocampal neurons, and decreased the survival rates both in hippocampal neurons and HT-22 cells. Pb accumulation in the choroid plexus and hippocampus, which are key areas associated with brain structure and function alterations in AD patients, could induce brain damage[71]. Pb has also been found to disrupt neurotransmitter balance and synaptic transmission, impacting hippocampal synaptic plasticity and resulting in cognitive function deficiencies[72, 73]. Our previous study demonstrated that sub-acute Pb exposure damaged the hippocampal structures, while Sodium para-aminosalicylic acid (PAS-Na) treatment recovered the cognitive deficits of rats induced by Pb[74]. The present study demonstrated that subchronic Pb exposure increased murine escape latency and swimming distance, while decreased the number of platform crossings, suggesting that Pb could induce cognitive impairment, consistent with previous studies[75].
Necroptosis challenges the traditional view on necrosis as a passive process[33]. This discovery opens new possibilities for treating clinical conditions linked to necroptosis, such as neurodegenerative diseases. A previous study found necroptosis was activated in AD patients’ brains, and that activation of necroptosis negatively correlates with cognitive scores[76]. Recently, necroptosis has been demonstrated to be essential for the neurodegenerative damage induced by heavy metals, including aluminum and manganese. TNF-R1, a crucial receptor for TNF-α toxicity, plays a pivotal role in mediating cell death via complex I formation. Inhibition of caspase-8 can activate TNF-R1, subsequently triggering the RIPK1/RIPK3-MLKL pathway[77, 78], highlighting its significance in cell necroptosis. TNF-α is implicated in neuronal cell damage through neuroinflammation and is significantly related to the development of neurodegenerative diseases[14, 79]. Pb-induced neuroinflammation has been studied, with a focus on the important role of TNF-α[80]. Both in vitro and vivo studies demonstrated that Pb exposure elevated TNF-α expression in BV-2 cells[81] and rats[82]. Additionally, an epidemiological investigation indicated a significant correlation between blood Pb level and TNF-α level in occupational populations with blood Pb concentrations of > or = 2.51 mg/dL [83]. Notably, our present study indicated that Pb increased TNF-R1 protein expression in mice hippocampus and HT-22 cells, shedding light on the potential mechanistic link between Pb exposure and TNF-α-mediated neuroinflammation. A previously published in vivo study suggested that TNF-α can induce necroptosis in hippocampal neurons via CYLD-RIPK1-RIPK3-MLKL signaling pathway, leading to neurotoxic effects[84]. RIPK1, RIPK3 and MLKL are involved in the execution of necroptosis. In this study, we found that Pb activated RIPK1 and RIPK3 protein expression and activated the phosphorylation of RIPK1 in HT-22 cells and mouse hippocampal neurons, accompanied by a rise in necroptosis signature protein MLKL expression and its phosphorylation. Moreover, consistent with prior research, the in vivo study also demonstrated that Pb inhibits the protein expression of caspase-8 in mice hippocampal tissues, while activated proteins expressions of RIPK1 and RIPK3, ultimately leading to the elevation of MLKL protein and its phosphorylation. Overall, Pb exposure increased RIPK1, p-RIPK1, MLKL, p-MLKL, PARP, c-PARP, protein expression levels in HT-22 cells, primary mouse hippocampal neurons, and mouse hippocampal tissues. In addition, the expression of TNF-R1 and RIPK3 proteins was increased in HT-22 cells and mouse hippocampus. Pb exposure also decreased caspase-8 protein expression levels in mouse hippocampal tissue (Table 2).
Table 2.
The summary in the effects of Pb on TNF-R1-RIPK1/RIPK3 pathway
| Protein | HT-22 cell | primary mouse hippocampal neurons | Mice |
|---|---|---|---|
| TNF-R1 | ↑ | - | ↑ |
| RIPK1 | ↑ | ↑ | ↑ |
| P-RIPK1 | ↑ | ↑ | ↑ |
| RIPK3 | ↑ | - | ↑ |
| MLKL | ↑ | ↑ | ↑ |
| p-MLKL | ↑ | ↑ | ↑ |
| PARP | ↑ | ↑ | ↑ |
| c-PARP | ↑ | ↑ | ↑ |
| caspase-8 | / | / | ↓ |
Note: (−) No change, (/) Not acquire.
P<0.05 and
P<0.01: compared to the Control group.
The present study revealed that Pb exposure increased PARP-1 protein expression, a factor associated with necroptosis. PARP-1, recognized as a DNA repair enzyme, plays a crucial role in maintaining chromosome structural integrity, DNA replication, transcription, genome stability and cell death mechanisms[85, 86]. Excessive DNA damage can hyperactivate PARP-1, prompting mitochondria to release apoptosis-inducing factor, which migrates to the nucleus, causing DNA fragmentation. Subsequently, DNA damage can further activate PARP-1, resulting in a significant reduction in nicotinamide-adenine dinucleotide (NAD+), inhibiting glycolysis, and resulting in a sharp decline in intracellular ATP levels and subsequent necroptosis[87]. The present study indicated that Pb elevated murine primary hippocampal neurons PARP-1 protein expression, as well as PARP-1 and c-PARP-1 protein, suggesting that PARP-1 may serve pivotal regulatory functions in Pb-induced necroptosis in hippocampal neurons. Notably, PARP-1 has been shown to be involved in another mode of programmed cell death-Parthanatos, also known as PARP-1 dependent cell death. Parthanatos specifically depends on PARP1 hyperactivation and is independent of caspase[88]. This also suggests that there may be multiple modes of cell death in Pb-induced hippocampal neuronal death.
There are limitations to our study. First, the exact mechanism of Pb-induced necroptosis in hippocampal neurons remains unclear and needs to be further investigated by applying inhibitors or knockout techniques, as the results obtained by detecting changes in necroptosis markers only in cellular versus mouse models can only serve as relevant descriptions and interpretations. In addition, although TNF-α/TNFR1-mediated activation of RIPK1 is the most comprehensively studied signaling pathway, there is a need to measure other inflammatory markers as they contribute to the study of the underlying mechanisms between Pb exposure and TNF-α-mediated neuroinflammation. Last but not least, since Pb exposure levels in animal studies often exceed actual exposure levels in humans, the possibility of oversaturation effects from high-dose exposures should be considered.
5. Conclusion
The present study suggests that Pb activated the necroptosis pathway RIPK1/RIPK3-MLKL through TNF-R1(Table 2), resulting in neuronal necroptosis characterized by the activation of necroptosis signature protein MLKL and its phosphorylated forms, ultimately resulting in neurotoxic effects that contribute to cognitive impairment in mice.
Acknowledgments
The authors gratefully acknowledge the financial supports by the National Natural Science Foundation of China and Guangxi Natural Science Foundation.
Funding
This study was supported by grants from the National Natural Science Foundation of China under Grant NSFC82160626, NSFC81803281; Guangxi Natural Science Foundation under Grant 2018GXNSFBA050060; And Guangxi Natural Science Found for Innovation Research Team under Grant 2019GXNSFGA245002.
Footnotes
Competing Interests
The authors declare no competing interests.
Ethical Approval
All animal procedures performed in this study were performed strictly according to the international standards of animal care guidelines and have been approved by the Animal Care and Use Committee of Guangxi Medical University.
Consent to publish
The paper has been approved by all authors for publication.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
- 1.Mitra A, Chatterjee S, Kataki S, Rastogi RP, Gupta DK (2021) Bacterial tolerance strategies against lead toxicity and their relevance in bioremediation application. Environ Sci Pollut R 28:14271–14284. 10.1007/s11356-021-12583-9. [DOI] [PubMed] [Google Scholar]
- 2.Cheema AI, Liu GJ, Yousaf B, Abbas Q, Zhou HH (2020) A comprehensive review of biogeochemical distribution and fractionation of lead isotopes for source tracing in distinct interactive environmental compartments. Science of the Total Environment 719:135658. 10.1016/j.scitotenv.2019.135658. [DOI] [PubMed] [Google Scholar]
- 3.Lamas GA, Bhatnagar A, Jones MR, Mann KK, Nasir K, Tellez-Plaza M, Ujueta F, Navas-Acien A, Council AHA, Nursing CCS, Hlth CLC, Dis CPV, Dis CKC (2023) Contaminant Metals as Cardiovascular Risk Factors: A Scientific Statement From the American Heart Association. J Am Heart Assoc 12:e029852. 10.1161/Jaha.123.029852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harari F, Sallsten G, Christensson A, Petkovic M, Hedblad B, Forsgard N, Melander O, Nilsson PM, Borné Y, Engström G, Barregard L (2018) Blood Lead Levels and Decreased Kidney Function in a Population-Based Cohort. Am J Kidney Dis 72:381–389. 10.1053/j.ajkd.2018.02.358. [DOI] [PubMed] [Google Scholar]
- 5.Dey KK, Kamila S, Das T, Chattopadhyay A (2024) Lead induced genotoxicity and hepatotoxicity in zebrafish (Danio rerio) at environmentally relevant concentration: Nrf2-Keap1 regulated stress response and expression of biomarker genes. Environmental toxicology and pharmacology:104396–104396. 10.1016/j.etap.2024.104396. [DOI] [PubMed] [Google Scholar]
- 6.Wu C, Wang J, Luo X, Wang B, Zhang X, Song Y, Zhang K, Zhang X, Sun M (2024) Lead exposure induced transgenerational developmental neurotoxicity by altering genome methylation in Drosophila melanogaster. Ecotox Environ Safe 271:115991. 10.1016/j.ecoenv.2024.115991. [DOI] [PubMed] [Google Scholar]
- 7.Mitra P, Sharma S, Purohit P, Sharma P (2017) Clinical and molecular aspects of lead toxicity: An update. Crit Rev Cl Lab Sci 54:506–528. 10.1080/10408363.2017.1408562. [DOI] [PubMed] [Google Scholar]
- 8.Ge YM, Chen LL, Sun XH, Yin ZH, Song XC, Li C, Liu JW, An ZX, Yang XF, Ning HM (2018) Lead-induced changes of cytoskeletal protein is involved in the pathological basis in mice brain. Environ Sci Pollut R 25:11746–11753. 10.1007/s11356-018-1334-6. [DOI] [PubMed] [Google Scholar]
- 9.Liu JX, Liao GZ, Tu HW, Huang Y, Peng T, Xu YJ, Chen XH, Huang ZB, Zhang YY, Meng XJ, Zou F (2019) A protective role of autophagy in Pb-induced developmental neurotoxicity in zebrafish. Chemosphere 235:1050–1058. 10.1016/j.chemosphere.2019.06.227. [DOI] [PubMed] [Google Scholar]
- 10.Ji XN, Wang BK, Paudel YN, Li ZH, Zhang SS, Mou L, Liu KC, Jin M (2021) Protective Effect of Chlorogenic Acid and Its Analogues on Lead-Induced Developmental Neurotoxicity Through Modulating Oxidative Stress and Autophagy. Front Mol Biosci 8:655549. 10.3389/fmolb.2021.655549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalid M, Abdollahi M (2019) Epigenetic modifications associated with pathophysiological effects of lead exposure. J Environ Sci Heal C 37:235–287. 10.1080/10590501.2019.1640581. [DOI] [PubMed] [Google Scholar]
- 12.Wang T, Zhang J, Xu Y (2020) Epigenetic Basis of Lead-Induced Neurological Disorders. Int J Env Res Pub He 17:4878. 10.3390/ijerph17134878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb A, Spiers K, Falkenberg G, Gu H, Du Y, Zheng W, Nie H (2020) Distribution of Pb and Se in mouse brain following subchronic Pb exposure by using synchrotron X-ray fluorescence. Med Phys 88:106–115. 10.1016/j.neuro.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chibowska K, Korbecki J, Gutowska I, Metryka E, Tarnowski M, Goschorska M, Barczak K, Chlubek D, Baranowska-Bosiacka I (2020) Pre- and Neonatal Exposure to Lead (Pb) Induces Neuroinflammation in the Forebrain Cortex, Hippocampus and Cerebellum of Rat Pups. Int J Mol Sci 21:1083. 10.3390/ijms21031083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafaiee R, Khastar H, Garmabi B, Taleb M, Norouzi P, Khaksari M (2021) Hydrogen sulfide protects hippocampal CA1 neurons against lead mediated neuronal damage via reduction oxidative stress in male rats. J Chem Neuroanat 112:101917. 10.1016/j.jchemneu.2020.101917. [DOI] [PubMed] [Google Scholar]
- 16.Baranowska-Bosiacka I, Falkowska A, Gutowska I, Gassowska M, Kolasa-Wolosiuk A, Tarnowski M, Chibowska K, Goschorska M, Lubkowska A, Chlubek D (2017) Glycogen metabolism in brain and neurons - astrocytes metabolic cooperation can be altered by pre- and neonatal lead (Pb) exposure. Toxicology 390:146–158. 10.1016/j.tox.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Saleh HA, Abd El-Aziz GS, Mustafa HN, El-Fark M, Mal A, Aburas M, Deifalla AH (2019) Thymoquinone ameliorates oxidative damage and histopathological changes of developing brain neurotoxicity. J Histotechnol 42:116–127. 10.1080/01478885.2019.1619654. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad F, Haque S, Ravinayagam V, Ahmad A, Kamli MR, Barreto GE, Ashraf GM (2020) Developmental lead (Pb)-induced deficits in redox and bioenergetic status of cerebellar synapses are ameliorated by ascorbate supplementation. Toxicology 440:152492. 10.1016/j.tox.2020.152492. [DOI] [PubMed] [Google Scholar]
- 19.Shilpa O, Anupama KP, Antony A, Gurushankara HP (2021) Lead (Pb) induced Oxidative Stress as a Mechanism to Cause Neurotoxicity in Drosophila melanogaster. Toxicology 462:152959. 10.1016/j.tox.2021.152959. [DOI] [PubMed] [Google Scholar]
- 20.Gassowska M, Baranowska-Bosiacka I, Moczydlowska J, Tarnowski M, Pilutin A, Gutowska I, Struzynska L, Chlubek D, Adamczyk A (2016) Perinatal exposure to lead (Pb) promotes Tau phosphorylation in the rat brain in a GSK-3β and CDK5 dependent manner: Relevance to neurological disorders. Toxicology 347:17–28. 10.1016/j.tox.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Pang SM, Li YS, Chen W, Li YB, Yang MY, Zhao LJ, Shen QW, Cheng N, Wang Y, Lin XQ, Ma JM, Wu HH, Zhu GC (2019) Pb exposure reduces the expression of SNX6 and Homer1 in offspring rats and PC12 cells. Toxicology 416:23–29. 10.1016/j.tox.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Shefa ST, Héroux P (2017) Both physiology and epidemiology support zero tolerable blood lead levels. Toxicol Lett 280:232–237. 10.1016/j.toxlet.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Park W, Wei S, Kim BS, Kim B, Bae SJ, Chae YC, Ryu D, Ha KT (2023) Diversity and complexity of cell death: a historical review. Exp Mol Med 55:1573–1594. 10.1038/s12276-023-01078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharifi AM, Baniasadi S, Jorjani M, Rahimi F, Bakhshayesh M (2002) Investigation of acute lead poisoning on apoptosis in rat hippocampus in vivo. Neurosci Lett 329:45–48. 10.1016/s0304-3940(02)00576-1. [DOI] [PubMed] [Google Scholar]
- 25.Gu XZ, Han MM, Du Y, Wu YL, Xu Y, Zhou XX, Ye DL, Wang HL (2019) Pb disrupts autophagic flux through inhibiting the formation and activity of lysosomes in neural cells. Toxicol in Vitro 55:43–50. 10.1016/j.tiv.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Cao YS, Zhao WJ, Zhong YQ, Jiang XF, Mei HY, Chang YJ, Wu DQ, Dou JR, Vasquez E, Shi X, Yang JT, Jia ZT, Tan XC, Li Q, Dong YY, Xie RJ, Gao J, Wu Y, Liu YY (2024) Effects of chronic low-level lead (Pb) exposure on cognitive function and hippocampal neuronal ferroptosis: An integrative approach using bioinformatics analysis, machine learning, and experimental validation. Science of the Total Environment 917:170317. 10.1016/j.scitotenv.2024.170317. [DOI] [PubMed] [Google Scholar]
- 27.Villa-Cedillo SA, Nava-Hernández MP, Soto-Domínguez A, Hernández-Ibarra JA, Perez-Trujillo JJ, Saucedo-Cárdenas O (2019) Neurodegeneration, demyelination, and astrogliosis in rat spinal cord by chronic lead treatment. Cell Biol Int 43:706–714. 10.1002/cbin.11147. [DOI] [PubMed] [Google Scholar]
- 28.Meng HT, Wang L, He JH, Wang ZF (2016) The Protective Effect of Gangliosides on Lead (Pb)-Induced Neurotoxicity Is Mediated by Autophagic Pathways. Int J Env Res Pub He 13:365. 10.3390/ijerph13040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bihaqi SW (2019) Early life exposure to lead (Pb) and changes in DNA methylation: relevance to Alzheimer’s disease. Rev Environ Health 34:187–195. 10.1515/reveh-2018-0076. [DOI] [PubMed] [Google Scholar]
- 30.Gassowska-Dobrowolska M, Chlubek M, Kolasa A, Tomasiak P, Korbecki J, Skowronska K, Tarnowski M, Masztalewicz M, Baranowska-Bosiacka I (2023) Microglia and Astroglia-The Potential Role in Neuroinflammation Induced by Pre- and Neonatal Exposure to Lead (Pb). Int J Mol Sci 24:9903. 10.3390/ijms24129903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu L, Li S, Pang SL, Zhang B, Wang JR, He B, Lv LY, Wang WX, Zhao N, Zhang YS (2021) Effects of lead exposure on the activation of microglia in mice fed with high-fat diets. Environ Toxicol 36:1923–1931. 10.1002/tox.23312. [DOI] [PubMed] [Google Scholar]
- 32.Degterev A, Huang ZH, Boyce M, Li YQ, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan JY (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119. 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 33.Morgan MJ, Kim YS (2022) Roles of RIPK3 in necroptosis, cell signaling, and disease. Exp Mol Med 54:1695–1704. 10.1038/s12276-022-00868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy JM (2020) The Killer Pseudokinase Mixed Lineage Kinase Domain-Like Protein (MLKL). Csh Perspect Biol 12:a036376. 10.1101/cshperspect.a036376a036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton K, Strasser A, Kayagaki N, Dixit VM (2024) Cell death. Cell 187:235–256. 10.1016/j.cell.2023.11.044. [DOI] [PubMed] [Google Scholar]
- 36.Yuan JY, Amin P, Ofengeim D (2019) Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci 20:19–33. 10.1038/s41583-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoury MK, Gupta K, Franco SR, Liu B (2020) Necroptosis in the Pathophysiology of Disease. Am J Pathol 190:272–285. 10.1016/j.ajpath.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onal T, Ozgul-Onal M, Chefetz I (2023) Mixed lineage kinase domain-like pseudokinase: Conventional (necroptosis) and unconventional (necroptosis-independent) functions and features. Advances in protein chemistry and structural biology 134:225–243. 10.1016/bs.apcsb.2022.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Tkachenko A, Havranek O (2024) Erythronecroptosis: an overview of necroptosis or programmed necrosis in red blood cells. Molecular and cellular biochemistry 10.1007/s11010-024-04948-8:Advance online publication. 10.1007/s11010-024-04948-8. [DOI] [PubMed] [Google Scholar]
- 40.Dionísio PA, Amaral JD, Rodrigues CMP (2020) Molecular mechanisms of necroptosis and relevance for neurodegenerative diseases. Int Rev Cel Mol Bio 353:31–82. 10.1016/bs.ircmb.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Jantas D, Lason W (2021) Preclinical Evidence for the Interplay between Oxidative Stress and RIP1-Dependent Cell Death in Neurodegeneration: State of the Art and Possible Therapeutic Implications. Antioxidants-Basel 10:1518. 10.3390/antiox10101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miao ZR, Miao ZY, Shi X, Wu H, Yao YJ, Xu SW (2022) The antagonistic effect of selenium on lead-induced apoptosis and necroptosis via P38/JNK/ERK pathway in chicken kidney. Ecotox Environ Safe 231:113176. 10.1016/j.ecoenv.2022.113176. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JY, Hao XF, Xu SW (2020) Selenium Prevents Lead-Induced Necroptosis by Restoring Antioxidant Functions and Blocking MAPK/NF-κB Pathway in Chicken Lymphocytes. Biol Trace Elem Res 198:644–653. 10.1007/s12011-020-02094-y. [DOI] [PubMed] [Google Scholar]
- 44.Chang XR, Niu SY, Shang MT, Li JY, Guo MH, Zhang WL, Sun ZY, Li YJ, Zhang R, Shen X, Tang M, Xue YY (2023) ROS-Drp1-mediated mitochondria fission contributes to hippocampal HT22 cell apoptosis induced by silver nanoparticles. Redox Biol 63:102739. 10.1016/j.redox.2023.102739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Zhang YY, Zheng X, Fang TY, Yang X, Luo X, Guo AL, Newell KA, Huang XF, Yu YH (2018) Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J Neuroinflamm 15(1):112. 10.1186/s12974-018-1141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li ZC, Wang LL, Zhao YS, Peng DJ, Chen J, Jiang SY, Zhao L, Aschner M, Li SJ, Jiang YM (2022) Sodium para-aminosalicylic acid ameliorates lead-induced hippocampal neuronal apoptosis by suppressing the activation of the IP3R-Ca2+-ASK1-p38 signaling pathway Ecotox Environ Safe 241:113829. 10.1016/j.ecoenv.2022.113829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao YS, Li JY, Li ZC, Wang LL, Gan CL, Chen J, Jiang SY, Aschner M, Ou SY, Jiang YM (2023) Sodium Para-aminosalicylic Acid Inhibits Lead-Induced Neuroinflammation in Brain Cortex of Rats by Modulating SIRT1/HMGB1/NF-κB Pathway. Neurochem Res 48:238–249. 10.1007/s11064-022-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng DJ, Li JY, Deng Y, Zhu XJ, Zhao L, Zhang YW, Li ZC, Ou SY, Li SJ, Jiang YM (2020) Sodium para-aminosalicylic acid inhibits manganese-induced NLRP3 inflammasome-dependent pyroptosis by inhibiting NF-κB pathway activation and oxidative stress. J Neuroinflamm 17:343. 10.1186/s12974-020-02018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pilat D, Paumier JM, García-González L, Louis L, Stephan D, Manrique C, Khrestchatisky M, Di Pasquale E, Baranger K, Rivera S (2022) MT5-MMP promotes neuroinflammation, neuronal excitability and Aβ production in primary neuron/astrocyte cultures from the 5xFAD mouse model of Alzheimer’s disease. J Neuroinflamm 19:65. 10.1186/s12974-022-02407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slota M, Wasik M, Stoltny T, Machon-Grecka A, Kasperczyk S (2022) Effects of environmental and occupational lead toxicity and its association with iron metabolism. Toxicol Appl Pharm 434:115794. 10.1016/j.taap.2021.115794. [DOI] [PubMed] [Google Scholar]
- 51.Yang JL, Juhasz AL, Li MY, Ding J, Xue XM, Zhou DM, Ma LQ, Li HB (2023) Chronic Exposure to Drinking Water As, Pb, and Cd at Provisional Guideline Values Reduces Weight Gain in Male Mice via Gut Microflora Alterations and Intestinal Inflammation. Environ Sci Technol 57:12981–12990. 10.1021/acs.est.3c02388. [DOI] [PubMed] [Google Scholar]
- 52.Albasher G, Al Kahtani S, Alwahibi MS, Almeer R (2020) Effect of Moringa oleifera Lam. methanolic extract on lead-induced oxidative stress-mediated hepatic damage and inflammation in rats. Environ Sci Pollut R 27:19877–19887. 10.1007/s11356-020-08525-6. [DOI] [PubMed] [Google Scholar]
- 53.Winiarska-Mieczan A (2014) Cumulative rate and distribution of Cd and Pb in the organs of adult male Wistar rats during oral exposure. Environmental Toxicology and Pharmacology 38:751–760. 10.1016/j.etap.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Bergdahl IA (2023) Correspondence on “Endocytosis-Mediated Transport of Pb in Rat Blood Cells”. Environ Sci Technol 57:15134–15135. 10.1021/acs.est.3c06212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J, Lee Y, Yang M (2014) Environmental Exposure to Lead (Pb) and Variations in Its Susceptibility. J Environ Sci Heal C 32:159–185. 10.1080/10590501.2014.907461. [DOI] [PubMed] [Google Scholar]
- 56.Levin R, Vieira CLZ, Rosenbaum MH, Bischoff K, Mordarski DC, Brown MJ (2021) The urban lead (Pb) burden in humans, animals and the natural environment. Environ Res 193:110377. 10.1016/j.envres.2020.110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McFarland MJ, Hauer ME, Reuben A (2022) Half of US population exposed to adverse lead levels in early childhood. P Natl Acad Sci USA 119:2118631119. 10.1073/pnas.2118631119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reuben A, Elliott ML, Abraham WC, Broadbent J, Houts RM, Ireland D, Knodt AR, Poulton R, Ramrakha S, Hariri AR, Caspi A, Moffitt TE (2020) Association of Childhood Lead Exposure With MRI Measurements of Structural Brain Integrity in Midlife. Jama-J Am Med Assoc 324:1970–1979. 10.1001/jama.2020.19998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW (2018) Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health 3:E177–E184. 10.1016/S2468-2667(18)30025-2. [DOI] [PubMed] [Google Scholar]
- 60.Carlson K, Neitzel RL (2018) Hearing loss, lead (Pb) exposure, and noise: a sound approach to ototoxicity exploration. J Toxicol Env Heal B 21:335–355. 10.1080/10937404.2018.1562391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angrand RC, Collins G, Landrigan PJ, Thomas VM (2022) Relation of blood lead levels and lead in gasoline: an updated systematic review. Environ Health-Glob 21:138. 10.1186/s12940-022-00936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ortega DR, Esquivel DFG, Ayala TB, Pineda B, Manzo SG, Quino JM, Mora PC, de la Cruz VP (2021) Cognitive Impairment Induced by Lead Exposure during Lifespan: Mechanisms of Lead Neurotoxicity. Toxics 9:23. 10.3390/toxics9020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahman A, Rao MS, Khan KM (2018) Intraventricular infusion of quinolinic acid impairs spatial learning and memory in young rats: a novel mechanism of lead-induced neurotoxicity. J Neuroinflamm 15:263. 10.1186/s12974-018-1306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bihaqi SW, Zawia NH (2013) Enhanced taupathy and AD-like pathology in aged primate brains decades after infantile exposure to lead (Pb). Neurotoxicology 39:95–101. 10.1016/j.neuro.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bihaqi SW, Bahmani A, Subaiea GM, Zawia NH (2014) Infantile exposure to lead and late-age cognitive decline: Relevance to AD. Alzheimers Dement 10:187–195. 10.1016/j.jalz.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bihaqi SW, Eid A, Zawia NH (2017) Lead exposure and tau hyperphosphorylation: An in vitro study. Neurotoxicology 62:218–223. 10.1016/j.neuro.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 67.Aminzadeh A, Salarinejad A (2019) Citicoline protects against lead-induced oxidative injury in neuronal PC12 cells. Biochem Cell Biol 97:715–721. 10.1139/bcb-2018-0218. [DOI] [PubMed] [Google Scholar]
- 68.Karri V, Kumar V, Ramos D, Oliveira E, Schuhmacher M (2018) An in vitro cytotoxic approach to assess the toxicity of heavy metals and their binary mixtures on hippocampal HT-22 cell line. Toxicol Lett 282:25–36. 10.1016/j.toxlet.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Rahman A, Al-Qenaie S, Rao MS, Khan KM, Guillemin GJ (2019) Memantine Is Protective against Cytotoxicity Caused by Lead and Quinolinic Acid in Cultured Rat Embryonic Hippocampal Cells. Chem Res Toxicol 32:1134–1143. 10.1021/acs.chemrestox.8b00421. [DOI] [PubMed] [Google Scholar]
- 70.Xie JK, Wu SC, Szadowski H, Min SH, Yang Y, Bowman AB, Rochet JC, Freeman JL, Yuan CL (2023) Developmental Pb exposure increases AD risk via altered intracellular Ca2+homeostasis in hiPSC-derived cortical neurons. J Biol Chem 299:105023. 10.1016/j.jbc.2023.105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R (2019) Low-Level Environmental Lead Exposure and Children’s Intellectual Function: An International Pooled Analysis Environ Health Persp 127:894–899. 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang MY, Li YB, Hu L, Luo D, Zhang Y, Xiao X, Li GL, Zhang LX, Zhu GC (2018) Lead exposure inhibits expression of SV2C through NRSF. Toxicology 398:23–30. 10.1016/j.tox.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 73.Oliveira ACA, Dionizio A, Teixeira FB, Bittencourt LO, Miranda GHN, Lopes GO, Varela ELP, Nabiça M, Ribera P, Dantas K, Leite A, Buzalaf MAR, Monteiro MC, Maia CSF, Lima RR (2020) Hippocampal Impairment Triggered by Long-Term Lead Exposure from Adolescence to Adulthood in Rats: Insights from Molecular to Functional Levels. Int J Mol Sci 21:6937. 10.3390/ijms21186937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu L-l, Zhang Y-w, Li Z-c, Fang Y-y, Wang L-l, Zhao Y-s, Li S-j, Ou S-y, Aschner M, Jiang Y-m (2022) Therapeutic Effects of Sodium Para-Aminosalicylic Acid on Cognitive Deficits and Activated ERK1/2-p90RSK/NF-κB Inflammatory Pathway in Pb-Exposed Rats. Biol Trace Elem Res 200:2807–2815. 10.1007/s12011-021-02874-0. [DOI] [PubMed] [Google Scholar]
- 75.Mansouri MT, Naghizadeh B, López-Larrubia P, Cauli O (2013) Behavioral deficits induced by lead exposure are accompanied by serotonergic and cholinergic alterations in the prefrontal cortex. Neurochem Int 62:232–239. 10.1016/j.neuint.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Caccamo A, Branca C, Piras IS, Ferreira E, Huentelman MJ, Liang WS, Readhead B, Dudley JT, Spangenberg EE, Green KN, Belfiore R, Winslow W, Oddo S (2017) Necroptosis activation in Alzheimer’s disease. Nat Neurosci 20:1236–+. 10.1038/nn.4608. [DOI] [PubMed] [Google Scholar]
- 77.Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in inflammation. Nature 517:311–320. 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 78.Newton K (2015) RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol 25:347–353. 10.1016/j.tcb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Jayaraman A, Htike TT, James R, Picon C, Reynolds R (2021) TNF-mediated neuroinflammation is linked to neuronal necroptosis in Alzheimer’s disease hippocampus. Acta Neuropathol Com 9:159. 10.1186/s40478-021-01264-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fischer R, Maier O (2015) Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxidative Medicine and Cellular Longevity 2015:610813. 10.1155/2015/610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen AQ, Fang Z, Chen XL, Yang S, Zhou YF, Mao L, Xia YP, Jin HJ, Li YN, You MF, Wang XX, Lei H, He QW, Hu B (2019) Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis 10:487. 10.1038/s41419-019-1716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lalrindika L, Khushboo M, Bhanushree B, Dinata R, Das M, Nisa N, Lalrinzuali S, Manikandan B, Saeed-Ahmed L, Sanjeev S, Gurusubramanian G, Bidanchi RM, Murthy MK, Roy VK (2022) Antioxidative, anti-inflammatory and anti-apoptotic action of ellagic acid against lead acetate induced testicular and hepato-renal oxidative damages and pathophysiological changes in male Long Evans rats. Environ Pollut 302:119048. 10.1016/j.envpol.2022.119048. [DOI] [PubMed] [Google Scholar]
- 83.Kim JH, Lee KH, Yoo DH, Kang D, Cho SH, Hong YC (2007) GSTM1 and TNF-alpha gene polymorphisms and relations between blood lead and inflammatory markers in a non-occupational population. Mutat Res-Gen Tox En 629:32–39. 10.1016/j.mrgentox.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 84.Liu S, Wang X, Li Y, Xu L, Yu XL, Ge L, Li J, Zhu YJ, He SD (2014) Necroptosis Mediates TNF-Induced Toxicity of Hippocampal Neurons. Biomed Res Int 2014:290182. 10.1155/2014/290182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruan QW, Ruan J, Zhang WB, Qian F, Yu ZW (2018) Targeting NAD + degradation: The therapeutic potential of flavonoids for Alzheimer’s disease and cognitive frailty (vol 128, pg 345, 2018). Pharmacol Res 132:242–244. 10.1016/j.phrs.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 86.Pandey N, Black BE (2021) Rapid Detection and Signaling of DNA Damage by PARP-1. Trends in Biochemical Sciences 46:744–757. 10.1016/j.tibs.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fouquerel E, Sobol RW (2014) ARTD1 (PARP1) activation and NAD in DNA repair and cell death. DNA Repair 23:27–32. 10.1016/j.dnarep.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou YX, Liu LH, Tao SF, Yao YH, Wang YL, Wei QC, Shao AW, Deng YC (2021) Parthanatos and its associated components: Promising therapeutic targets for cancer. Pharmacol Res 163:105299. 10.1016/j.phrs.2020.105299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


