Abstract
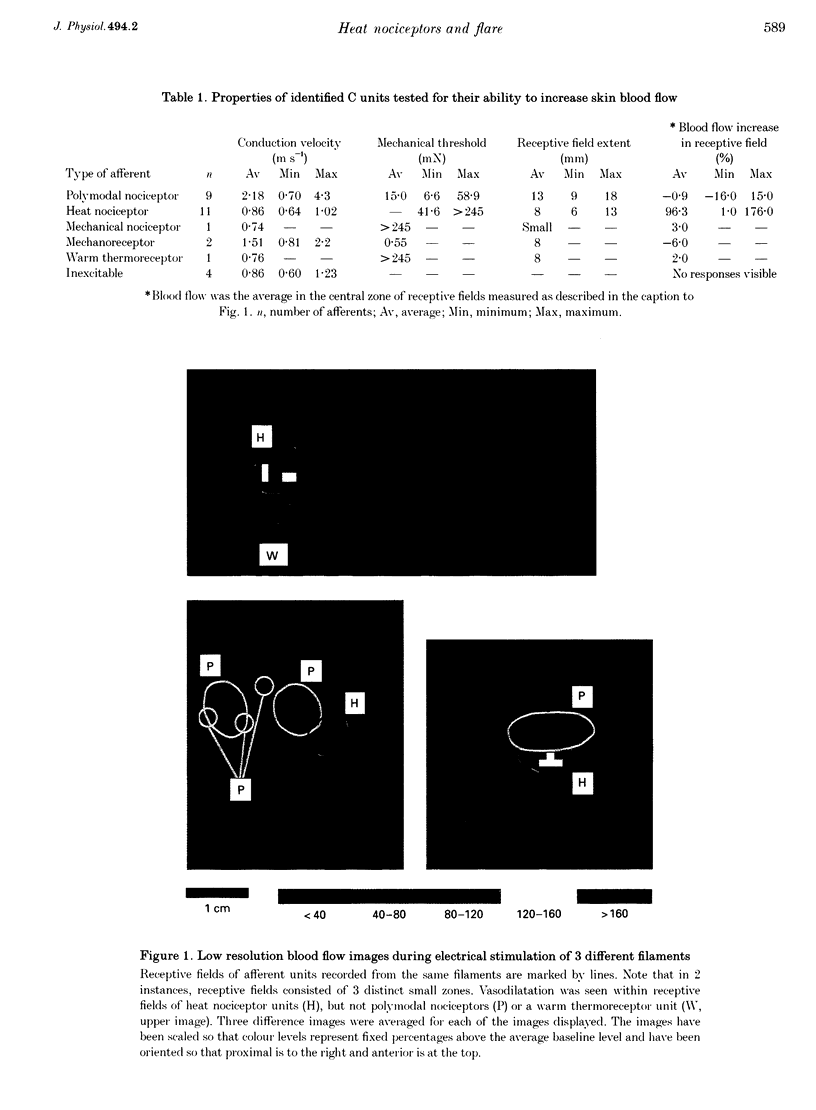
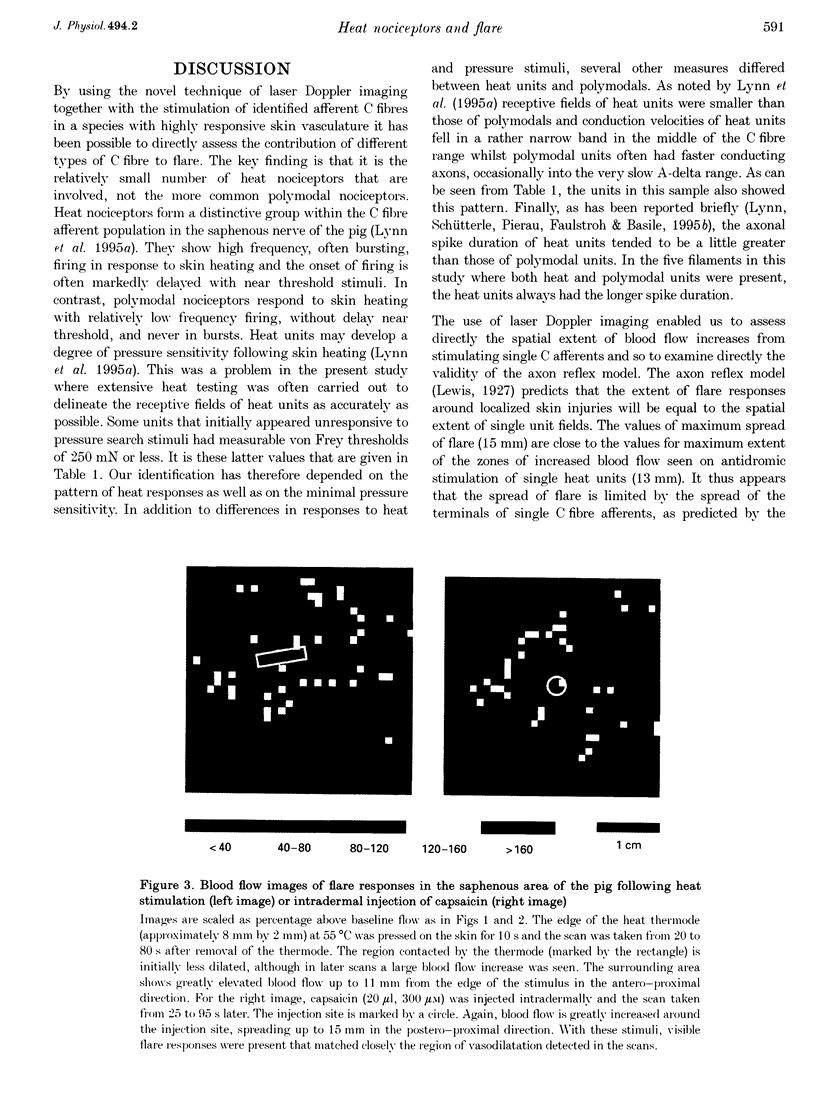
1. Skin blood flow has been imaged during stimulation of fine nerve filaments containing small numbers of identified C fibre units. Filaments were dissected from the saphenous nerve of anaesthetized pigs. 2. Stimulation of filaments containing C heat nociceptor units gave small areas of elevated blood flow (average increase 96%, n = 11) restricted to the afferent receptive field. The extent of the areas of raised blood flow was imaged completely for 8 units. The average extent of vasodilatation in the direction of greatest spread was 8 mm and the maximum spread in any unit was 13 mm. 3. Stimulation of C polymodal nociceptor units never caused increases in blood flow in or near their receptive fields. 4. Localized noxious stimuli (55 degrees C or intradermal injection of capsaicin) caused flare extending 7-15 mm in the same skin region. 5. In agreement with the axon reflex model, spread of flare was restricted to the zone innervated by the terminals of single C fibre units. 6. It is concluded that the C heat nociceptor units are the major class of afferent involved in the flare reaction in the skin of the pig. C polymodal nociceptor units do not appear to be involved in flare in this species. The probable situation in human skin, which is also innervated by heat nociceptors, is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann T. K., Simone D. A., Shain C. N., LaMotte R. H. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991 Jul;66(1):212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Bessou P., Perl E. R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Bharali L. A., Lisney S. J. The relationship between unmyelinated afferent type and neurogenic plasma extravasation in normal and reinnervated rat skin. Neuroscience. 1992;47(3):703–712. doi: 10.1016/0306-4522(92)90178-5. [DOI] [PubMed] [Google Scholar]
- Fleischer E., Handwerker H. O., Joukhadar S. Unmyelinated nociceptive units in two skin areas of the rat. Brain Res. 1983 May 9;267(1):81–92. doi: 10.1016/0006-8993(83)91041-7. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenins P. Identification of the unmyelinated sensory nerves which evoke plasma extravasation in response to antidromic stimulation. Neurosci Lett. 1981 Sep 1;25(2):137–141. doi: 10.1016/0304-3940(81)90321-9. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Fields H. L., Basbaum A. I. Peptides and the primary afferent nociceptor. J Neurosci. 1993 Jun;13(6):2273–2286. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Lynn B., Cotsell B. Blood flow increases in the skin of the anaesthetized rat that follow antidromic sensory nerve stimulation and strong mechanical stimulation. Neurosci Lett. 1992 Mar 30;137(2):249–252. doi: 10.1016/0304-3940(92)90415-4. [DOI] [PubMed] [Google Scholar]
- Lynn B., Faulstroh K., Pierau F. K. The classification and properties of nociceptive afferent units from the skin of the anaesthetized pig. Eur J Neurosci. 1995 Mar 1;7(3):431–437. doi: 10.1111/j.1460-9568.1995.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Lynn B. Neurogenic inflammation. Skin Pharmacol. 1988;1(4):217–224. doi: 10.1159/000210778. [DOI] [PubMed] [Google Scholar]
- Moskowitz M. A., Brody M., Liu-Chen L. Y. In vitro release of immunoreactive substance P from putative afferent nerve endings in bovine pia arachnoid. Neuroscience. 1983 Aug;9(4):809–814. doi: 10.1016/0306-4522(83)90269-5. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Schmelz M., Forster C., Ringkamp M., Torebjörk E., Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995 Jan;15(1 Pt 1):333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. H., Barker J. N., Morris R. W., MacDonald D. M., Lee T. H. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J Immunol. 1993 Sep 15;151(6):3274–3282. [PubMed] [Google Scholar]
- Szolcsányi J. Antidromic vasodilatation and neurogenic inflammation. Agents Actions. 1988 Feb;23(1-2):4–11. doi: 10.1007/BF01967170. [DOI] [PubMed] [Google Scholar]
- Wårdell K., Naver H. K., Nilsson G. E., Wallin B. G. The cutaneous vascular axon reflex in humans characterized by laser Doppler perfusion imaging. J Physiol. 1993 Jan;460:185–199. doi: 10.1113/jphysiol.1993.sp019466. [DOI] [PMC free article] [PubMed] [Google Scholar]