Abstract
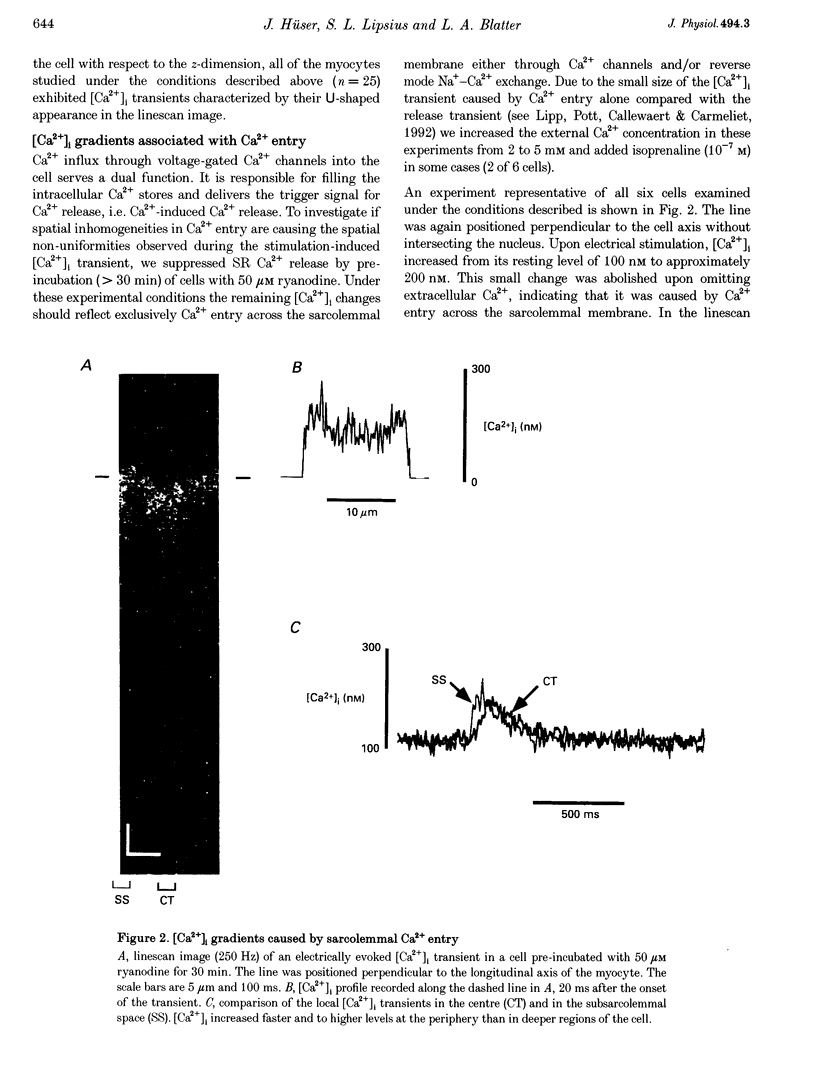
1. Confocal microscopy in combination with the calcium-sensitive fluorescent probe fluo-3 was used to study spatial aspects of intracellular Ca2+ signals during excitation-contraction coupling in isolated atrial myocytes from cat heart. 2. Imaging of [Ca2+]i transients evoked by electrical stimulation revealed that Ca2+ release started at the periphery and subsequently spread towards the centre of the myocyte. 3. Blocking sarcoplasmic reticulum (SR) Ca2+ release with 50 microM ryanodine unmasked spatial inhomogeneities in the [Ca2+]i was higher in the periphery than in central regions of the myocyte. 4. Positive (or negative) staircase or postrest potentiation of the 'whole-cell' [Ca2+] transients were paralleled by characteristic changes in the spatial profile of the [Ca2+]i signal. With low SR Ca2+ load [Ca2+]i transients in the subsarcolemmal space were small and no Ca2+ release in the centre of the cell was observed. Loading of the SR increased subsarcolemmal [Ca2+]i transient amplitude and subsequently triggered further release in more central regions of the cell. 5. Spontaneous Ca2+ release from functional SR units, i.e. Ca2+ sparks, occurred at higher frequency in the subsarcolemmal space than in more central regions of the myocyte. 6. Visualization of the surface membrane using the membrane-selective dye Di-8-ANEPPS demonstrated that transverse tubules (t-tubules) were absent in atrial cells. 7. It is concluded that in atrial myocytes voltage-dependent Ca2+ entry triggers Ca2+ release from peripheral coupling SR that subsequently induces further Ca2+ release from stores in more central regions of the myocyte. Spreading of Ca2+ release from the cell periphery to the centre accounts for [Ca2+]i gradients underlying the whole-cell [Ca2+]i transient. The finding that cat atrial myocytes lack t-tubules demonstrates the functional importance of Ca2+ release from extended junctional (corbular) SR in these cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Budde T., Lipp P., Pott L. Measurement of Ca2(+)-release-dependent inward current reveals two distinct components of Ca2+ release from sarcoplasmic reticulum in guinea-pig atrial myocytes. Pflugers Arch. 1991 Feb;417(6):638–644. doi: 10.1007/BF00372963. [DOI] [PubMed] [Google Scholar]
- Callewaert G. Excitation-contraction coupling in mammalian cardiac cells. Cardiovasc Res. 1992 Oct;26(10):923–932. doi: 10.1093/cvr/26.10.923. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994 Nov;67(5):1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. The control of calcium release in heart muscle. Science. 1995 May 19;268(5213):1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Carl S. L., Felix K., Caswell A. H., Brandt N. R., Ball W. J., Jr, Vaghy P. L., Meissner G., Ferguson D. G. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J Cell Biol. 1995 May;129(3):673–682. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Cannell M. B., Lederer W. J. Propagation of excitation-contraction coupling into ventricular myocytes. Pflugers Arch. 1994 Oct;428(3-4):415–417. doi: 10.1007/BF00724526. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Dolber P. C., Sommer J. R. Corbular sarcoplasmic reticulum of rabbit cardiac muscle. J Ultrastruct Res. 1984 May;87(2):190–196. doi: 10.1016/s0022-5320(84)80078-7. [DOI] [PubMed] [Google Scholar]
- Harkins A. B., Kurebayashi N., Baylor S. M. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophys J. 1993 Aug;65(2):865–881. doi: 10.1016/S0006-3495(93)81112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- KOCH-WESER J., BLINKS J. R. THE INFLUENCE OF THE INTERVAL BETWEEN BEATS ON MYOCARDIAL CONTRACTILITY. Pharmacol Rev. 1963 Sep;15:601–652. [PubMed] [Google Scholar]
- Lewartowski B., Pytkowski B. Cellular mechanism of the relationship between myocardial force and frequency of contractions. Prog Biophys Mol Biol. 1987;50(2):97–120. doi: 10.1016/0079-6107(87)90005-8. [DOI] [PubMed] [Google Scholar]
- Lipp P., Pott L., Callewaert G., Carmeliet E. Calcium transients caused by calcium entry are influenced by the sarcoplasmic reticulum in guinea-pig atrial myocytes. J Physiol. 1992 Aug;454:321–338. doi: 10.1113/jphysiol.1992.sp019266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Pott L., Callewaert G., Carmeliet E. Simultaneous recording of Indo-1 fluorescence and Na+/Ca2+ exchange current reveals two components of Ca2(+)-release from sarcoplasmic reticulum of cardiac atrial myocytes. FEBS Lett. 1990 Nov 26;275(1-2):181–184. doi: 10.1016/0014-5793(90)81467-3. [DOI] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995 May 19;268(5213):1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local, stochastic release of Ca2+ in voltage-clamped rat heart cells: visualization with confocal microscopy. J Physiol. 1994 Oct 1;480(Pt 1):21–29. doi: 10.1113/jphysiol.1994.sp020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Fawcett D. W. The ultrastructure of the cat myocardium. II. Atrial muscle. J Cell Biol. 1969 Jul;42(1):46–67. doi: 10.1083/jcb.42.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechmann S., Pott L. Identification of Na-Ca exchange current in single cardiac myocytes. Nature. 1986 Feb 13;319(6054):597–599. doi: 10.1038/319597a0. [DOI] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y. Role of ryanodine receptors. Crit Rev Biochem Mol Biol. 1994;29(4):229–274. doi: 10.3109/10409239409083482. [DOI] [PubMed] [Google Scholar]
- Shacklock P. S., Wier W. G., Balke C. W. Local Ca2+ transients (Ca2+ sparks) originate at transverse tubules in rat heart cells. J Physiol. 1995 Sep 15;487(Pt 3):601–608. doi: 10.1113/jphysiol.1995.sp020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. G., Lipsius S. L. beta-Adrenergic stimulation induces acetylcholine to activate ATP-sensitive K+ current in cat atrial myocytes. Circ Res. 1995 Sep;77(3):565–574. doi: 10.1161/01.res.77.3.565. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Vereecke J., Carmeliet E., Lipsius S. L. Ionic currents activated during hyperpolarization of single right atrial myocytes from cat heart. Circ Res. 1991 Apr;68(4):1059–1069. doi: 10.1161/01.res.68.4.1059. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Lipsius S. L. Na(+)-Ca2+ exchange current in latent pacemaker cells isolated from cat right atrium. J Physiol. 1993 Jul;466:263–285. [PMC free article] [PubMed] [Google Scholar]








