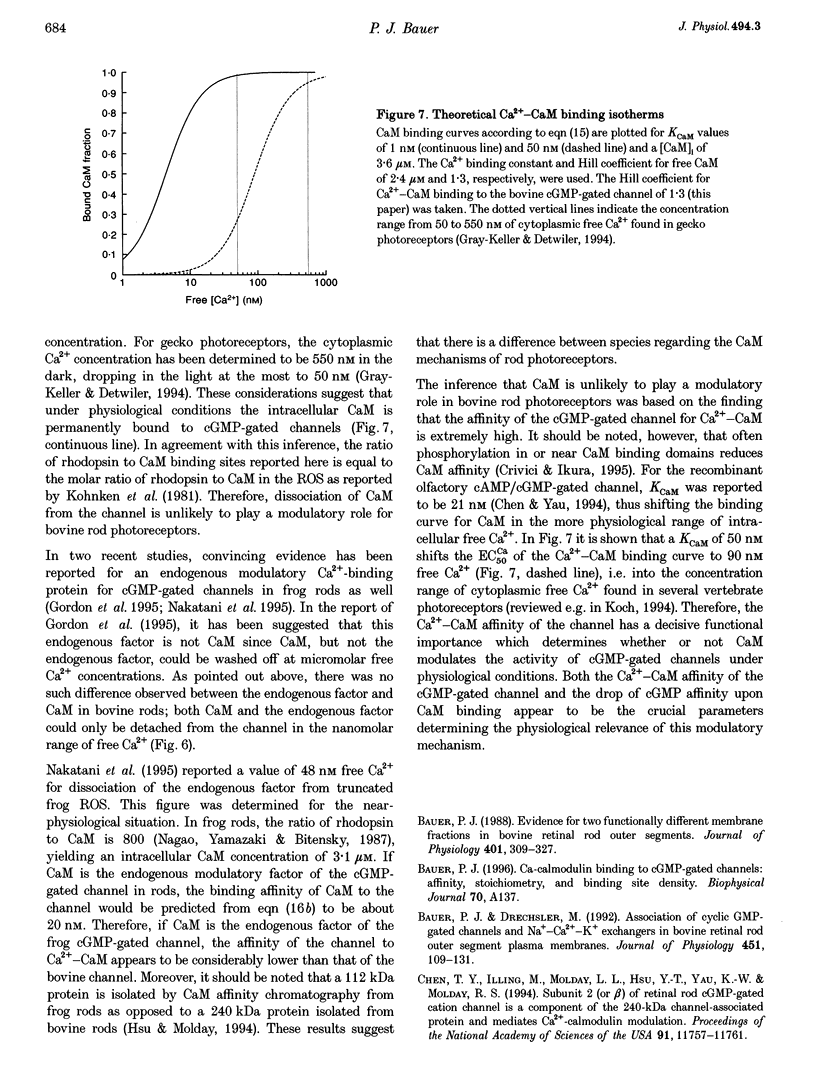
Abstract
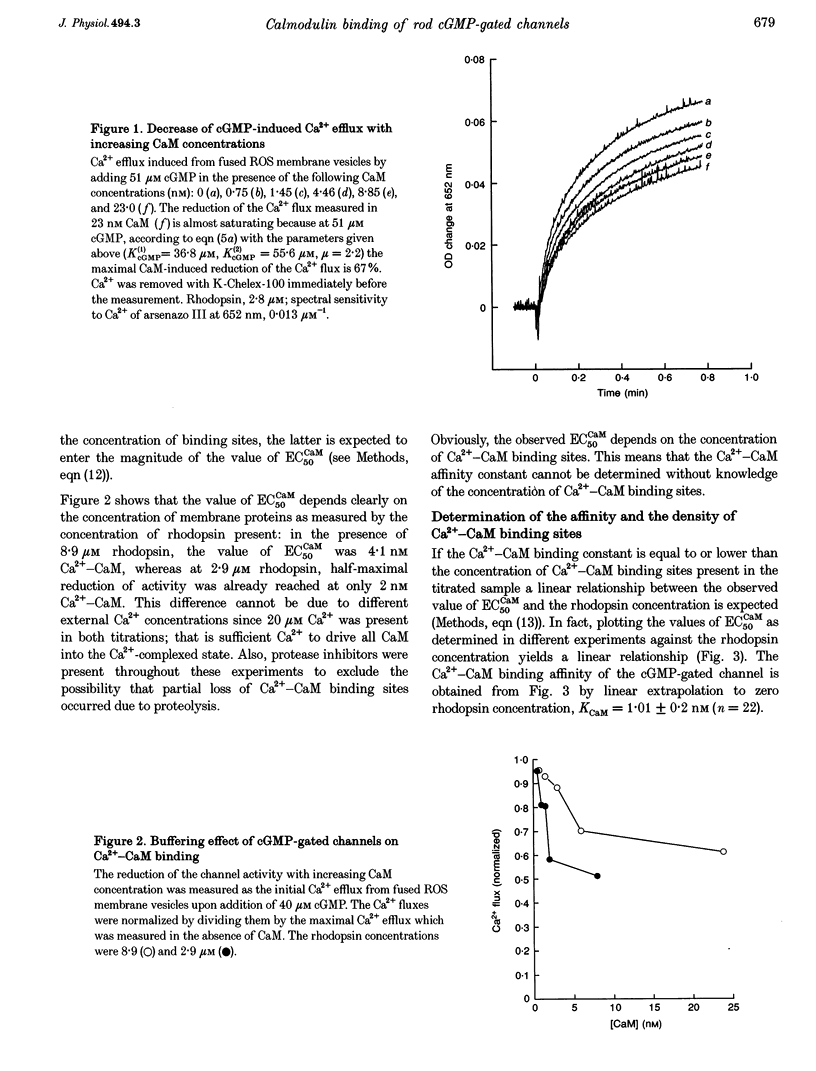
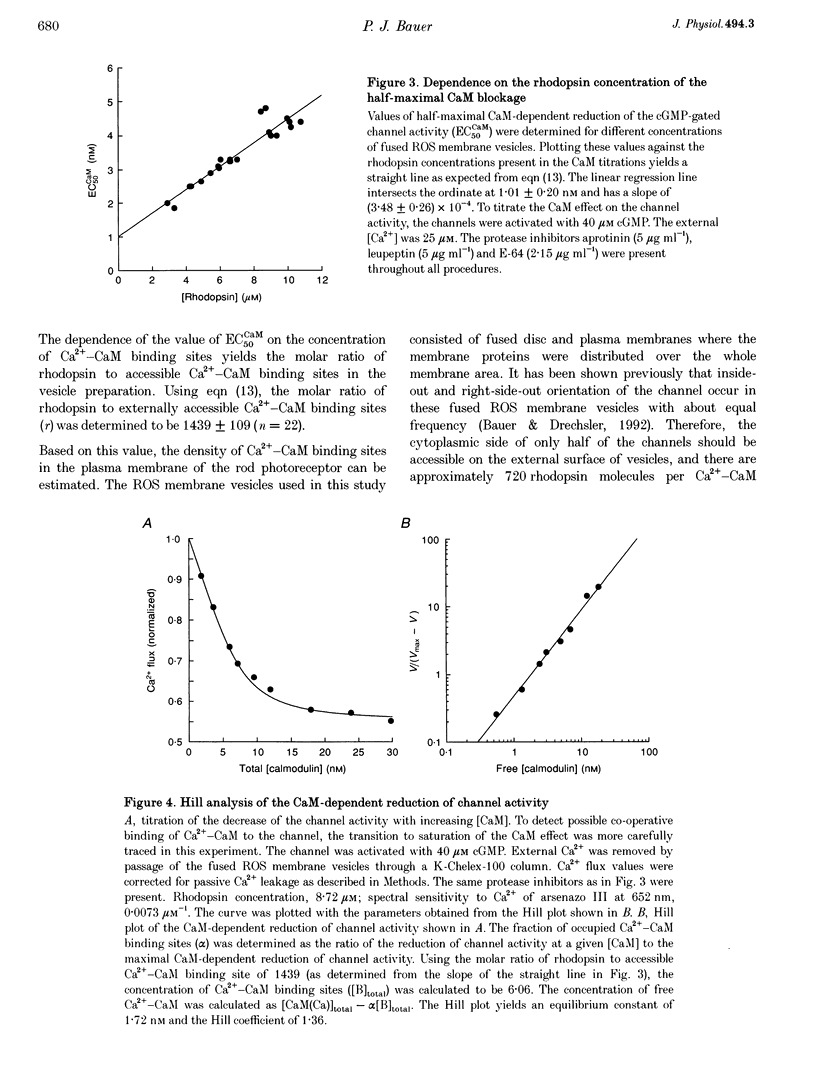
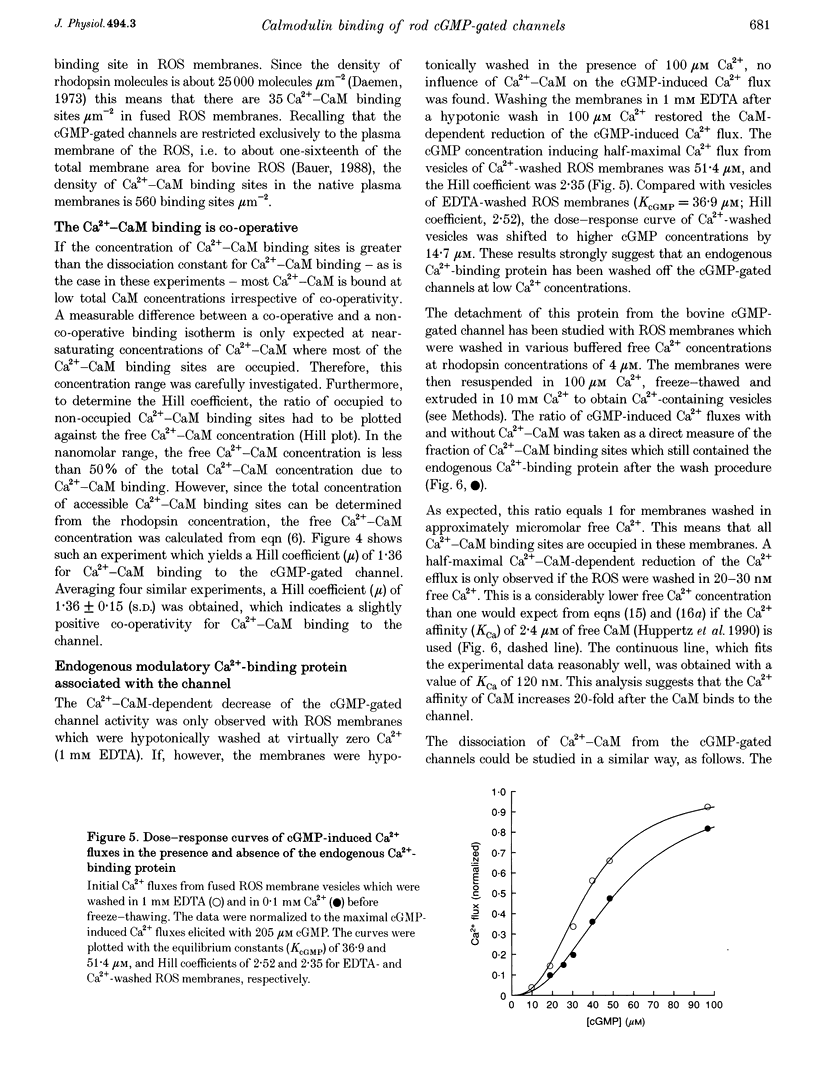
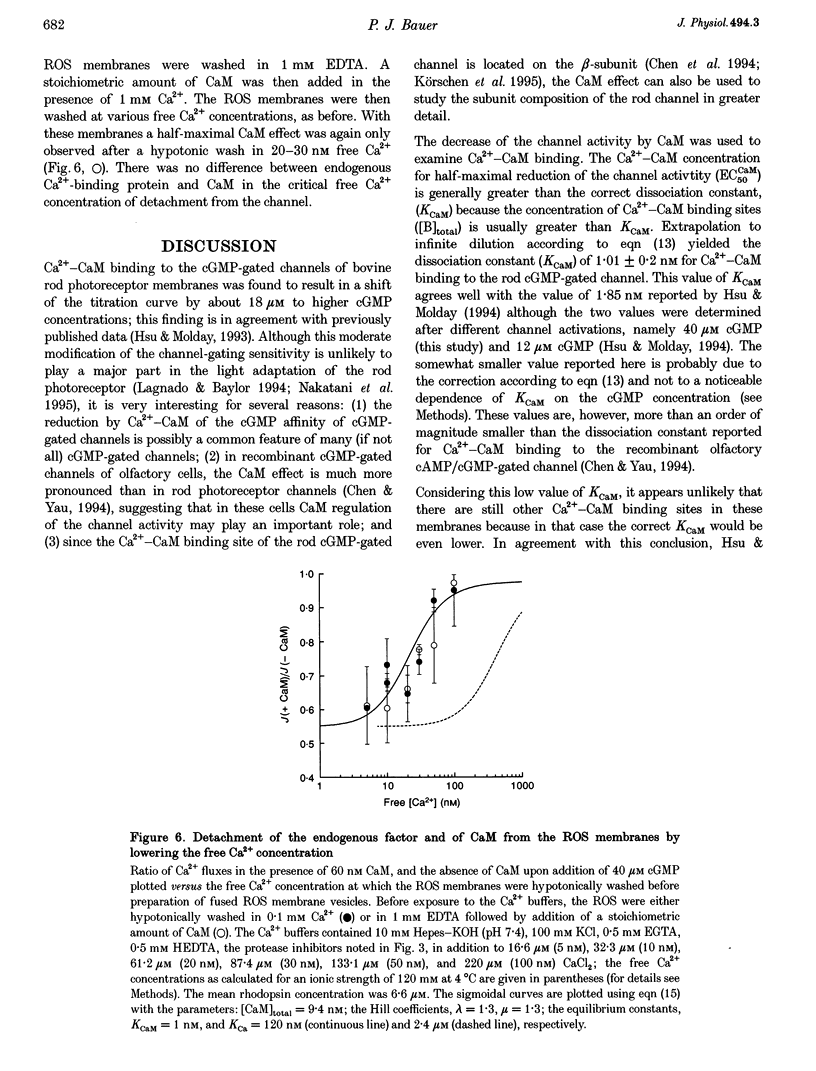
1. Ca(2+)-loaded vesicles of bovine rod outer segment (ROS) membranes were used to examine the influence of Ca(2+)-calmodulin (Ca(2+)-CaM) on the activity of cGMP-gated channels. 2. In vesicles prepared from ROS membranes which were washed at zero free Ca2+, Ca(2+)-CaM reduced the Ca2+ flux to maximally 40%. The dose-response curve for activation of the cGMP-gated channel had a half-maximal value of 36.8 +/- 2 microM in the CaM-free state, and of 55.6 +/- 5.2 microM in the Ca(2+)-CaM-bound state. In both cases the Hill coefficients were 2.2 +/- 0.2. 3. In vesicles prepared from ROS membranes which were washed at 100 microM Ca2+, the dose-response curve was identical to the Ca(2+)-CaM-bound state. 4. Titration of the Ca(2+)-CaM-dependent decrease of the channel activity upon addition of 40 microM cGMP yielded half-maximal Ca(2+)-CaM concentrations (EC50CaM) which were linearly correlated with the concentration of membrane vesicles. Extrapolation of EC50CaM to infinite dilution of vesicles yielded a Ca(2+)-CaM affinity constant for the cGMP-gated channel of 1.01 +/- 0.20 nM. Hill analysis of the Ca(2+)-CaM titrations resulted in a Hill coefficient of 1.36 +/- 0.15. 5. From the slope of the linear regression of EC50CaM plotted vs. the rhodopsin concentration, the molar ratio of rhodopsin to externally accessible Ca(2+)-CaM binding sites of fused ROS membranes was determined to be 1439 +/- 109. Therefore, there are about 720 molecules of rhodopsin per Ca(2+)-CaM binding site present in ROS. 6. Based on these data, a density of 560 Ca(2+)-CaM binding sites micron-2 is estimated for the plasma membrane of bovine ROS, suggesting that there are two Ca(2+)-CaM binding sites per channel. 7. The Ca(2+)-CaM effect did not become noticeable until the ROS membranes were hypotonically washed at free [Ca2+] below 100 nM, suggesting that an endogenous Ca(2+)-binding protein was washed off in the absence of Ca2+. 8. If the endogenous Ca(2+)-binding protein of bovine ROS membranes was washed off at zero Ca2+ and then Ca(2+)-CaM added, Ca(2+)-CaM could only be washed off again at free [Ca2+] below 100 nM. 9. These findings strongly suggest that the endogenous Ca(2+)-binding protein of the bovine cGMP-gated channel is CaM.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer P. J., Drechsler M. Association of cyclic GMP-gated channels and Na(+)-Ca(2+)-K+ exchangers in bovine retinal rod outer segment plasma membranes. J Physiol. 1992;451:109–131. doi: 10.1113/jphysiol.1992.sp019156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P. J. Evidence for two functionally different membrane fractions in bovine retinal rod outer segments. J Physiol. 1988 Jul;401:309–327. doi: 10.1113/jphysiol.1988.sp017164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. Y., Illing M., Molday L. L., Hsu Y. T., Yau K. W., Molday R. S. Subunit 2 (or beta) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca(2+)-calmodulin modulation. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11757–11761. doi: 10.1073/pnas.91.24.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. Y., Yau K. W. Direct modulation by Ca(2+)-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994 Apr 7;368(6471):545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Cook N. J., Molday L. L., Reid D., Kaupp U. B., Molday R. S. The cGMP-gated channel of bovine rod photoreceptors is localized exclusively in the plasma membrane. J Biol Chem. 1989 Apr 25;264(12):6996–6999. [PubMed] [Google Scholar]
- Crivici A., Ikura M. Molecular and structural basis of target recognition by calmodulin. Annu Rev Biophys Biomol Struct. 1995;24:85–116. doi: 10.1146/annurev.bb.24.060195.000505. [DOI] [PubMed] [Google Scholar]
- Crouch T. H., Klee C. B. Positive cooperative binding of calcium to bovine brain calmodulin. Biochemistry. 1980 Aug 5;19(16):3692–3698. doi: 10.1021/bi00557a009. [DOI] [PubMed] [Google Scholar]
- Daemen F. J. Vertebrate rod outer segment membranes. Biochim Biophys Acta. 1973 Nov 28;300(3):255–288. doi: 10.1016/0304-4157(73)90006-3. [DOI] [PubMed] [Google Scholar]
- Dedman J. R., Potter J. D., Jackson R. L., Johnson J. D., Means A. R. Physicochemical properties of rat testis Ca2+-dependent regulator protein of cyclic nucleotide phosphodiesterase. Relationship of Ca2+-binding, conformational changes, and phosphodiesterase activity. J Biol Chem. 1977 Dec 10;252(23):8415–8422. [PubMed] [Google Scholar]
- Gordon S. E., Downing-Park J., Zimmerman A. L. Modulation of the cGMP-gated ion channel in frog rods by calmodulin and an endogenous inhibitory factor. J Physiol. 1995 Aug 1;486(Pt 3):533–546. doi: 10.1113/jphysiol.1995.sp020832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller M. P., Detwiler P. B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994 Oct;13(4):849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Hsu Y. T., Molday R. S. Interaction of calmodulin with the cyclic GMP-gated channel of rod photoreceptor cells. Modulation of activity, affinity purification, and localization. J Biol Chem. 1994 Nov 25;269(47):29765–29770. [PubMed] [Google Scholar]
- Hsu Y. T., Molday R. S. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993 Jan 7;361(6407):76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Huppertz B., Weyand I., Bauer P. J. Ca2+ binding capacity of cytoplasmic proteins from rod photoreceptors is mainly due to arrestin. J Biol Chem. 1990 Jun 5;265(16):9470–9475. [PubMed] [Google Scholar]
- Kaupp U. B., Koch K. W. Role of cGMP and Ca2+ in vertebrate photoreceptor excitation and adaptation. Annu Rev Physiol. 1992;54:153–175. doi: 10.1146/annurev.ph.54.030192.001101. [DOI] [PubMed] [Google Scholar]
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993 Apr 29;362(6423):855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- Koch K. W. Calcium as modulator of phototransduction in vertebrate photoreceptor cells. Rev Physiol Biochem Pharmacol. 1994;125:149–192. doi: 10.1007/BFb0030910. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Kohnken R. E., Chafouleas J. G., Eadie D. M., Means A. R., McConnell D. G. Calmodulin in bovine rod outer segments. J Biol Chem. 1981 Dec 10;256(23):12517–12522. [PubMed] [Google Scholar]
- Körschen H. G., Illing M., Seifert R., Sesti F., Williams A., Gotzes S., Colville C., Müller F., Dosé A., Godde M. A 240 kDa protein represents the complete beta subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron. 1995 Sep;15(3):627–636. doi: 10.1016/0896-6273(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. A. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature. 1994 Jan 20;367(6460):273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. Signal flow in visual transduction. Neuron. 1992 Jun;8(6):995–1002. doi: 10.1016/0896-6273(92)90122-t. [DOI] [PubMed] [Google Scholar]
- Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988 Jul 7;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- Nagao S., Yamazaki A., Bitensky M. W. Calmodulin and calmodulin binding proteins in amphibian rod outer segments. Biochemistry. 1987 Mar 24;26(6):1659–1665. doi: 10.1021/bi00380a026. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Koutalos Y., Yau K. W. Ca2+ modulation of the cGMP-gated channel of bullfrog retinal rod photoreceptors. J Physiol. 1995 Apr 1;484(Pt 1):69–76. doi: 10.1113/jphysiol.1995.sp020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and light adaptation in retinal rods and cones. Nature. 1988 Jul 7;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Schoenmakers T. J., Visser G. J., Flik G., Theuvenet A. P. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992 Jun;12(6):870-4, 876-9. [PubMed] [Google Scholar]
- Yau K. W. Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):9–32. [PubMed] [Google Scholar]


