Abstract
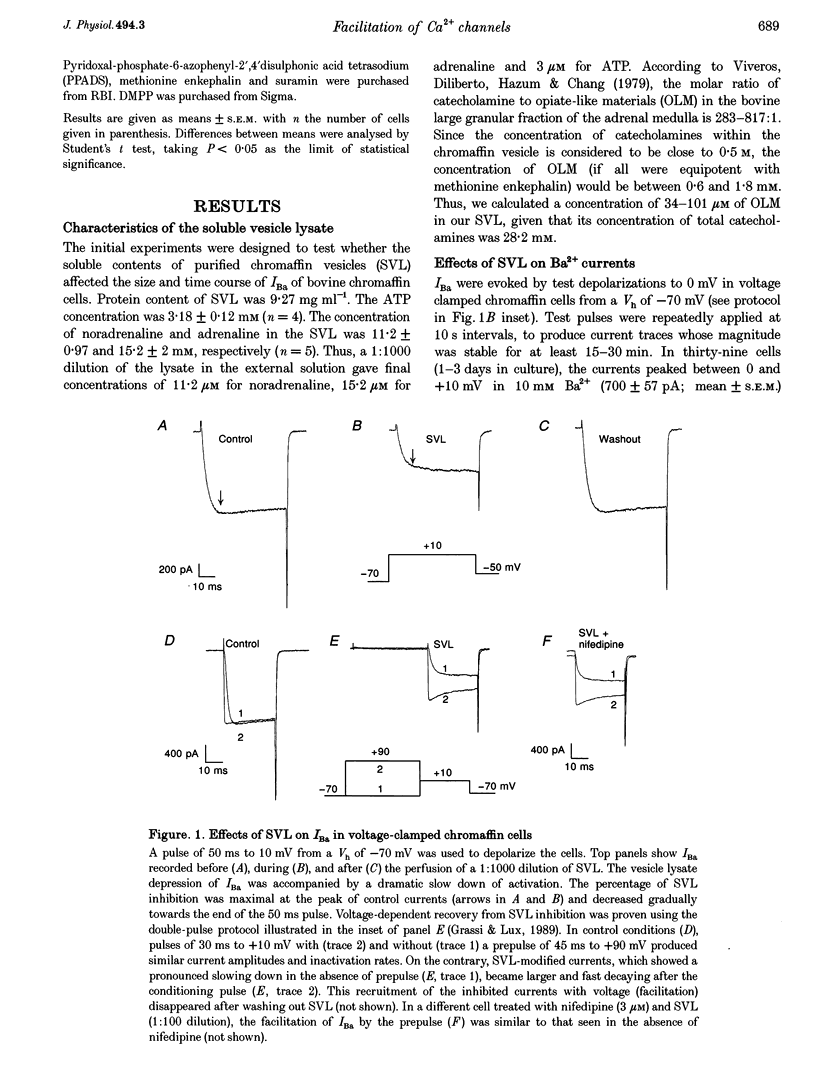
1. This study was planned to clarify the mechanism of Ca2+ channel facilitation by depolarizing prepulses given to voltage-clamped bovine chromaffin cells. The hypothesis for an autocrine modulation of such channels was tested by studying the effects of a soluble vesicle lysate (SVL) on whole-cell Ba2+ currents (IBa). 2. SVL was prepared from a bovine adrenal medullary homogenate. The ATP content in this concentrated SVL amounted to 3.18 +/- 0.12 mM (n = 4). The concentration of noradrenaline and adrenaline present in the SVL was 11.2 +/- 0.97 and 15.2 +/- 2 mM, respectively (n = 5). A 1:1000 dilution of SVL in the external solution halved the magnitude of IBa and produced a 7-fold slowing of its activation kinetics. The blocking effects of SVL were concentration dependent and quickly reversed upon washout. 3. Inhibition and slowing of the kinetics of IBa by SVL could be partially reversed by strong depolarizing prepulses (+90 mV, 45 ms). This reversal of inhibition, called Ca2+ channel facilitation, persisted in the presence of 3 microM nifedipine. 4. Intracellular dialysis of GDP-beta-S (0.5 mM) or pretreatment of the cells with pertussis toxin (100 ng ml-1 for 18-24 h) prevented the reduction in peak current caused by a 1:100 dilution of SVL; no prepulse facilitation could be observed under these conditions. 5. The receptor blockers naloxone (10 microM) or suramin (100 microM) and PPADS (100 microM) largely antagonized the effects of SVL. Treatment of SVL with alkaline phosphatase or dialysis against a saline buffer to remove low molecular mass materials (< 10 kDa) considerably reduced the activity of SVL. 6. Stopping the flow of the external solution (10 mM Ba2+) gradually reduced the size, and slowed down the activation phase, of the current. Prepulse facilitation of IBa was absent or weak in a superfused cell, but was massive upon flow-stop conditions in the presence or absence of 3 microM nifedipine. 7. Our experiments suggest that facilitation by prepulses of whole-cell current through Ca2+ channels is due to the suppression of an autoinhibitory autocrine loop present in bovine chromaffin cells. By acting at least on purinergic and opiate receptors, the exocytotic release of ATP and opiates will cause a tonic inhibition of the current through a G-protein-mediated mechanism. Such a mechanism will be removed by strong depolarizing prepulses, and will involve preferentially non-L-type channels. In the light of these and other recent results, previously held views on the selective recruitment by prepulses of dihydropyridine-sensitive Ca2+ channels are not tenable.
Full text
PDF

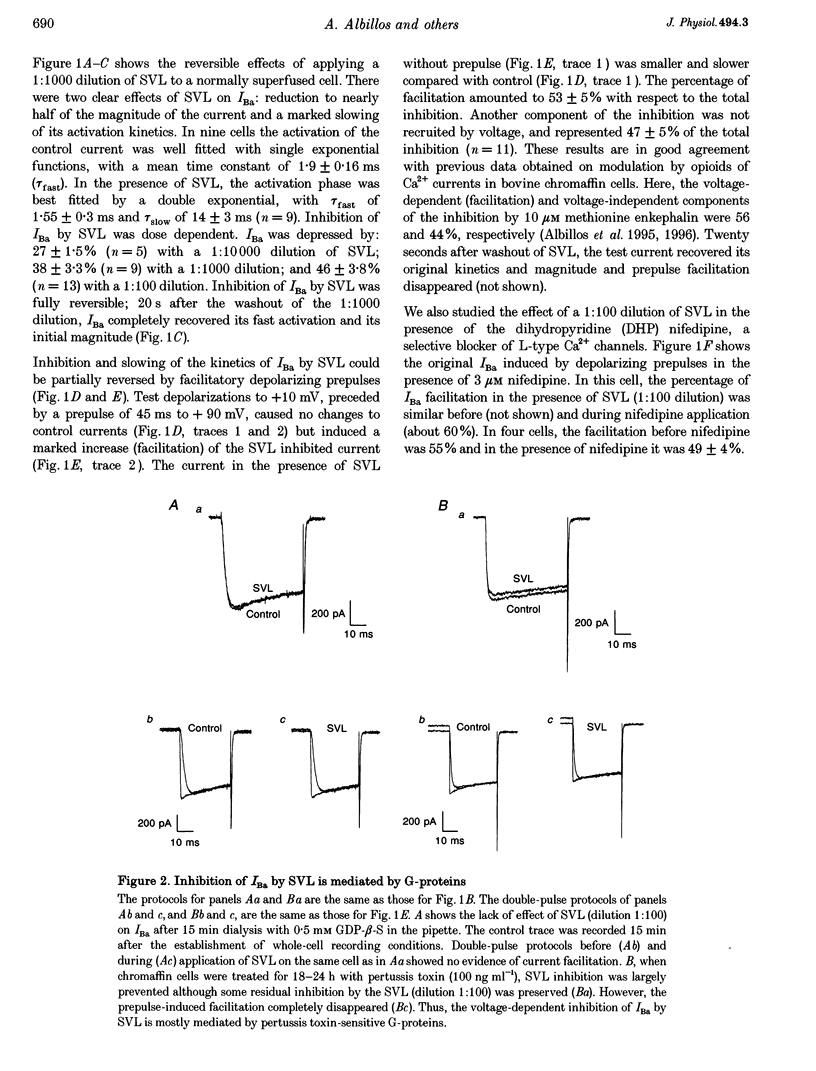


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
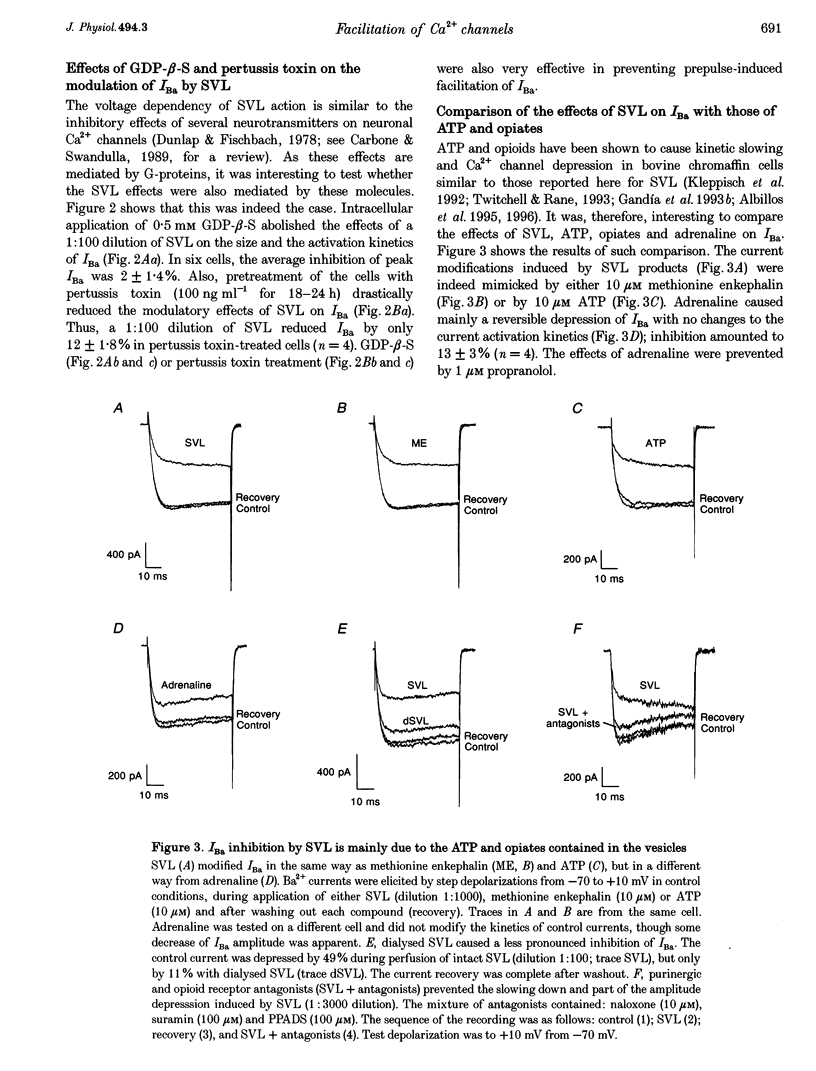
- Albillos A., Artalejo A. R., López M. G., Gandía L., García A. G., Carbone E. Calcium channel subtypes in cat chromaffin cells. J Physiol. 1994 Jun 1;477(Pt 2):197–213. doi: 10.1113/jphysiol.1994.sp020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albillos A., García A. G., Gandía L. omega-Agatoxin-IVA-sensitive calcium channels in bovine chromaffin cells. FEBS Lett. 1993 Dec 27;336(2):259–262. doi: 10.1016/0014-5793(93)80815-c. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Adams M. E., Fox A. P. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994 Jan 6;367(6458):72–76. doi: 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Ariano M. A., Perlman R. L., Fox A. P. Activation of facilitation calcium channels in chromaffin cells by D1 dopamine receptors through a cAMP/protein kinase A-dependent mechanism. Nature. 1990 Nov 15;348(6298):239–242. doi: 10.1038/348239a0. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Dahmer M. K., Perlman R. L., Fox A. P. Two types of Ca2+ currents are found in bovine chromaffin cells: facilitation is due to the recruitment of one type. J Physiol. 1991 Jan;432:681–707. doi: 10.1113/jphysiol.1991.sp018406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo C. R., Perlman R. L., Fox A. P. Omega-conotoxin GVIA blocks a Ca2+ current in bovine chromaffin cells that is not of the "classic" N type. Neuron. 1992 Jan;8(1):85–95. doi: 10.1016/0896-6273(92)90110-y. [DOI] [PubMed] [Google Scholar]
- Ballesta J. J., Palmero M., Hidalgo M. J., Gutierrez L. M., Reig J. A., Viniegra S., Garcia A. G. Separate binding and functional sites for omega-conotoxin and nitrendipine suggest two types of calcium channels in bovine chromaffin cells. J Neurochem. 1989 Oct;53(4):1050–1056. doi: 10.1111/j.1471-4159.1989.tb07394.x. [DOI] [PubMed] [Google Scholar]
- Borges R., Sala F., García A. G. Continuous monitoring of catecholamine release from perfused cat adrenals. J Neurosci Methods. 1986 Jun;16(4):289–300. doi: 10.1016/0165-0270(86)90054-3. [DOI] [PubMed] [Google Scholar]
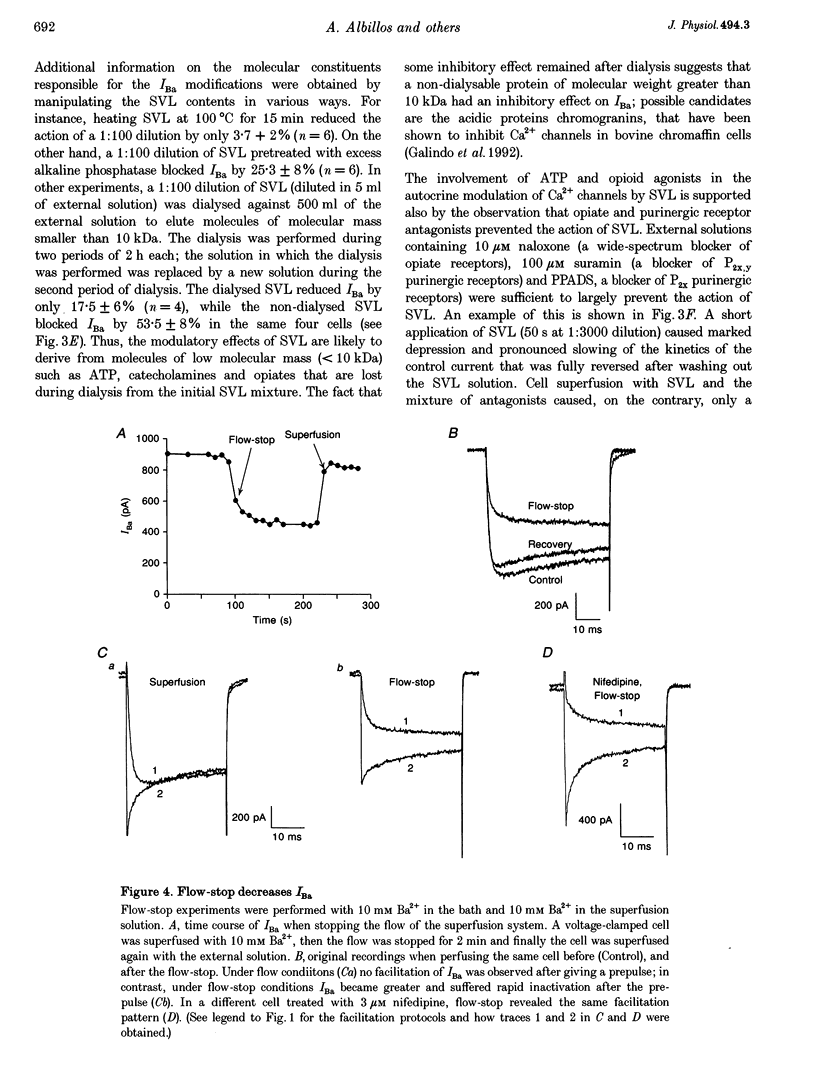
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Johnson R. G., Morad M. Regulation of the secretory response in bovine chromaffin cells. Am J Physiol. 1991 Apr;260(4 Pt 1):C851–C860. doi: 10.1152/ajpcell.1991.260.4.C851. [DOI] [PubMed] [Google Scholar]
- Carbone E., Sher E., Clementi F. Ca currents in human neuroblastoma IMR32 cells: kinetics, permeability and pharmacology. Pflugers Arch. 1990 Apr;416(1-2):170–179. doi: 10.1007/BF00370239. [DOI] [PubMed] [Google Scholar]
- Carbone E., Swandulla D. Neuronal calcium channels: kinetics, blockade and modulation. Prog Biophys Mol Biol. 1989;54(1):31–58. doi: 10.1016/0079-6107(89)90008-4. [DOI] [PubMed] [Google Scholar]
- Doupnik C. A., Pun R. Y. G-protein activation mediates prepulse facilitation of Ca2+ channel currents in bovine chromaffin cells. J Membr Biol. 1994 May;140(1):47–56. doi: 10.1007/BF00234485. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium ocmponent of sensory neurone action potentials. Nature. 1978 Dec 21;276(5690):837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo E., Mendez M., Calvo S., Gonzalez-Garcia C., Ceña V., Hubert P., Bader M. F., Aunis D. Chromostatin receptors control calcium channel activity in adrenal chromaffin cells. J Biol Chem. 1992 Jan 5;267(1):407–412. [PubMed] [Google Scholar]
- Gandía L., Albillos A., García A. G. Bovine chromaffin cells possess FTX-sensitive calcium channels. Biochem Biophys Res Commun. 1993 Jul 30;194(2):671–676. doi: 10.1006/bbrc.1993.1874. [DOI] [PubMed] [Google Scholar]
- Gandía L., García A. G., Morad M. ATP modulation of calcium channels in chromaffin cells. J Physiol. 1993 Oct;470:55–72. doi: 10.1113/jphysiol.1993.sp019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hans M., Illes P., Takeda K. The blocking effects of omega-conotoxin on Ca current in bovine chromaffin cells. Neurosci Lett. 1990 Jun 22;114(1):63–68. doi: 10.1016/0304-3940(90)90429-d. [DOI] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994 Dec;17(12):531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Rothlein J., Smith S. J. Facilitation of Ca2+-channel currents in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5871–5875. doi: 10.1073/pnas.81.18.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Smith S. J. Large depolarization induces long openings of voltage-dependent calcium channels in adrenal chromaffin cells. J Neurosci. 1987 Feb;7(2):571–580. doi: 10.1523/JNEUROSCI.07-02-00571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Puig M. Effect of flow-stop on noradrenaline release from normal spleens and spleens treated with cocaine, phentolamine or phenoxybenzamine. Br J Pharmacol. 1971 Oct;43(2):359–369. [PMC free article] [PubMed] [Google Scholar]
- Kleppisch T., Ahnert-Hilger G., Gollasch M., Spicher K., Hescheler J., Schultz G., Rosenthal W. Inhibition of voltage-dependent Ca2+ channels via alpha 2-adrenergic and opioid receptors in cultured bovine adrenal chromaffin cells. Pflugers Arch. 1992 Jun;421(2-3):131–137. doi: 10.1007/BF00374819. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980 Dec;32(4):337–362. [PubMed] [Google Scholar]
- Lipscombe D., Kongsamut S., Tsien R. W. Alpha-adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature. 1989 Aug 24;340(6235):639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- López M. G., Villarroya M., Lara B., Martínez Sierra R., Albillos A., García A. G., Gandía L. Q- and L-type Ca2+ channels dominate the control of secretion in bovine chromaffin cells. FEBS Lett. 1994 Aug 8;349(3):331–337. doi: 10.1016/0014-5793(94)00696-2. [DOI] [PubMed] [Google Scholar]
- Moro M. A., López M. G., Gandía L., Michelena P., García A. G. Separation and culture of living adrenaline- and noradrenaline-containing cells from bovine adrenal medullae. Anal Biochem. 1990 Mar;185(2):243–248. doi: 10.1016/0003-2697(90)90287-j. [DOI] [PubMed] [Google Scholar]
- Pollo A., Lovallo M., Sher E., Carbone E. Voltage-dependent noradrenergic modulation of omega-conotoxin-sensitive Ca2+ channels in human neuroblastoma IMR32 cells. Pflugers Arch. 1992 Oct;422(1):75–83. doi: 10.1007/BF00381516. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell W. A., Rane S. G. Opioid peptide modulation of Ca(2+)-dependent K+ and voltage-activated Ca2+ currents in bovine adrenal chromaffin cells. Neuron. 1993 Apr;10(4):701–709. doi: 10.1016/0896-6273(93)90171-m. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Diliberto E. J., Jr, Hazum E., Chang K. J. Opiate-like materials in the adrenal medulla: evidence for storage and secretion with catecholamines. Mol Pharmacol. 1979 Nov;16(3):1101–1108. [PubMed] [Google Scholar]
- Winkler H., Sietzen M., Schober M. The life cycle of catecholamine-storing vesicles. Ann N Y Acad Sci. 1987;493:3–19. doi: 10.1111/j.1749-6632.1987.tb27176.x. [DOI] [PubMed] [Google Scholar]
- von Rüden L., García A. G., López M. G. The mechanism of Ba(2+)-induced exocytosis from single chromaffin cells. FEBS Lett. 1993 Dec 20;336(1):48–52. doi: 10.1016/0014-5793(93)81606-z. [DOI] [PubMed] [Google Scholar]


