Abstract
Envafolimab is a novel inhibitor for programmed cell death protein-ligand 1 (PD-L1) that can be administered subcutaneously. It has been found to be effective and safe in the treatment of advanced high microsatellite unstable (MSI-H) / mismatch repair deficient (dMMR) solid tumors. Currently, the efficacy of programmed cell death protein-1 (PD-1) / programmed cell death protein-ligand 1 (PD-L1) inhibitors in the treatment of microsatellite stable (MSS) tumors is not clear. We report a case of advanced colon cancer with MSS metastases in bilateral clavicle, mediastinum, retroperitoneum, bilateral hilum, and left side of thoracic 11/12 vertebral body. After 8 months of Envafolimab treatment, there was a significant reduction in metastatic lesions. As of February 1st, 2024, the patient exhibited no significant adverse reactions. The current efficacy evaluation was the partial response (PR), and the overall survival (OS) was more than 12 months. Considering the safety and efficacy of Envafolimab observed in our case, we believe that Envafolimab may be a promising drug for the treatment of MSS metastatic colon cancer.
Keywords: colon cancer, Envafolimab, immunotherapy, case report
Introduction
Due to the poor prognosis of advanced colorectal cancer (CRC), including the presence of metastasis in the liver, lungs, and other multiple organs, 25−30% of patients with CRC are not able to undergo surgery when diagnosed, and thus are deprived of effective treatment.1,2 Therefore, research in this area has attracted widespread attention. The primary treatment methods include surgery, chemotherapy, radiotherapy, targeted therapy, immunotherapy, etc.3–6 Immune checkpoint inhibitors (ICIs) showed significant efficacy on advanced CRC with high microsatellite unstable (MSI-H) / mismatch repair deficient (dMMR). However, in patients with advanced CRC, most of them are microsatellite stable (MSS) / mismatch repair proficient (pMMR) type, but ICIs have not shown obvious effect on such tumors. Immunotherapy in MSS / pMMR advanced CRC faces some challenges, but it still has application prospects.7 With the breakthrough of cancer immunotherapy in recent years, new immune checkpoint inhibitors provide a new choice for the treatment of CRC. Envafolimab is a novel inhibitor for programmed cell death protein-ligand 1 (PD-L1) developed independently in China. It is a novel homodimer fusion of humanized single domain PD-L1 antibody and Fc fragment of human immunoglobulin G1 linked covalently through interchain disulfide bonds. Mechanistically, Envafolimab mainly blocks inhibitory signaling to T cells and promotes the activation of T cells by blocking PD-1/PD-L1 and PD-L1/ cluster of differentiation 80(CD80) signaling pathways, thereby inhibiting tumor growth.8–10 This drug was approved by the State Drug Administration of China on November 25, 2021 and approve for commercial use (approval number: national drug standard S20210046). Envafolimab is mainly used for the treatment of patients with solid tumors with advanced MSI-H/dMMR. It can be injected subcutaneously owing to its excellent structural stability, low molecular weight, and strong tissue permeability. Compared with other intravenously injected PD-1/PD-L1 inhibitors, Envafolimab provides patients with a more convenient and flexible choice of treatment mode, which may completely alter the mode of immunotherapy in the future.10 We report a case of MSS advanced colon cancer characterized by multiple systemic metastases. The operation opportunity has been lost because the tumor has multiple systemic metastases, such as in the bilateral clavicular region, mediastinum, bilateral hilum, and retroperitoneum. Following a multidisciplinary discussion in our department, the patient was treated with Envafolimab. After treatment for 8 months, positron emission tomography (PET) and computed tomography (PET-CT) reexamination showed a significant reduction of the primary tumor lesions, bilateral clavicular region, mediastinum, bilateral hilum, left paravertebral, and lymph nodes in the retroperitoneal region of thoracic 11/12 vertebrae were. At present, the curative effect was evaluated as partial response (PR), and overall survival (OS) was more than 12 months.
Case Presentation
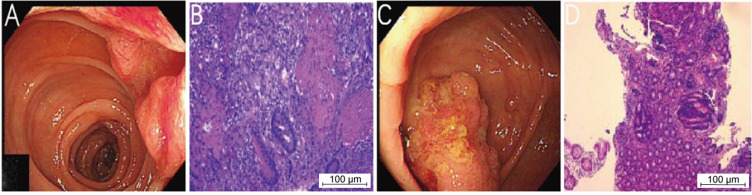
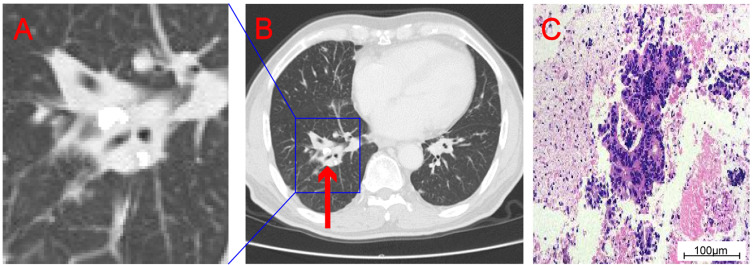
A patient (72-year-old male) was admitted to our hospital due to intermittent diarrhea for more than 5 months. Colonoscopy revealed a lesion of about 2.5×2.5 cm in size in the transverse colon (65 cm from the anus) that grew into the intestinal cavity; this organism can be seen in the ileocecal part, a wide basal type. An endoscopic biopsy was performed. Pathological examination of the biopsy revealed transverse colon adenocarcinoma, ileocecal adenoma with high-grade dysplasia (Figure 1). Chest CT showed a shadow of soft tissue density in the right hilum of about 35×20 mm in size, consistent with metastasis. Pathological results of fibrobronchoscopic biopsy suggested adenocarcinoma, which was considered to metastasize from the primary lesion of the colon (Figure 2). PET-CT examination further revealed a nodular hyperdense shadow in the right hilum, of about 2.8×2.2 cm size and tended to be malignant. Multiple nodules were observed in both lungs, the larger one of about 1.5×0.9 cm size was located in the anterior basal segment of the right lower lung. Considering the metastatic tumor, multiple enlarged lymph nodes were observed in the bilateral clavicular region, mediastinum and hilum, the left side of thoracic 11 and 12 vertebral bodies, and retroperitoneal lymph nodes, with the largest being about 2.3×1.6 cm, and was suspected of metastatic tumor (Figure 3). Genetic analysis of colon cancer tissue biopsy revealed TP53 (+), MSS (Table 1), diagnosed as colon adenocarcinoma with systemic metastasis, cT3N1bM1b IVB stage.
Figure 1.
Colonoscopy revealed a new organism in the transverse colon (A). Histopathological examination showed adenocarcinoma (B). Colonoscopy revealed ileocecal mass (C). Histopathological examination showed adenoma with high-grade dysplasia (D).
Figure 2.
Enlarge the density shadow of soft tissue in the right pulmonary hilum displayed on CT (A). CT showed soft tissue density shadow at the right pulmonary hilum (the “red arrow” indicates lesion) (B). Histopathological examination showed adenocarcinoma (C).
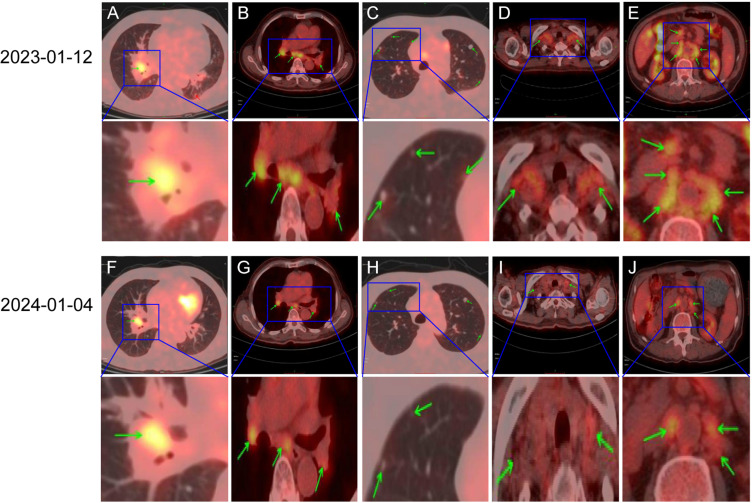
Figure 3.
Changes in lesions observed by PET-CT scan (the ‘green arrows’ indicates tumor metastasis). The mediastinum, bilateral hilar and bilateral pulmonary multiple lymph nodes were significantly reduced (A and F, B and G, C and H). Bilateral clavicular lymph nodes were significantly reduced (D and I). The left paravertebral and retroperitoneal multiple lymph nodes in t11/12 vertebrae were significantly reduced (E and J).
Table 1.
Genetic Test Results of Patients
| Genes | Result | Concrete Result | Specific Value | Methodology |
|---|---|---|---|---|
| ALK | Negative | — | — | |
| BRAF | Negative | — | — | |
| EGFR | Negative | — | — | |
| ERB2 | Negative | — | — | |
| KRAS | Negative | — | — | NGS |
| MET | Negative | — | — | |
| RET | Negative | — | — | |
| ROS1 | Negative | — | — | |
| MSI | MSS | — | — | |
| TP53 | Positive | c.641_644delinsTp.His214_Ser215delinsLeu | 7.92% |
Note: Genetic testing was performed using next-generation sequencing (NGS).
Abbreviations: ALK, anaplastic lymphoma kinase; BRAF, This gene is located on human chromosome 7 (NC_000007.14 (140719327.140924928, complement))and encodes the serine/threonine protein kinase of the RAF family; EGFR, epidermal growth factor receptor; ERBB2, receptor tyrosine kinase 2; KRAS, Kirsten-rous avian sarcoma; MET, This gene is located on chromosome 7q21-q31, and its encoded met protein belongs to tyrosine kinase receptor; RET, RET proto-oncogene; ROS1, ROS proto-oncogene 1 receptor tyrosine kinase; MSI, microsatellite instability; MSS, microsatellite stable; TP53, Tumor Protein P53.
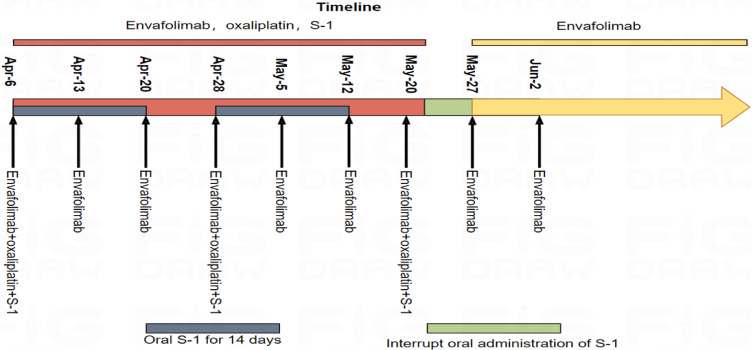
Considering that surgical resection of the lesion was difficult, after multidisciplinary (MDT) discussion, the patient received the first doses of Envafolimab combined with the first cycle of SOX (Envafolimab SC 150 mg QW, oxaliplatin 150 mg IVGTT D1, S-1 40 mg/m2 Po Bid D1-14) from April 6, 2023. Figure 4 illustrates the entire treatment process. The treatment proceeded smoothly with no obvious adverse reactions. The NRS2002 score was 3 points, and the Eastern Cooperative Oncology Group (ECOG) score was 0 points. On April 13 and April 20, 2023, the patients received the second and third dose of Envafolimab SC 150 mg QW. On April 28, 2023, the patients received the fourth dose of Envafolimab combined with the second cycle of safety of S-1 and oxaliplatin (SOX) neoadjuvant chemotherapy. The treatment dose was the same as before. During the treatment, nausea and vomiting, liver function damage, alanine transaminase (ALT) 90.20 U/L, and aspartate transaminase (AST) 106.40 U/L occurred, and the symptoms were relieved upon symptomatic treatment. On May 5 and 12, 2023, the patients were treated with Envafolimab fifth and sixth times, and on May 20, 2023, the seventh dose of Envafolimab combined with the third cycle of SOX neoadjuvant chemotherapy was given. During the treatment, the patient had severe symptoms of gastrointestinal disturbances, such as nausea and vomiting, liver function damage, and neutrophil decline, with ALT 201.72 U/L, AST 235.10 U/L, NEU 0.37×109/L. After treatment with symptomatic antiemetic, liver protection, granulocyte growth factor, and nutritional support, as well as withdrawal of S-1, the adverse reactions were alleviated, the white blood cell count increased, and liver function improved. The patient could not tolerate chemotherapy, and therefore, he refused to continue the SOX regimen. Upon discussion, the patient suspended the use of the SOX regimen and switched to single drug therapy with Envafolimab. Besides anti-tumor treatment, nutritional support was provided. The nutrition scheme was as follows: if the patient could eat, no enteral nutrition intervention was carried out. If the amount of food intake did not reach half of the normal amount, enteral nutrition was taken orally. During enteral nutrition, the proportion of energy from carbohydrates (30–35 kcal/kg) was reduced appropriately, and the proportion of energy supply was increased to egg white (1.5 g/kg), fat (1.3 g/kg), and carbohydrate (3 g/kg), in which carbohydrates accounted for 30%-50%, fat accounted for 25%-40%, and protein, for 15%-30%. Subsequently, the patient continued to receive monotherapy of Envafolimab SC 150 mg QW regularly.
Figure 4.
The treatment procedures.
After 8 months of treatment, the patient underwent PET-CT scan on January 4, 2024. Compared with PET-CT examination on January 12, 2023, there was a significant reduction in the number of multiple lymph nodes in bilateral clavicle, mediastinum, bilateral hilum, left paravertebral body of thoracic 11/12 and retroperitoneal. Multiple small lymph nodes were observed in the mesenteric region of abdomen and pelvis. The nodular hyperdense shadow in the right hilum of the lung was 1.4×2.2 cm, smaller than before. Multiple nodules were still found in both lungs, some of which were relatively reduced and narrowed compared with the previous ones, while the remaining portion of the lungs exhibited patchy and strip-shaped, dense shadows that were reduced and absorbed compared with the previous ones, with unclear boundaries (Figure 3). The evaluation of curative effect suggested PR. At the time of submission of this manuscript, the patient was in good overall condition, with NRS2002 score of 2 points and ECOG score of 1 point. The overall survival (OS) was more than 1 year, and he continued to receive single drug therapy with Envafolimab.
Discussion
Based on various treatment guidelines for CRC in our country and abroad, surgical treatment is the only radical treatment. However, patients with metastatic colorectal cancer (mCRC) are unable to undergo radical surgery, so surgery is not the best choice.11–13 Many patients with advanced CRC have successfully achieved tumor phase reduction through neoadjuvant chemotherapy, radiotherapy, immunotherapy, targeted therapy and other treatment types. Some even obtained the opportunity of radical surgery, prolonging their survival time.14–16 However, the effect of treatment on some patients with tumor metastasis is still limited, and adverse reactions are common. Currently, the 5-year survival rate of CRC patients in China is 62%, and the 5-year survival rate of patients with distant metastasis is reduced to 14%.17,18 The current treatment approach for advanced CRC is not satisfactory, and new treatment options need further exploration.
At the time of diagnosis, this patient with advanced transverse colon cancer presented with multiple metastases in bilateral clavicle, bilateral lungs, mediastinum, thoracic 11 and 12 left paravertebral body, and retroperitoneal space. Genetic analysis showed that it was an MSS tumor. Previously, immunotherapy has proven ineffective for MSS tumors. Given that the patients with advanced cancer do not have enough time to choose treatment, with limited available drugs, we made a personalized treatment plan according to the condition of the patient. For advanced colon cancer with multiple metastases in MSS, various guidelines recommend the consideration of participation in a suitable clinical trial. Nonetheless, patients refuse to be included in the clinical trial because they are concerned about being categorized into the placebo group. Our current treatment plan combines the latest relevant clinical research in our country and abroad with our clinical experience, and selects the only PD-L1 inhibitor - Envafolimab as an adjuvant to SOX chemotherapy - to be subcutaneously injected. The patient trusts us and agrees to the treatment plan.
The reasons for using Envafolimab combined with SOX regimen are: 1. Capecitabine combined with oxaliplatin (XELOX) is the first-line chemotherapy for mCRC. Relevant studies have pointed out that S-1 combined with oxaliplatin (SOX) is also effective as a chemotherapy for mCRC. A non inferiority meta-analysis based on S-1 versus 5-fluorouracil(5-FU) or capecitabine in the treatment of mCRC patients found that S-1 based chemotherapy regimens have been proven to be at least as effective as 5-FU/capecitabine based chemotherapy regimens in terms of efficacy.19 2.PET-CT examination at our center revealed that the patient had multiple metastases in the whole body. To maximize the survival time of the patient, in combination with the gene test results of the patient, a personalized treatment plan was necessary. At the same time, the patients lost confidence in the follow-up treatment and refused to come to the hospital for further treatment. Envafolimab can be injected subcutaneously; it can greatly alleviate the psychological anxiety of patients with long-term hospitalization treatment and is easy to use. 3. PD-1/PD-L1 inhibitors can benefit patients with advanced CRC having MSI-H. The efficacy of PD-1/PD-L1 inhibitors is still controversial for patients with advanced colon cancer having MSS and multiple metastases. A literature review revealed that PD-1/PD-L1 inhibitors can benefit some solid tumor patients with MSS/pMMR.20 PD-1/PD-L1 inhibitors combined with chemotherapy has become the first-line standard treatment for many solid tumors. The possible mechanisms include synergy, immune cell increase and immune memory formation. Immunotherapy combined with chemotherapy has brought hope to MSS/pMMR CRC patients, which may improve the prognosis of such patients.7 Zhu et al21 reported the treatment of a patient with advanced colon cancer having pMMR through Sintilimab combined with XELOX regimen and observed PCR. Yang et al22 reported that one patient with advanced MSS/pMMR colon cancer developed disease progression after first-line chemotherapy, palliative surgery, and second-line chemotherapy in combination with targeted therapy. After the combination treatment of PD-1 inhibitor with radiotherapy and granulocyte-macrophage colony-stimulating factor, progression-free survival exceeded two years, and a complete response was obtained. A study of Pembrolizumab combined with chemotherapy in the treatment of MSS/pMMR mCRC found that Pembrolizumab combined with chemotherapy in the first-line and second-line treatment of MSS/pMMR mCRC were both safe and effective.23 The use of Envafolimab may be one of the few treatment approaches, and its subcutaneous administration method has achieved greater acceptability and treatment compliance. After three cycles of treatment, the gastrointestinal symptoms such as nausea and vomiting were obvious in the patients, and the adverse reactions were alleviated after stopping the SOX regimen. Upon discussion with the patient, the SOX regimen was suspended, and instead, the single drug therapy of Envafolimab was used. After 8 months of anti-PD-L1 treatment, PET-CT reexamination showed that retroperitoneal multiple metastases in the bilateral clavicle, bilateral lungs, mediastinum, left paravertebral body of thoracic 11 and 12 were significantly smaller than before, and the curative effect of the patients reached PR, accompanied with less adverse reactions, proving that our treatment options were effective.
We developed a good nutritional support program, and the curative effect of chemotherapy, immunotherapy, and other comprehensive anti-tumor treatment approaches for patients with good nutritional status was better than that of patients having poor nutrition.24 However, there is no consensus on the standard treatment for patients with advanced colon cancer having multiple systemic metastases. How long does it take for Envafolimab to be effective? What is the optimal therapeutic dose? What is the specific mechanism of Envafolimab on MSS advanced colon cancer? These questions do not have clear answers and need further study. Envafolimab has been found to exhibit good efficacy in adult patients having MSI-H or dMMR advanced solid tumors and consequently improved their survival rate. A Phase I study on Envafolimab treatment of advanced solid tumors showed that its subcutaneous injection made a good breakthrough in both efficacy and tolerance in patients with advanced solid tumors who had undergone other drug treatments in the past and preliminarily proved Envafolimab potentially a better choice than other intravenous PD-L1 inhibitors.25 In addition, the results of another phase I study involving Japanese patients with advanced solid tumors showed that Envafolimab was safe in Japanese patients and confirmed the convenience of its application.10 The results of the CN006 study showed significantly improved overall response rate and OS of patients with advanced dMMR/MSI-H tumor who had earlier undergone treatment of subcutaneous injection of Envafolimab.9 However, considering the inconsistent sample size, dosage, and interval of different clinical trials, further large-scale studies are needed to confirm the effectiveness and safety of Envafolimab. Although there is no report yet of the results of Phase III clinical trials of Envafolimab for treating advanced solid tumors, Envafolimab undoubtedly provides a new treatment option for patients with MSI-H or dMMR advanced solid tumors. The efficacy of Envafolimab in the treatment of advanced colon cancer with MSS is not clear currently. The treatment approach we formulated for patients is undoubtedly novel. In fact, tumor metastases reduced significantly after using Envafolimab, accompanied by good efficacy and no obvious adverse reactions. Although this was only a case report, it also provides a reference for other PD-1/PD-L1 inhibitors in the treatment of patients with advanced colon cancer with MSS.
In summary, Envafolimab provides a new treatment option for patients with advanced colon cancer, especially in colon cancer with MSS. However, more studies are needed to verify its efficacy and safety.
Funding Statement
The current work was supported by the National Natural Science Foundation of China (No.82360116) and the Guizhou Provincial Science and Technology Planning Project (Grant No. qiankehejichu-ZK [2022] general 444).
Abbreviations
PD-1, programmed cell death protein-1; PD-L1, programmed cell death protein-ligand 1; MSS, microsatellite stable; ICIs, Immune checkpoint inhibitors; MSI-H, high microsatellite unstable; dMMR, mismatch repair deficient; PR, partial response; CRC, colorectal cancer; mCRC, metastatic colorectal cancer; pMMR, mismatch repair proficient; OS, overall survival; ECOG, Eastern Cooperative Oncology Group; IVGTT, intravenously guttae; Po, peros; S-1, tegafur-gimeracil-oteracil; SC, Subcutaneous Injection; QW, Once Weekly; Bid, twice a day.
Ethics Approval and Informed Consent
Institutional approval was not required to publish the case details. The patient has agreed to the publication of this article. The study was approved by the ethics committee of The Affiliated Hospital of the Guizhou Medical University. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave approval to the final version to be published; have agreed on the journal to which the article has been submitted; and agree to be held accountable for all aspects of the work.
Consent for Publication
We obtained consent to the publication of medical data.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. doi: 10.1001/jama.2021.0106 [DOI] [PubMed] [Google Scholar]
- 2.Johdi NA, Sukor NF. Colorectal cancer immunotherapy: options and strategies. Front Immunol. 2020;11:1624. doi: 10.3389/fimmu.2020.01624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrobel P, Ahmed S. Current status of immunotherapy in metastatic colorectal cancer. Int J Colorectal Dis. 2019;34(1):13–25. doi: 10.1007/s00384-018-3202-8 [DOI] [PubMed] [Google Scholar]
- 4.Saeed A, Park R, Pathak H, et al. Clinical and biomarker results from a Phase II trial of combined cabozantinib and durvalumab in patients with chemotherapy-refractory colorectal cancer (CRC): CAMILLA CRC cohort. Nat Commun. 2024;15(1):1533. doi: 10.1038/s41467-024-45960-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized Phase III Study. J Clin Oncol. 2023;41(21):3663–3669. doi: 10.1200/jco.22.02760 [DOI] [PubMed] [Google Scholar]
- 6.Li A, Huang T, Zheng R, et al. Preoperative chemoradiotherapy with capecitabine and triweekly oxaliplatin versus capecitabine monotherapy for locally advanced rectal cancer: a propensity-score matched study. BMC Cancer. 2022;22(1):789. doi: 10.1186/s12885-022-09855-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Fan W, Du Y, et al. Application of immune combination therapy in MSS/pMMR-type colorectal cancer: current status and future perspectives. Zhonghua Zhong Liu Za Zhi. 2024;46(8):725–736. doi: 10.3760/cma.j.cn112152-20230904-00116 [DOI] [PubMed] [Google Scholar]
- 8.Markham A. Envafolimab: first approval. Drugs. 2022;82(2):235–240. doi: 10.1007/s40265-022-01671-w [DOI] [PubMed] [Google Scholar]
- 9.Li J, Deng Y, Zhang W, et al. Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors. J Hematol Oncol. 2021;14(1):95. doi: 10.1186/s13045-021-01095-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu T, Nakajima TE, Yamamoto N, et al. Phase I study of envafolimab (KN035), a novel subcutaneous single-domain anti-PD-L1 monoclonal antibody, in Japanese patients with advanced solid tumors. Invest New Drugs. 2022;40(5):1021–1031. doi: 10.1007/s10637-022-01287-7 [DOI] [PubMed] [Google Scholar]
- 11.Morris VK, Kennedy EB, Baxter NN, et al. Treatment of metastatic colorectal cancer: ASCO guideline. J Clin Oncol. 2022. doi: 10.1200/jco.22.01690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mi M, Weng S, Xu Z, Hu H, Wang Y, Yuan Y. CSCO guidelines for colorectal cancer version 2023: updates and insights. Chin J Cancer Res. 2023;35(3):233–238. doi: 10.21147/j.issn.1000-9604.2023.03.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42. doi: 10.1007/s10147-019-01485-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhai M, Zhang Z, Wang H, et al. Efficacy and safety of radiotherapy combined with anti-angiogenic therapy and immune checkpoint inhibitors in MSS/pMMR metastatic colorectal cancer. Cancer Med. 2023;13(1). doi: 10.1002/cam4.6820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen B, Zhao H, Huang J, et al. Efficacy of regorafenib combined with PD-1 inhibitors in elderly patients with advanced metastatic colorectal cancer. BMC Geriatr. 2022;22(1):987. doi: 10.1186/s12877-022-03637-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou S, Wang C, Shen L, et al. Regorafenib alone or in combination with high/low-dose radiotherapy plus toripalimab as third-line treatment in patients with metastatic colorectal cancer: protocol for a prospective, randomized, controlled phase II clinical trial (SLOT). Front Oncol. 2023;13:1274487. doi: 10.3389/fonc.2023.1274487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang R, Lian J, Wang X, et al. Survival rate of colorectal cancer in China: a systematic review and meta-analysis. Front Oncol. 2023;13:1033154. doi: 10.3389/fonc.2023.1033154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233–254. doi: 10.3322/caac.21772 [DOI] [PubMed] [Google Scholar]
- 19.Derksen J, Smit K, May A, Punt C. Systematic review and non-inferiority meta-analysis of randomised phase II/III trials on S-1-based therapy versus 5-fluorouracil- or capecitabine-based therapy in the treatment of patients with metastatic colorectal cancer. Eur J Cancer. 2022;166:73–86. doi: 10.1016/j.ejca.2022.02.004 [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Yang Z, An Y, et al. Clinical benefits of PD-1/PD-L1 inhibitors in patients with metastatic colorectal cancer: a systematic review and meta-analysis. World J Surg Oncol. 2022;20(1):93. doi: 10.1186/s12957-022-02549-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J, Li G, Zhang Z, Wang Y. A case of complete remission in Proficient Mismatch Repair (pMMR) advanced colon cancer treated with sintilimab and XELOX. Immuno Target Ther. 2023;12:17–23. doi: 10.2147/itt.S393526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Xing P, Kong Y, Xu M, Zhang L. PD-1 inhibitor combined with radiotherapy and GM-CSF in MSS/pMMR metastatic colon cancer: a case report. Front Oncol. 2023;13:1078915. doi: 10.3389/fonc.2023.1078915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim R, Tehfe M, Kavan P, et al. Pembrolizumab plus mFOLFOX7 or FOLFIRI for microsatellite stable/mismatch repair-proficient metastatic colorectal cancer: KEYNOTE-651 cohorts B and D. Clin Colorectal Cancer. 2024;23(2):118–127.e6. doi: 10.1016/j.clcc.2024.03.001 [DOI] [PubMed] [Google Scholar]
- 24.Qin Y, Chen D. Nutritional support of tumor patients with chemotherapy. Cell Biochem Biophys. 2015;72(2):633–636. doi: 10.1007/s12013-015-0515-x [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulos KP, Harb W, Peer CJ, et al. First-in-human phase I study of envafolimab, a novel subcutaneous single-domain Anti-PD-L1 antibody. Patients Adv Solid Tumors Oncol. 2021;26(9):e1514–e1525. doi: 10.1002/onco.13817 [DOI] [PMC free article] [PubMed] [Google Scholar]