Abstract
Purpose
Small fiber neuropathy (SFN) is characterized by neuropathic pain, associated with decreased quality of life (QOL). It remains unclear which psychosocial factors play a role in SFN. The experience sampling method (ESM) allows a profound understanding of the real-time fluctuations in reaction to events. The main goal of this study was to increase knowledge of the interrelationships between pain intensity, physical activity, and psychosocial factors in patients with SFN in daily practice over time.
Patients and Methods
A prospective observational ESM study with the PsyMate© application (smart-eHealth GmbH, Luxembourg) was conducted at the Adelante location of Maastricht University Medical Center+ in the Netherlands. Participants with idiopathic SFN, older than 18 years, with an indication for rehabilitation, were included. Pain intensity, fatigue, positive and negative affect, physical activity, avoidance behavior, and pain catastrophic thoughts were incorporated into the ESM questions. Participants received 10 beep signals per day followed by the above-mentioned questions, for consecutive 7 days. The results were analyzed with linear mixed-effect models.
Results
Twenty-one participants were included with a mean age of 48.24 (SD ± 13.89) years, of whom 76.2% were female. More pain (now) resulted in more physical activity (later) (a) and more physical activity (now) resulted in more pain (later) (b). The first association (a) is influenced by pain catastrophic thoughts and fatigue, and the second (b) by an increase in affective states and a lower level of avoidance behavior.
Conclusion
In idiopathic SFN, pain intensity, and physical activity showed a 2-sided association, influenced by catastrophic thoughts, fatigue, affective states, and avoidance behavior.
Keywords: chronic pain, rehabilitation therapy, biopsychosocial factors, small fiber neuropathy
Introduction
Small fiber neuropathy (SFN) is a chronic neuropathic pain condition, due to damage to the small nerve fibers.1 Current (pharmacological) pain treatment is mostly insufficient with a lot of side effects and decreased quality of life (QOL).2,3 More understanding of SFN is necessary to develop a personalized and effective treatment modality that helps patients increase QOL.4–8 Chronic pain has a great impact on physical functioning and QOL.8–10 The reverse, influence of physical functioning on the pain experience is also established.11,12 The exact coherency of pain and physical functioning in SFN has not been clarified.
Pain fluctuates over time and days and is influenced by psychosocial factors such as pain catastrophizing, physical functioning, depression, anxiety, fatigue, and pain-related fear, as observed in several chronic pain conditions.13–32 These psychosocial factors also influence physical functioning, and the development of pain-related disability.13–30 The hypothesis that pain intensity will result in a decrease in physical functioning, and vice versa is explained by the fear-avoidance model which states that an activity might be interpreted as extremely painful or harmful, resulting in fear of more pain or harm which on its turn can lead to the avoidance of activities.33,34 On the other hand, not every chronic pain patient will develop a pain-related disability.35–37
The assessment of psychosocial factors and pain usually occurs with once or twice-completed questionnaires to compare the outcome measurements and to gather a summative overview of (past) complaints.38–40 The retrospective character of this kind of assessments is disadvantageous and persistently influences the outcome measurements due to recall and memory bias.38,39,41–48 Recall bias is the inaccurate remembrance of past events or experiences, and memory bias is the alteration of memory.49 Both will lead to the remembrance of more intense complaints and negative memories.38,39,41–48 Nonetheless, a more accurate and real-life assessment exists to avoid these types of bias.50 Collecting real-life assessments and dynamics is possible with the experience sampling method (ESM), which is a smartphone-based application with repeated measurements in the natural environment.51–55 A deeper and more reliable understanding of dynamic processes and disease-related fluctuations can be observed, especially, the variability of symptomatology and pain intensity,38,51,56,57 avoiding memory and recall bias.50 ESM is labeled as a reliable and valid tool to assess daily life fluctuations and dynamics in chronic pain disorders.32,38,39 These momentary assessments can be important for patients and caregivers to understand and be aware of the fluctuations of complaints over time.32,58
The relation between physical activity and pain and the influence of aforementioned psychosocial factors is not clear yet in SFN. For this reason, we conducted a prospective observational ESM study that aimed to gain more information on the patterns of pain intensity and physical activity in SFN. These insights may clarify the dynamics of psychosocial factors with pain intensity and physical activity in patients with SFN.
Material and Methods
Study Design
A prospective, observational study with ESM was conducted at the rehabilitation department of Adelante location of Maastricht University Medical Center+ in the Netherlands. The study was approved by the medical ethical committee of Zuyderland Medical Center (METCZ20210022). This study complies with the Declaration of Helsinki.
Study Population
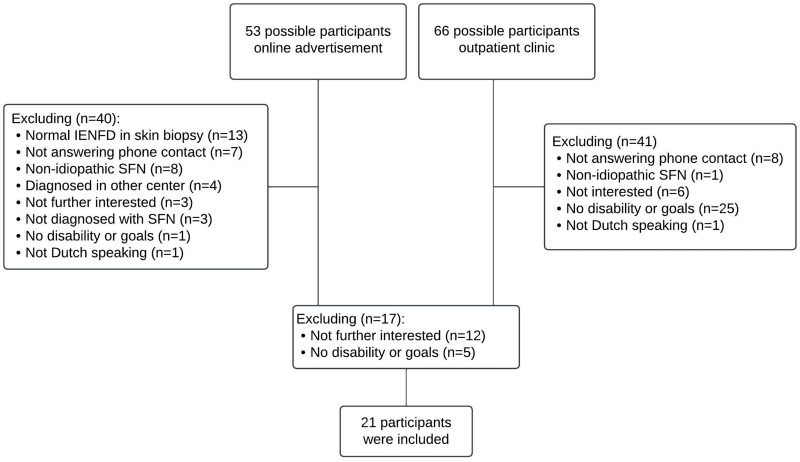
The Department of Neurology of the Maastricht University Medical Center+ is a tertiary referral center for diagnosing SFN and treatment advice. Participants were recruited between September 2021 and March 2022. Through an online advertisement, potential participants were informed and could approach the research team. The research team contacted possible participants by phone to check the in- and exclusion criteria, after getting permission to check their electronic patient file. Potential participants were then referred to the Department of Rehabilitation Medicine. Interested participants of the outpatient neurological clinic were also referred to the Department of Rehabilitation Medicine to be screened by the rehabilitation physician. The inclusion criteria were diagnosed with idiopathic SFN according to the Besta criteria,59 age 18 years or older, and an indication for rehabilitation treatment (eg, experiencing pain-related disability in daily life). The exclusion criteria were the presence of psychiatric comorbidity and insufficient understanding of the Dutch language to fill in ESM and questionnaires. When the physician and participants approved, enrollment in the study started. A research assistant contacted participants for further information about the study and to give information about the smartphone application PsyMate©. All patients provided informed consent. Then, baseline characteristics, such as SFN diagnostics, were retrieved from the electronic patients’ files. Figure 1 shows the study flowchart.
Figure 1.
Study flowchart. 119 possible participants were approached, of whom 81 participants were excluded by the research team due to several reasons. In a second step, a rehabilitation physician invited 38 potential participants for a consultation to inform patients about rehabilitation treatment possibilities and check the indication for treatment. With this additional information, 17 persons refrained from treatment. At the end, 21 participants were included.
Abbreviations: IENFD, intra-epidermal nerve fiber density; SFN, small fiber neuropathy.
ESM
Repeated measurements were assessed with the PsyMate© application (smart-eHealth GmbH, Luxembourg). PsyMate© is an application on the smartphone, compatible with Android and iOS, which can be easily downloaded. We used PsyMate© to gain more information about pain intensity, physical activity, and potential psychosocial factors, such as positive and negative affect, pain catastrophizing, fear avoidance beliefs, and avoidance behavior of participants with SFN.
PsyMate© was programmed to generate 10 beep signals a day between 7:30 AM and 10:30 PM, for 7 consecutive days.54 A random schedule, with a minimum interval of 15 minutes and a maximum interval of 90 minutes between two beeps, was set. After each beep signal, around 14 short questions and statements appeared. Participants had 15 minutes to fill out each question and statement, otherwise, it disappeared. A couple of statements are original, adopted from PsyMate©, and other statements were added by our research group, see Table 1.60 All questions, except for pain and fatigue, could be answered on a 7-point Likert scale (1: not at all, 7: very much), which is the standardized answer option in PsyMate©. Questions about pain and fatigue consisted of an 11-point numeric rating scale because both questions have been taken over from the standardized pain and fatigue questionnaires. All questions in PsyMate© have adequate psychometric properties to measure change over time.61 The following questions had open-ended answer options “What am I doing?”, “Where am I?”, and “Who is joining me?”. Table 1 provides an overview of all ESM questions.
Table 1.
Overview of ESM Questions with Scoring
| ESM Question | Score | Original or Self-Designed Statements |
|---|---|---|
| Pain intensity | ||
| I am in pain | 0 = no pain, 10 = worst pain | Original |
| Fatigue | ||
| I am tired | 0 = not tired, 10 = very tired | Original |
| Affect | ||
| I am cheerful | 1 = not, 7 = very | Original |
| I am relaxed | 1 = not, 7 = very | Original |
| I am sad | 1 = not, 7 = very | Original |
| I am anxious | 1 = not, 7 = very | Original |
| Physical activity | ||
| I was physical active since the last beep signal | 1 = not, 7 = very | Additional |
| Pain catastrophizing | ||
| I want the pain to stop | 1 = not, 7 = very | Additional |
| I am not thinking clear | 1 = not, 7 = very | Additional |
| Pain thoughts and beliefs | ||
| I try to move less | 1 = not, 7 = very | Additional |
| The pain determines what I am doing | 1 = not, 7 = very | Additional |
| General open questions | ||
| What am I doing? | Work, housekeeping-related activities, food-related activities, selfcare, care for others, resting, social contact, nothing, use of social media, sports, relaxing, something else. | Original |
| Where am I? | Home, visitation, at work, public place, on my way to, somewhere else. | Original |
| Who is joining me? | No one, partner, family, friends, coworkers, acquaintances, strangers. | Original |
Within the standardized procedure prior to the rehabilitation physician visit, a set of questionnaires was completed including Pain Catastrophizing Scale (PCS), Hospital Anxiety and Depression Scale (HADS), SF-12, and SFN-specific questionnaires: SFN-RODS (SFN-specific Rasch built Overall Disability Scale) and SFN-SIQ (SFN - Symptom Inventory Questionnaire.62 The PCS questionnaire inventories the presence of catastrophic thoughts about pain with 13 questions. A score higher than 30 indicates the presence of catastrophic thoughts.60 HADS questionnaire is used to measure depressive and anxious symptoms.63 The questionnaire is divided into two subscales, with each 7 items, related to either anxiety or depression, with the following scores: “normal” between 0 and 7, “mild” between 8 and 10, and “moderate” higher than 11.64 The highest score on the subscale is 21. The SF-12 questionnaire is estimating the health status with 8 items.65 The outcome ranges between 0 and 100, in which a higher score than 50 indicates better physical and mental health functioning than the general population.66 The SFN-RODS is a 32-item questionnaire, measuring the ability in daily life activities in patients with SFN.67 Three different answer options are available. The total score ranges between 0 and 100. A higher score indicates more disability. The SFN-SIQ is a 13-item SFN-related questionnaire, measuring mainly the autonomic complaints in SFN.67 Four different answer options are available. The total score ranges between 0 and 100, in which a higher score indicates more autonomic complaints. All mentioned questionnaires are valid and reliable.60,65,67,68 Questionnaires were filled in before installing the ESM application.
All included patients were diagnosed with SFN according to the Besta criteria: the combination of at least two clinical signs and symptoms, abnormal temperature thresholds in quantitative sensory testing (QST), and/or reduced intra-epidermal nerve fiber density (IENFD) in skin biopsy, with no involvement of large fibers.59,69
Statistical Analysis
Baseline characteristics were calculated with descriptive statistics. The categorical variables were calculated as frequencies and proportions. Continuous or dichotomous variables were calculated as means and standard deviation. Normality assumptions were checked with SPSS (Version 27.0, SPSS Inc., Chicago, IL, USA).
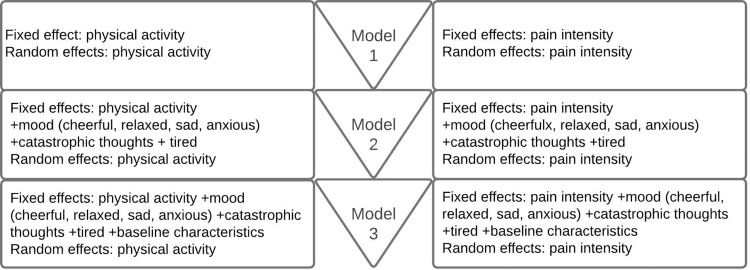
The first aim was to gain more information on the association between pain intensity and physical activity (first direction) and between physical activity and pain intensity (second direction) over time. The ESM data were analyzed with linear mixed-effects models on 3 levels; patients, days, and beeps. In the following steps, the model was built 2-sided (pain intensity vs physical activity, and physical activity vs pain intensity), with random and fixed effects. The relation between pain intensity and physical activity was analyzed with the following variables: “I am in pain” and “I was physically active since the last beep”. This is the first model (model 1), see Figure 2.
Figure 2.
Structure of linear mixed-effects model.
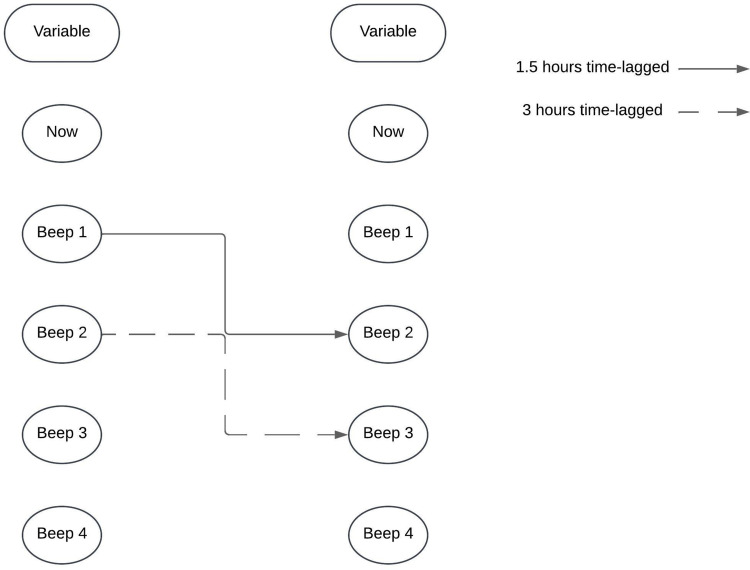
The second aim was to identify the impact of several psychosocial factors in the association between pain intensity and physical activity (model 1). In order to identify the factors that influence model 1, several additional steps were taken. In the second model (model 2), psychosocial factors including “I am sad”, “I am anxious”, “I am cheerful”, “I am relaxed”, “I want the pain to stop”, “I can’t think clearly”, “The pain determines what I am doing”, and “I try to move less” were added. In the third model (model 3), baseline variables of baseline characteristics and outcomes of the baseline questionnaires were included. This process is illustrated in Figure 2 and was carried out using a backward stepwise regression analysis. Only statistically significant factors were added to the final model after each step. Both models were analyzed with a time-lagged interval, meaning that pain intensity (now) was linked to physical activity plus or minus 1.5 hours later, and vice versa, as shown in Figure 3. The models were also analyzed with a longer time-lagged interval of plus or minus 3 hours.
Figure 3.
Time-lagged analysis.
Analyses were performed with SPSS (Version 27.0, SPSS Inc., Chicago, IL, USA). A significance level of 0.05 was used.
Results
Baseline Characteristics
In total, 119 possible participants were approached. Eighty-one participants were excluded due to several reasons by the expert team, see Figure 1. Thirty-eight (interested) participants were referred to the rehabilitation physician, of whom 21 were included (see Figure 1). The total study population consisted of 16 females (76.2%), with a mean age of 48.24 ± 13.89 years. More than half of the participants had a partner. In addition, 50% of the participants were unemployed. The outcome of the questionnaires and the baseline characteristics are presented in Table 2.
Table 2.
Patient and Psychosocial Characteristics of Small Fiber Neuropathy Patients (N = 21)
| Sociodemographic Characteristics | |
|---|---|
| N (%) | |
| Gender, female, n (%) | 16 (76.2) |
| Age in years, mean (SD) | 48.24 (13.89) |
| Marital status, n (%) | |
| Relationship | 14 (66.6) |
| No relationship | 7 (33.4) |
| Employment, n (%) | |
| Unemployed | 11 (52.4) |
| Employed (paid) | 10 (47.6) |
| SFN characteristics | |
| Decreased IENFD in skin biopsy (%) | 20 (95.2) |
| Abnormal QST (%) | 14 (66.7) |
| SFN duration (years) | 2.71 (2.80) |
| Psychosocial characteristics | |
| PCS, total score, mean (SD)* (0–52) | 18.48 (11.90) |
| HADS, subscale anxiety, mean (SD)^ (0–21) | 7.71 (4.44) |
| HADS, subscale depression, mean (SD)^ (0–21) | 7.90 (3.55) |
| SF-12, subscale general total health#(0–100) | 11.00 (2.17) |
| SF-12, subscale mental health# (0–100) | 27.29 (4.43) |
| SF-12, subscale physical functioning# (0–100) | 16.29 (3.55) |
| SFN-RODS, mean (SD)$ (0–100) | 66.18 (16.66) |
| SFN-SIQ (CM), mean (SD)+ (0–100) | 49.71 (9.12) |
Notes: *a higher score indicates more catastrophic thoughts, ^score between 0–7 is normal, 8–10 is borderline, ≥11 is abnormal, #a higher score indicates a lower QOL, $a higher score indicates more physical disability, +a higher score indicates more autonomic complaints.
Abbreviations: n, number; SD, standard deviation; SFN, small fiber neuropathy; IENFD, intra-epidermal nerve fiber density; QST, quantitative sensory testing; PCS, Pain catastrophizing scale; HADS, Hospital anxiety and depression scale; SF-12, short form 12 questions; PIPS, Psychological inflexibility in pain scale; SFN-RODS, SFN specific Rasch-built overall disability scale; SFN-SIQ, SFN specific symptom inventory questionnaire; CM, centile metric.
Some ESM questions had a lower response rate, including “What am I doing?”, “Where am I?”, and “Who is joining me?”. Therefore, these ESM questions could not be included in the analysis.
The Impact of Pain Intensity on Physical Activity (a)
Physical activity influences the pain intensity (p-value = 0.035, 95% CI [0.00–0.14]), indicating an increase of 1 point on physical activity resulted in an increase of 0.07 in pain intensity, see Table 3. In model 3, we accounted for pain catastrophic thoughts (“I want the pain to stop”) (p-value < 0.001, 95% CI [0.35–0.54], coefficient 0.45) and fatigue (p-value < 0.001, 95% CI [0.19–0.32], coefficient 0.25). No affective states were statistically significant in this analysis nor did the time-lagged analysis reveal any statistically significant association.
Table 3.
Overview of Model 1 and Model 3 with Associations Between Pain Intensity and Physical Activity
| Model 1 AIC = 1919.06 | Model 3 AIC = 1767.40 | |||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | p-value | Estimate | 95% CI | p-value | |
| Intercept | 5.31 | 4.57–6.04 | <0.001 | 2.01 | 1.23–2.80 | <0.001 |
| Physical activity | 0.08 | 0.00–1.17 | 0.052 | 0.07 | 0.00–0.14 | 0.035 |
| Pain catastrophic thoughts | 0.45 | 0.35–0.54 | <0.001 | |||
| Fatigue | 0.25 | 0.19–0.32 | <0.001 | |||
The Impact of Physical Activity on Pain Intensity (b)
Pain intensity also influenced physical activity (p-value = 0.029, 95% CI [0.02–0.30]), whereby an increase of 1 point in pain intensity increased the physical activity with 0.16, see Table 4. Model 3 accounted for different moods, such as: cheerful and anxious affective state (p-value < 0.001, 95% CI [0.21–0.51] coefficient 0.36, p-value = 0.045, 95% CI [0.00–0.35] coefficient) 0.17 and avoidance behavior ((“I try to move less”) (p-value = 0.017, 95% CI [−0.24- −0.02] coefficient −0.13). Analyzing physical activity as a time-lagged variable showed no statistically significant association.
Table 4.
Overview of Model 1 and Model 3 with Associations Between Physical Activity and Pain Intensity
| Model 1 AIC = 2442.17 | Model 3 AIC = 2421.87 | |||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P-value | Estimate | 95% CI | P-value | |
| Intercept | 2.63 | 1.77–3.49 | <0.001 | 0.96 | −0.24–2.16 | 0.116 |
| Pain intensity | 0.13 | −0.00–0.28 | 0.059 | 0.16 | 0.02–0.30 | 0.029 |
| Mood: cheerful | 0.36 | 0.21–0.51 | <0.001 | |||
| Mood: anxious | 0.17 | 0.00–0.35 | 0.045 | |||
| Avoidance behavior | −0.13 | −0.24 - −0.02 | 0.017 | |||
Discussion
In this study, we used ESM to gain more information on the patterns of pain intensity and physical activity in patients with SFN. We also wanted to identify possible influencing factors on the relationship between pain intensity and physical activity. This is the first study to investigate such patterns in SFN. Results show that pain intensity and physical activity have a 2-sided association, indicating that more pain (now) resulted in more physical activity (later) (a), and more physical activity (now) resulted in more pain (later) (b). The first association (a) is accounted for by pain catastrophic thoughts (“I want the pain to stop”) and fatigue. The second association (b) is accounted for by an increase in affective states, including cheerfulness and anxiousness, and a lower level of avoidance behavior (“I try to move less”). The importance of these observations, as well as, the differences between the associations will be discussed.
The Impact of Pain Intensity on Physical Activity, and Related Factors (a)
More pain (now) resulting in more physical activity (later), was a non-expected result. Based on previous studies, it was hypothesized that an increase in pain intensity results in a decrease in physical activity.70,71 In earlier SFN research, investigators used paper diaries to understand pain dynamics. SFN patients did not report maximum pain during exercise; however, they reported a higher pain at rest, during sleep, and while standing.9 The impact of psychological factors was not taken into account. It could be that recall bias influenced the pain scores during rest, sleep, and while standing, due to distraction. Earlier SFN research has observed the use of distraction as a method to forget the pain.10 Scientific evidence shows a significant reduction in pain intensity during distraction.72,73 The direct relation between pain intensity and physical activity could be different in neuropathic pain, compared to other pain disorders in which the musculoskeletal system is more involved, eg, joint disorders or osteoarthritis.31,33,74,75 Research on the effectiveness of exercise on neuropathic pain indeed indicated a pain-reducing effect of exercise, as patients had persistent pain for a longer time, they might have learned by experiencing the effect of being active.76–79
The association between physical activity and pain intensity was influenced and supported by pain catastrophic thoughts and fatigue. This means that an increase in physical activity leads to an increase in pain catastrophic thoughts and fatigue, which contributes to the increased pain intensity. First, pain catastrophizing has been defined as a heightened negative state of mind, especially in painful circumstances.17 Pain catastrophic thoughts are almost always present in chronic pain,80,81 resulting in fluctuations in pain intensity and a decrease in physical activity.16,18,80,82–86 This is in concordance with the fear-avoidance model.33,87 Our findings are in line with the pain literature, in which pain catastrophizing has been observed in SFN.88
Regarding fatigue, the increase in pain intensity, due to more physical activity, resulted in more fatigue. In this association, the level of pain intensity was correlated with the level of physical activity. Chronic pain seems to be associated with fatigue, which is present in 64% of the chronic pain populations.89–91 How fatigue develops throughout the day remains unclear. Different reasons are mentioned, first that patients are getting more tired throughout the day due to increased physical activity, and second that patients are mentally tired by thinking all day about their pain.92 However, we only observed the relation with the increase in pain and physical activity, independent of fatigue.
The Impact of Physical Activity on Pain Intensity, and Related Factors (b)
More physical activity (now) resulted in more pain (later). This is in line with other chronic pain conditions, such as fibromyalgia.70,93,94 Based on this, patients with chronic pain will eventually set a couple of (daily) restrictions.33 In painful polyneuropathy, an increase in disability has been observed due to the experienced pain.7,95 Earlier research described the restrictions of patients with SFN in daily life, resulting in a change in identity and former way of living.10 The pain increased by 0.07 points on a scale of 0–10. Questions could arise about the clinical relevance of this pain increase. However, it seems that a change in pain intensity, independent of the level, is relevant and noticed by patients. These findings should be taken into account in the future treatment of patients with SFN.
Physical activity and pain intensity were influenced by two affective states, including cheerfulness and anxiety.96 An increase in physical activity leading to a more cheerful state was also found by other researchers.97 Cheerfulness could be related to the fact that patients felt able to be (more) physically active contrary to a period before and were able to perform an activity that they like or consider valuable. In contrast, feelings of anxiety were also related to the increase in physical activity. Chronic pain is mostly accompanied by (increased) negative affect, such as anxiety and depressive thoughts, also in SFN.80,88,98,99 Fear and pain catastrophic thoughts seem to be mediating the association between pain intensity and physical disability.100 It is plausible that patients experienced pain-related fear due to an increase in physical activity, being anxious for the possible increase in pain intensity that will follow. If a patient would learn to disconnect (or uncouple) the association between physical activity and their pain levels, in order to accept pain and build up committed action.101 This is more promising than primarily trying to control pain. However, a difference in fear avoidance has been observed between men and women, in which only women were associated with an increase in pain avoidance.102
However, avoidance behavior (“I try to move less”) decreased in relation to physical activity and pain intensity. Our results suggest that the increase in pain intensity elevated the amount of physical activity, in which avoidance behavior was not present. The fear-avoidance model indicates that in the presence of higher levels of pain, patients who fear an injury or fear an increase in pain will tend to avoid further activities they fear which can lead to a decrease in (daily) activities (f.e. work, sport, household).33 The difference with other chronic pain disorders could be in the fact that SFN is a neuropathic pain disorder and not a primary musculoskeletal pain disorder. Patients know that they cannot damage muscles or joints by being active, so they would be probably less fear-avoidant. However, patients who do not know this fact will be precautious.103 In addition, the activity itself has been found to have neuroprotective effects by strengthening neurogenesis and reducing the inflammatory response.76 As patients learn this during their efforts to cope with their pain, they may not decrease their activity levels when in pain.
The findings of our study are remarkably different from previous chronic (neuropathic) pain studies. The differences and similarities with previous chronic pain research were discussed above. A lot of hypotheses have been developed throughout the years, of which the fear-avoidance is the most used. As described earlier, in chronic pain conditions, pain catastrophizing and pain intensity influence the level of physical activity, inducing disability.33 In SFN, the opposite has been observed, which is an interesting finding. Why are SFN patients more active when their pain is increasing? Is it due to distraction or may it be due to a different pathophysiological mechanism in which the musculoskeletal system is not directly involved in SFN, therefore resulting in the increase in pain levels at a later moment when activity stops and the positive effects of being active diminish? This is not observed and described in earlier research. Another hypothesis is the gate control theory, in which the pathway of pain throughout small and large fibers toward the spinal cord is described. The small and large fibers function as a gate-controlling mechanism, whereas the large fibers inhibit activity, and the small fibers facilitate activity.104 It may be hypothesized in SFN that the pain stimulus reaches the spinal cord, however, by activation of endogenous factors due to large fiber activation by exercise pain hyperactivity in the dorsal horn is reduced, and therefore, more repeated stimulation is necessary for the pain stimulus to reach the spinal cord. Maybe, this is the reason why SFN patients increase their physical activity rather than avoid activity. However, when the period of activity ends, inhibition diminishes and pain can flare up. A focus group in SFN revealed similar findings, where patients explained that they have to plan their (physical) activities, so they can take a day off the day after.10 In polyneuropathy, patients reported an immediate increase in pain intensity after an activity,105 confirming our findings. Earlier experimental research observed that a painful shock, not related to physical activity, resulted in global fear of everything.106 However, more extensive research is necessary to establish our findings, in order to offer patients a more suitable treatment approach.
In addition to pain, patients with SFN experience more complaints and obstacles in daily life, suggesting an undertreatment when current treatment options solely focus on pain reduction.107 If so, would SFN-related disability decrease if patients were adequately treated and educated, including education about the impact of psychosocial factors and contextual factors including physical activity characteristics? In other chronic pain disorders, rehabilitation treatments addressing psychosocial factors resulted in improvements in physical activity.108–110 Knowing and understanding the dynamics between the psychosocial factors with pain intensity and physical activity is necessary. This knowledge provides a new dimension in future treatment modalities with a personalized treatment approach. The same has been done in other chronic pain conditions, such as painful diabetic neuropathy and complex regional pain syndrome type 1.109,110 ESM could be used in a clinical setting to gather information before treatment starts, and patients have their actual appointment with the clinician. This information could guide the clinician in understanding the personal dynamics of pain intensity and physical activity that can be integrated into personalized pain education. However, ESM could also be used in a treatment setting to monitor the effectiveness of a treatment modality throughout time, avoiding memory and recall bias.111,112 ESM is widely used as a therapeutic monitoring device, especially in mental health disorders.112 Moreover, ESM could give patients insights into their own disease-related fluctuations. With self-monitoring and self-insight, a more efficacious treatment outcome could be obtained.111,113
This is the first study investigating the patterns and complexity of pain intensity and physical activity with disease-related symptoms in SFN. Moreover, the statements and questions of ESM have been extensively validated in different kinds of chronic pain conditions.53 This ensures the validity and reproducibility of ESM in primary and secondary chronic pain conditions. Another strength is the study design. Repeated measurements allow a more reliable and deeper understanding of disease-related fluctuations and symptoms contrary to traditional questionnaires.53,56 However, the results may not be applicable to a wider population of neuropathic and musculoskeletal pain but only to patients with SFN. There are also some limitations. The sample size is small with an unequal division of male-female. However, chronic pain and SFN are more common in females. The small sample size could result in selection bias. However, due to multilevel analysis with the repeated measurements a possible bias is prevented. In ESM, calculating a power analysis for the sample size is difficult and still investigated.114 The aim of this study was also not to search for causality but to gain more information on the patterns of pain intensity and physical activity in SFN and to identify possible influencing factors and relationships in the patient’s environment.
Conclusion
In conclusion, we found that pain intensity and physical activity are associated in a bidirectional manner. Catastrophic thoughts (“I want the pain to stop”) and fatigue influence the impact of pain intensity on physical activity. Affective states, including cheerfulness and anxiousness, and avoidance behavior (“I try to move less”) influence the impact of physical activity on pain intensity. These influencing factors may be targets for personalized treatment of SFN in the future. ESM allows us to understand the dynamics between environment, behavior, and affective state with disease-related symptoms.
Funding Statement
The present study was funded by the Prinses Beatrix Spierfonds, [grant number W.OK17-09]. This research is executed within the European Reference Network for Neuromuscular Diseases.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, AD, upon reasonable request.
Disclosure
Dr. Hoeijmakers received grants from the Prinses Beatrix Spierfonds (W.OK17-09 and W.TR22-01). Dr. Faber was awarded grants from the European Union’s Horizon 2020 research, the innovation programme Marie Sklodowska-Curie grant for PAIN-Net, a grant from the Molecule-to-man pain network (grant no. 721841), grants from Grifols and Lamepro for a trial on IVIg in small fibre neuropathy, and grants from the Prinses Beatrix Spierfonds. She has also been a member of steering committees/advisory boards for studies in small fiber neuropathy of Biogen/Convergence, Vertex, Sangamo and OliPass, outside the submitted work. Dr. Verbunt received grants for pain rehabilitation from ZonMW project (10390102210026) and a grant from insurance companies Menzis, Zilveren Kruis and CZ. Dr. Köke, Dr. Damci, Dr. den Hollander and Dr. Waardenburg declared no conflicts of interest in this work. Dr van Laake-Geelen received a grant from zonMW for pain rehabilitation in spinal cord injury (09032212110056).
References
- 1.Sopacua M, Hoeijmakers JGJ, Merkies ISJ, Lauria G, Waxman SG, Faber CG. Small-fiber neuropathy: expanding the clinical pain universe. J Peripher Nerv Syst. 2018;24:19–23. [DOI] [PubMed] [Google Scholar]
- 2.de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy - A large cohort study and review of the literature. Eur J Neurol. 2018;25(2):348–355. doi: 10.1111/ene.13508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.den Hollander M, Heijnders N, de Jong JR, Vlaeyen JWS, Smeets R, Goossens M. Exposure in Vivo Versus Pain-Contingent Physical Therapy in Complex Regional Pain Syndrome Type I: a Cost-Effectiveness Analysis. Int J Technol Assess Health Care. 2018;34(4):400–409. doi: 10.1017/S0266462318000429 [DOI] [PubMed] [Google Scholar]
- 5.de Jong JR, Vlaeyen JW, Onghena P, Cuypers C, den Hollander M, Ruijgrok J. Reduction of pain-related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. Pain. 2005;116(3):264–275. doi: 10.1016/j.pain.2005.04.019 [DOI] [PubMed] [Google Scholar]
- 6.Girach A, Julian TH, Varrassi G, Paladini A, Vadalouka A, Zis P. Quality of Life in Painful Peripheral Neuropathies: a Systematic Review. Pain Res Manag. 2019;2019:2091960. doi: 10.1155/2019/2091960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geelen CC, Kindermans HP, van den Bergh JP, Verbunt JA. Perceived Physical Activity Decline as a Mediator in the Relationship Between Pain Catastrophizing, Disability, and Quality of Life in Patients with Painful Diabetic Neuropathy. Pain Pract. 2017;17(3):320–328. doi: 10.1111/papr.12449 [DOI] [PubMed] [Google Scholar]
- 8.Bakkers MFC, Hoeijmakers JGJ, Lauria G, Merkies ISJ, Merkies ISJ. Small fibers, large impact: quality of life in small-fiber neuropathy. Muscle Nerve. 2014;49(3):329–336. doi: 10.1002/mus.23910 [DOI] [PubMed] [Google Scholar]
- 9.Brouwer BA, van Kuijk SMJ, Bouwhuis A, et al. The Pain Dynamics of Small Fiber Neuropathy. J Pain. 2018;20(6):655–663. doi: 10.1016/j.jpain.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 10.Damci A, Hoeijmakers JGJ, de Jong J, et al. Living with small fiber neuropathy: insights from qualitative focus group interviews. J Int Med Res. 2022;50(11):3000605221132463. doi: 10.1177/03000605221132463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster NE, Pincus T, Underwood MR, Vogel S, Breen A, Harding G. Understanding the process of care for musculoskeletal conditions--why a biomedical approach is inadequate. Rheumatology. 2003;42(3):401–404. [PubMed] [Google Scholar]
- 12.Taylor AM, Phillips K, Patel KV, et al. Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations. Pain. 2016;157(9):1836–1850. doi: 10.1097/j.pain.0000000000000577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with Rheumatoid-Arthritis Pain - Catastrophizing as a Maladaptive Strategy. Pain. 1989;37(1):51–56. doi: 10.1016/0304-3959(89)90152-8 [DOI] [PubMed] [Google Scholar]
- 14.Turk D, Rudy TE. Cognitive factors and persistent pain: a glimpse into Pandora’s box. Cognitive Theory Res. 1992;16(2):99–122. doi: 10.1007/BF01173484 [DOI] [Google Scholar]
- 15.Main CJ, Waddell G. A Comparison of Cognitive Measures in Low-Back-Pain - Statistical Structure and Clinical Validity at Initial Assessment. Pain. 1991;46(3):287–298. doi: 10.1016/0304-3959(91)90112-B [DOI] [PubMed] [Google Scholar]
- 16.Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain. 2001;17(2):165–172. doi: 10.1097/00002508-200106000-00009 [DOI] [PubMed] [Google Scholar]
- 17.Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17(1):52–64. doi: 10.1097/00002508-200103000-00008 [DOI] [PubMed] [Google Scholar]
- 18.Sullivan MJL, Stanish W, Waite H, Sullivan M, Tripp DA. Catastrophizing, pain, and disability in patients with soft-tissue injuries. Pain. 1998;77(3):253–260. doi: 10.1016/S0304-3959(98)00097-9 [DOI] [PubMed] [Google Scholar]
- 19.Sullivan MJ, Stanish W, Sullivan ME, Tripp D. Differential predictors of pain and disability in patients with whiplash injuries. Pain Res Manag. 2002;7(2):68–74. doi: 10.1155/2002/176378 [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Calderon J, Jensen MP, Morales-Asencio JM, Luque-Suarez A. Pain Catastrophizing and Function In Individuals With Chronic Musculoskeletal Pain: a Systematic Review and Meta-Analysis. Clin J Pain. 2019;35(3):279–293. doi: 10.1097/AJP.0000000000000676 [DOI] [PubMed] [Google Scholar]
- 21.Martin MY, Bradley LA, Alexander RW, et al. Coping strategies predict disability in patients with primary fibromyalgia. Pain. 1996;68(1):45–53. doi: 10.1016/S0304-3959(96)03179-X [DOI] [PubMed] [Google Scholar]
- 22.McCracken LM, Gross RT. Does anxiety affect coping with chronic pain? Clin J Pain. 1993;9(4):253–259. doi: 10.1097/00002508-199312000-00006 [DOI] [PubMed] [Google Scholar]
- 23.Sullivan MJL, Deon JL. Relation between Catastrophizing and Depression in Chronic Pain Patients. J Abnormal Psychol. 1990;99(3):260–263. doi: 10.1037/0021-843X.99.3.260 [DOI] [PubMed] [Google Scholar]
- 24.Geisser ME, Robinson ME, Keefe FJ, Weiner ML. Catastrophizing, depression and the sensory, affective and evaluative aspects of chronic pain. Pain. 1994;59(1):79–83. doi: 10.1016/0304-3959(94)90050-7 [DOI] [PubMed] [Google Scholar]
- 25.Arnow BA, Hunkeler EM, Blasey CM, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68(2):262–268. doi: 10.1097/01.psy.0000204851.15499.fc [DOI] [PubMed] [Google Scholar]
- 26.McCracken LM, Gross RT. The role of pain-related anxiety reduction in the outcome of multidisciplinary treatment for chronic low back pain: preliminary results. J Occupatl Rehabil. 1998;8(3):179–189. doi: 10.1023/A:1021374322673 [DOI] [Google Scholar]
- 27.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–168. doi: 10.1016/0304-3959(93)90127-B [DOI] [PubMed] [Google Scholar]
- 28.Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80(1–2):329–339. doi: 10.1016/S0304-3959(98)00229-2 [DOI] [PubMed] [Google Scholar]
- 29.van den Hout JH, Vlaeyen JW, Houben RM, Soeters AP, Peters ML. The effects of failure feedback and pain-related fear on pain report, pain tolerance, and pain avoidance in chronic low back pain patients. Pain. 2001;92(1–2):247–257. doi: 10.1016/S0304-3959(01)00261-5 [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Calderon J, Flores-Cortes M, Morales-Asencio JM, Luque-Suarez A. Pain-Related Fear, Pain Intensity and Function in Individuals With Chronic Musculoskeletal Pain: a Systematic Review and Meta-Analysis. J Pain. 2019;20(12):1394–1415. doi: 10.1016/j.jpain.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 31.Lazaridou A, Martel MO, Cornelius M, et al. The Association Between Daily Physical Activity and Pain Among Patients with Knee Osteoarthritis: the Moderating Role of Pain Catastrophizing. Pain Med. 2019;20(5):916–924. doi: 10.1093/pm/pny129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villa M, Palsson T, Royo A, Bjarkam C, Boudreau S. Digital pain mapping and tracking in patients with chronic pain: longitudinal study. J Med Internet Res. 2020;22(10):e21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30(1):77–94. doi: 10.1007/s10865-006-9085-0 [DOI] [PubMed] [Google Scholar]
- 34.Vlaeyen JW, Kole-Snijders AM, Rotteveel AM, Ruesink R, Heuts PH. The role of fear of movement/(re)injury in pain disability. J Occup Rehabil. 1995;5(4):235–252. doi: 10.1007/BF02109988 [DOI] [PubMed] [Google Scholar]
- 35.Stubbs D, Krebs E, Bair M, et al. Sex Differences in Pain and Pain-Related Disability among Primary Care Patients with Chronic Musculoskeletal Pain. Pain Med. 2010;11(2):232–239. doi: 10.1111/j.1526-4637.2009.00760.x [DOI] [PubMed] [Google Scholar]
- 36.Raftery MN, Sarma K, Murphy AW, De la Harpe D, Normand C, McGuire BE. Chronic pain in the Republic of Ireland--community prevalence, psychosocial profile and predictors of pain-related disability: results from the Prevalence, Impact and Cost of Chronic Pain (PRIME) study, part 1. Pain. 2011;152(5):1096–1103. doi: 10.1016/j.pain.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 37.Scudds RJ, Mc DRJ. Empirical evidence of the association between the presence of musculoskeletal pain and physical disability in community-dwelling senior citizens. Pain. 1998;75(2–3):229–235. doi: 10.1016/S0304-3959(97)00224-8 [DOI] [PubMed] [Google Scholar]
- 38.Smyth J, Stone A. Ecological momentary assessment research in behavioral medicine. J Happiness Stud. 2003;4(1):35–52. doi: 10.1023/A:1023657221954 [DOI] [Google Scholar]
- 39.Gendreau M, Hufford MR, Stone AA. Measuring clinical pain in chronic widespread pain: selected methodological issues. Best Pract Res Clin Rheumatol. 2003;17(4):575–592. doi: 10.1016/S1521-6942(03)00031-7 [DOI] [PubMed] [Google Scholar]
- 40.Stone AA, Obbarius A, Junghaenel DU, Wen CKF, Schneider S. High-resolution, field approaches for assessing pain: ecological Momentary Assessment. Pain. 2021;162(1):4–9. doi: 10.1097/j.pain.0000000000002049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone AA, Broderick JE. Real-time data collection for pain: appraisal and current status. Pain Med. 2007;8(Suppl 3):S85–93. doi: 10.1111/j.1526-4637.2007.00372.x [DOI] [PubMed] [Google Scholar]
- 42.Fredrickson BL. Extracting meaning from past affective experiences: the importance of peaks, ends, and specific emotions. Cognition Emotion. 2000;14(4):577–606. doi: 10.1080/026999300402808 [DOI] [Google Scholar]
- 43.Kikuchi H, Yoshiuchi K, Miyasaka N, et al. Reliability of recalled self-report on headache intensity: investigation using ecological momentary assessment technique. Cephalalgia. 2006;26(11):1335–1343. doi: 10.1111/j.1468-2982.2006.01221.x [DOI] [PubMed] [Google Scholar]
- 44.Stone AA, Broderick JE, Schwartz JE, Shiffman S, Litcher-Kelly L, Calvanese P. Intensive momentary reporting of pain with an electronic diary: reactivity, compliance, and patient satisfaction. Pain. 2003;104(1–2):343–351. doi: 10.1016/S0304-3959(03)00040-X [DOI] [PubMed] [Google Scholar]
- 45.Feine JS, Lavigne GJ, Dao TTT, Morin C, Lund JP. Memories of chronic pain and perceptions of relief. Pain. 1998;77(2):137–141. doi: 10.1016/S0304-3959(98)00089-X [DOI] [PubMed] [Google Scholar]
- 46.Schwarz N, Sudman S Autobiographical memory and the valildity of retrospective reports. New York: Springer-Verlag; 1994. [Google Scholar]
- 47.Fredrickson BL, Kahneman D. Duration neglect in retrospective evaluations of affective episodes. J Pers Soc Psychol. 1993;65(1):45–55. doi: 10.1037/0022-3514.65.1.45 [DOI] [PubMed] [Google Scholar]
- 48.Smith WB, Safer MA. Effects of present pain level on recall of chronic pain and medication use. Pain. 1993;55(3):355–361. doi: 10.1016/0304-3959(93)90011-D [DOI] [PubMed] [Google Scholar]
- 49.Pincus T, Morley S. Cognitive-processing bias in chronic pain: a review and integration. Psychol Bull. 2001;127(5):599–617. doi: 10.1037/0033-2909.127.5.599 [DOI] [PubMed] [Google Scholar]
- 50.May M, Junghaenel DU, Ono M, Stone AA, Schneider S. Ecological Momentary Assessment Methodology in Chronic Pain Research: a Systematic Review. J Pain. 2018;19(7):699–716. doi: 10.1016/j.jpain.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myin-Germeys I, Oorschot M, Collip D, Lataster J, Delespaul P, van Os J. Experience sampling research in psychopathology: opening the black box of daily life. Psychol Med. 2009;39(9):1533–1547. doi: 10.1017/S0033291708004947 [DOI] [PubMed] [Google Scholar]
- 52.Myin-Germeys I, Kasanova Z, Vaessen T, et al. Experience sampling methodology in mental health research: new insights and technical developments. World Psychiatry. 2018;17(2):123–132. doi: 10.1002/wps.20513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verhagen S, Hasmi L, Drukker M, van Os J, Delespaul A. Use of experience sampling method in the context of clinical trails. Evid Based Ment Health. 2016;19(3):86–89. doi: 10.1136/ebmental-2016-102418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rintala A, Wampers M, Myin-Germeys I, Viechtbauer W. Momentary predictors of compliance in studies using the experience sampling method. Psychiatry Res. 2020;286:112896. doi: 10.1016/j.psychres.2020.112896 [DOI] [PubMed] [Google Scholar]
- 55.Damci A, Hoeijmakers JGJ, den Hollander M, et al. Acceptability, usability and feasibility of experienced sampling method in chronic secondary pain syndromes. Front Neurol. 2023;14:1219236. doi: 10.3389/fneur.2023.1219236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Page MG, Gauvin L, Sylvestre MP, Nitulescu R, Dyachenko A, Choiniere M. An Ecological Momentary Assessment Study of Pain Intensity Variability: ascertaining Extent, Predictors, and Associations With Quality of Life. Interference and Health Care Utilization Among Individuals Living with Chronic Low Back Pain J Pain. 2022;23(7):1151–1166. [DOI] [PubMed] [Google Scholar]
- 57.Vendrig AA, Lousberg R. Within-person relationships among pain intensity, mood and physical activity in chronic pain: a naturalistic approach. Pain. 1997;73(1):71–76. doi: 10.1016/S0304-3959(97)00075-4 [DOI] [PubMed] [Google Scholar]
- 58.Sorbi MJ, Peters ML, Kruise DA, et al. Electronic momentary assessment in chronic pain I: psychological pain responses as predictors of pain intensity. Clin J Pain. 2006;22(1):55–66. doi: 10.1097/01.ajp.0000148624.46756.fa [DOI] [PubMed] [Google Scholar]
- 59.Devigili G, Cazzato D, Lauria G. Clinical diagnosis and management of small fiber neuropathy: an update on best practice. Expert Rev Neurother. 2020;20(9):967–980. doi: 10.1080/14737175.2020.1794825 [DOI] [PubMed] [Google Scholar]
- 60.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):525–532. doi: 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 61.Verhagen SJW, Berben JA, Leue C, et al. Demonstrating the reliability of transdiagnostic mHealth Routine Outcome Monitoring in mental health services using experience sampling technology. PLoS One. 2017;12(10):e0186294. doi: 10.1371/journal.pone.0186294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koke AJ, Smeets RJ, Schreurs KM, et al. Dutch Dataset Pain Rehabilitation in daily practice: content, patient characteristics and reference data. Eur J Pain. 2017;21(3):434–444. doi: 10.1002/ejp.937 [DOI] [PubMed] [Google Scholar]
- 63.Spinhoven P, Ormel J, Sloekers PPA, Kempen GIJM, Speckens AEM, VanHemert AM. A validation study of the Hospital Anxiety and Depression Scale (Hads) in different groups of Dutch subjects. Psychological Medicine. 1997;27(2):363–370. doi: 10.1017/S0033291796004382 [DOI] [PubMed] [Google Scholar]
- 64.Breeman S, Cotton S, Fielding S, Jones GT. Normative data for the Hospital Anxiety and Depression Scale. Qual Life Res. 2015;24(2):391–398. doi: 10.1007/s11136-014-0763-z [DOI] [PubMed] [Google Scholar]
- 65.Ware Jr J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 66.Ware J, Kosinski M, Keller S. SF-12: How to Score the SF-12 Physical and Mental Summary Scales. Boston: New England Medical Center; 1995. [Google Scholar]
- 67.Brouwer BA, Bakkers M, Hoeijmakers JG, Faber CG, Merkies IS. Improving assessment in small fiber neuropathy. J Peripher Nerv Syst. 2015;20(3):333–340. doi: 10.1111/jns.12128 [DOI] [PubMed] [Google Scholar]
- 68.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/S0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 69.Devigili G, Rinaldo S, Lombardi R, et al. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain. 2019;142(12):3728–3736. doi: 10.1093/brain/awz333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Damsgard E, Thrane G, Anke A, Fors T, Roe C. Activity-related pain in patients with chronic musculoskeletal disorders. Disabil Rehabil. 2010;32(17):1428–1437. doi: 10.3109/09638280903567877 [DOI] [PubMed] [Google Scholar]
- 71.Lima LV, Abner TSS, Sluka KA. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J Physiol. 2017;595(13):4141–4150. doi: 10.1113/JP273355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rad A, Wippert P-M. Insights into pain distraction and the impact of pain catastrophizing on pain perception during different types of distraction tasks. Front Pain Res. 2024;5(1266974):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sluka KA, Frey-Law L, Hoeger Bement M. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain. 2018;159(Suppl 1):S91–S97. doi: 10.1097/j.pain.0000000000001235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDonough CM, Jette AM. The contribution of osteoarthritis to functional limitations and disability. Clin Geriatr Med. 2010;26(3):387–399. doi: 10.1016/j.cger.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Jong JR, Vlaeyen JW, Onghena P, Goossens ME, Geilen M, Mulder H. Fear of movement/(re)injury in chronic low back pain: education or exposure in vivo as mediator to fear reduction? Clin J Pain. 2005;21(1):9–17. doi: 10.1097/00002508-200501000-00002 [DOI] [PubMed] [Google Scholar]
- 76.Wang MJ, Jing XY, Wang YZ, et al. Exercise, Spinal Microglia and Neuropathic Pain: potential Molecular Mechanisms. Neurochem Res. 2024;49(1):29–37. doi: 10.1007/s11064-023-04025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matesanz-Garcia L, Billerot C, Fundaun J, Schmid AB. Effect of Type and Dose of Exercise on Neuropathic Pain After Experimental Sciatic Nerve Injury: a Preclinical Systematic Review and Meta-Analysis. J Pain. 2023;24(6):921–938. doi: 10.1016/j.jpain.2023.01.011 [DOI] [PubMed] [Google Scholar]
- 78.Ruimonte-Crespo J, Plaza-Manzano G, Diaz-Arribas MJ, et al. Aerobic Exercise and Neuropathic Pain: insights from Animal Models and Implications for Human Therapy. Biomedicines. 2023;11(12):3174. doi: 10.3390/biomedicines11123174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schreiber KL, Campbell C, Martel MO, et al. Distraction analgesia in chronic pain patients: the impact of catastrophizing. Anesthesiology. 2014;121(6):1292–1301. doi: 10.1097/ALN.0000000000000465 [DOI] [PubMed] [Google Scholar]
- 80.Frumkin MR, Rodebaugh TL. The role of affect in chronic pain: a systematic review of within-person symptom dynamics. J Psychosom Res. 2021;147:110527. doi: 10.1016/j.jpsychores.2021.110527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sullivan MJ, Rodgers WM, Kirsch I. Catastrophizing, depression and expectancies for pain and emotional distress. Pain. 2001;91(1–2):147–154. doi: 10.1016/S0304-3959(00)00430-9 [DOI] [PubMed] [Google Scholar]
- 82.Arnow BA, Blasey CM, Constantino MJ, et al. Catastrophizing, depression and pain-related disability. Gen Hosp Psychiatry. 2011;33(2):150–156. doi: 10.1016/j.genhosppsych.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 83.Radat F, Margot-Duclot A, Attal N. Psychiatric co-morbidities in patients with chronic peripheral neuropathic pain: a multicentre cohort study. Eur J Pain. 2013;17(10):1547–1557. doi: 10.1002/j.1532-2149.2013.00334.x [DOI] [PubMed] [Google Scholar]
- 84.Varallo G, Scarpina F, Giusti EM, et al. The Role of Pain Catastrophizing and Pain Acceptance in Performance-Based and Self-Reported Physical Functioning in Individuals with Fibromyalgia and Obesity. J Pers Med. 2021;11(8):810. doi: 10.3390/jpm11080810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martinez-Calderon J, Struyf F, Meeus M, Luque-Suarez A. The association between pain beliefs and pain intensity and/or disability in people with shoulder pain: a systematic review. Musculoskelet Sci Pract. 2018;37:29–57. doi: 10.1016/j.msksp.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 86.Millere A, Kalnberza-Ribule Z, Mezals M, Nulle A, Millere I, Deklava L. Disability, pain catastrophizing and stress coping of patients with low back pain in rehabilitation practice in Latvia. J Back Musculoskelet Rehabil. 2020;33(2):323–328. doi: 10.3233/BMR-170945 [DOI] [PubMed] [Google Scholar]
- 87.Elfving B, Andersson T, Grooten WJ. Low levels of physical activity in back pain patients are associated with high levels of fear-avoidance beliefs and pain catastrophizing. Physiother Res Int. 2007;12(1):14–24. doi: 10.1002/pri.355 [DOI] [PubMed] [Google Scholar]
- 88.Damci A, Schruers KRJ, Leue C, Faber CG, Hoeijmakers JGJ. Anxiety and depression in small fiber neuropathy. J Peripher Nerv Syst. 2022;27(4):291–301. doi: 10.1111/jns.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Creavin ST, Dunn KM, Mallen CD, Nijrolder I, van der Windt DA. Co-occurrence and associations of pain and fatigue in a community sample of Dutch adults. Eur J Pain. 2010;14(3):327–334. doi: 10.1016/j.ejpain.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 90.Fishbain D, Cole B, Cutler R, Lewis J, Rosomoff HL, Rosomoff RS. Is pain fatiguing? A structured evidence based review. Pain Med. 2003;4(1):51–62. doi: 10.1046/j.1526-4637.2003.03008.x [DOI] [PubMed] [Google Scholar]
- 91.Craig A, Tran Y, Siddall P, et al. Developing a model of associations between chronic pain, depressive mood, chronic fatigue, and self-efficacy in people with spinal cord injury. J Pain. 2013;14(9):911–920. doi: 10.1016/j.jpain.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 92.Van Damme S, Becker S, Van der Linden D. Tired of pain? Toward a better understanding of fatigue in chronic pain. Pain. 2018;159(1):7–10. doi: 10.1097/j.pain.0000000000001054 [DOI] [PubMed] [Google Scholar]
- 93.Dailey DL, Keffala VJ, Sluka KA. Do cognitive and physical fatigue tasks enhance pain, cognitive fatigue, and physical fatigue in people with fibromyalgia? Arthritis Care Res. 2015;67(2):288–296. doi: 10.1002/acr.22417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118(1–2):176–184. doi: 10.1016/j.pain.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 95.Liedberg GM, Vrethem M. Polyneuropathy, with and without neurogenic pain, and its impact on daily life activities--A descriptive study. Disabil Rehabil. 2009;31(17):1402–1408. doi: 10.1080/09638280802621382 [DOI] [PubMed] [Google Scholar]
- 96.Eccleston C. Chronic pain and distraction: an experimental investigation into the role of sustained and shifting attention in the processing of chronic persistent pain. Behav Res Ther. 1995;33(4):391–405. doi: 10.1016/0005-7967(94)00057-Q [DOI] [PubMed] [Google Scholar]
- 97.Richards J, Jiang X, Kelly P, Chau J, Bauman A, Ding D. Don’t worry, be happy: cross-sectional associations between physical activity and happiness in 15 European countries. BMC Public Health. 2015;15(1):53. doi: 10.1186/s12889-015-1391-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lerman SF, Rudich Z, Brill S, Shalev H, Shahar G. Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosom Med. 2015;77(3):333–341. doi: 10.1097/PSY.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 99.Parmelee PA, Behrens EA, Costlow Hill K, et al. Momentary Associations of Osteoarthritis Pain and Affect: depression as Moderator. J Gerontol B Psychol Sci Soc Sci. 2022;77(7):1240–1249. doi: 10.1093/geronb/gbab221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marshall PWM, Schabrun S, Knox MF. Physical activity and the mediating effect of fear, depression, anxiety, and catastrophizing on pain related disability in people with chronic low back pain. PLoS One. 2017;12(7):e0180788. doi: 10.1371/journal.pone.0180788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.den Hollander M, Smeets R, van Meulenbroek T, van Laake-Geelen CCM, Baadjou VA, Timmers I. Exposure in vivo as a treatment approach to target pain-related fear: theory and new insights from research and clinical practice. PTJ. 2022;102:1–9. [DOI] [PubMed] [Google Scholar]
- 102.Waardenburg S, Visseren L, van Daal E, et al. Do Men and Women Have a Different Association between Fear-Avoidance and Pain Intensity in Chronic Pain? An Experience Sampling Method Cohort-Study. J Clin Med. 2022;11(19):5515. doi: 10.3390/jcm11195515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shala R, Roussel N, Moseley GL, Osinski T, Puentedura EJ. Can we just talk our patients out of pain? Should pain neuroscience education be our only tool? J Man Manip Ther. 2021;29(1):1–3. doi: 10.1080/10669817.2021.1873259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5–12. doi: 10.1152/jn.00457.2012 [DOI] [PubMed] [Google Scholar]
- 105.Kanera IM, van Laake-Geelen CCM, Ruijgrok JM, et al. Living with painful diabetic neuropathy: insights from focus groups into fears and coping strategies. Psychol Health. 2019;34(1):84–105. doi: 10.1080/08870446.2018.1518526 [DOI] [PubMed] [Google Scholar]
- 106.Meulders A, Vlaeyen JWS. Reduction of fear of movement-related pain and pain-related anxiety: an associative learning approach using a voluntary movement paradigm. Pain. 2012;153(7):1504–1513. doi: 10.1016/j.pain.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 107.Resnik DB, Rehm M, Minard RB. The undertreatment of pain: scientific, clinical, cultural, and philosophical factors. Med Health Care Philos. 2001;4(3):277–288. doi: 10.1023/A:1012057403159 [DOI] [PubMed] [Google Scholar]
- 108.Leeuw M, Goossens ME, van Breukelen GJ, et al. Exposure in vivo versus operant graded activity in chronic low back pain patients: results of a randomized controlled trial. Pain. 2008;138(1):192–207. doi: 10.1016/j.pain.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 109.den Hollander M, Goossens M, de Jong J, et al. Expose or protect? A randomized controlled trial of exposure in vivo vs pain-contingent treatment as usual in patients with complex regional pain syndrome type 1. Pain. 2016;157(10):2318–2329. doi: 10.1097/j.pain.0000000000000651 [DOI] [PubMed] [Google Scholar]
- 110.van Laake-Geelen CCM, Smeets R, Goossens M, Verbunt JA. Effectiveness of Exposure in Vivo for Patients with Painful Diabetic Neuropathy: a Pilot Study of Effects on Physical Activity and Quality of Life. J Rehabil Med Clin Commun. 2021;4:1000046. doi: 10.2340/20030711-1000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Riese H, von Klipstein L, Schoevers RA, van der Veen DC, Servaas MN. Personalized ESM monitoring and feedback to support psychological treatment for depression: a pragmatic randomized controlled trial (Therap-i). BMC Psychiatry. 2021;21(1):143. doi: 10.1186/s12888-021-03123-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kramer I, Simons CJ, Hartmann JA, et al. A therapeutic application of the experience sampling method in the treatment of depression: a randomized controlled trial. World Psychiatry. 2014;13(1):68–77. doi: 10.1002/wps.20090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Os J, Verhagen S, Marsman A, et al. The experience sampling method as an mHealth tool to support self-monitoring, self-insight, and personalized health care in clinical practice. Depress Anxiety. 2017;34(6):481–493. doi: 10.1002/da.22647 [DOI] [PubMed] [Google Scholar]
- 114.Myin-Germeys I, Kuppens P. The Open Handbook of Experience Sampling Methodology. Leuven: REAL; 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, AD, upon reasonable request.