Abstract
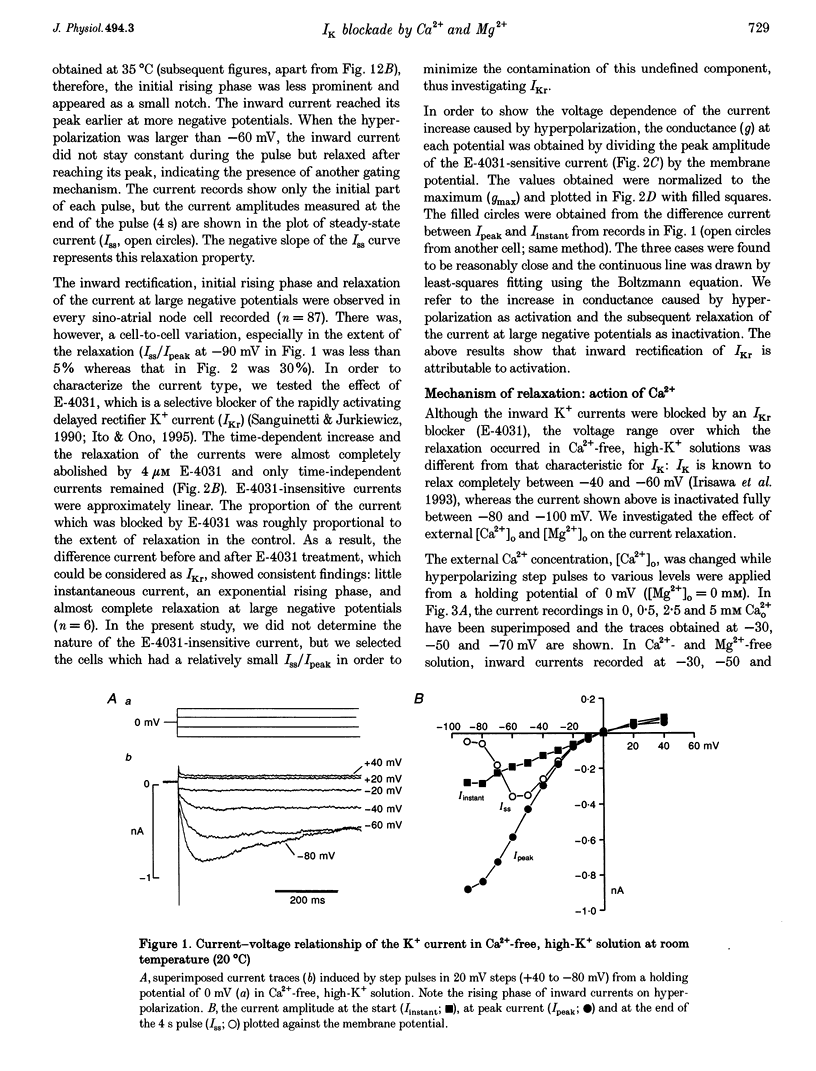
1. The properties of the delayed rectifier K+ current (IK) of rabbit isolated sino-atrial node cells were investigated in high (140 mM) [K+]o using the whole-cell-clamp technique. 2. Hyperpolarizing clamp pulses from 0 mV induced an instantaneous current jump (I-V relation linear) followed by a time-dependent increase in inward current to a peak, whereas depolarizing clamp pulses induced little outward current. The peak I-V relation showed a strong inward rectification. The inwardly rectifying current was blocked by E-4031. 3. The inward K+ current induced by hyperpolarizing clamp pulses from 0 mV relaxed after reaching its peak. The rate of the relaxation increased as the membrane potential became more negative and concentrations of external Ca2+ or Mg2+ were increased. The steady-state current was smaller as the relaxation of the current accelerated on increasing [Ca2+]o or [Mg2+]o. 4. Depolarizing clamp pulses from -80 mV induced an increase in inward current, reaching a steady state. The amplitude of the steady-state current became smaller and the rate of current increase became slower as [Ca2+]o or [Mg2+]o was increased. 5. The effects of Ca2+ and Mg2+ are well explained by a time- and voltage-dependent blockade of the K+ channel by these ions. The fractional electrical distance of the binding site calculated from the voltage dependence of the blocking rate constant is 0.69 for Ca2+ and 0.88 for Mg2+. The blocking rate constant at 0 mV for Ca2+ is about 15 times faster than that for Mg2+, indicating stronger effects of Ca2+. 6. A re-interpretation of IK in sino-atrial node cells is proposed: there are two independent gates (an activation gate which opens on hyperpolarization and an inactivation gate which closes on hyperpolarization) and a binding site for Ca2+ and Mg2+ inside the channel. Binding of these ions, which is facilitated by hyperpolarization, causes channel blockade, resulting in the observed voltage dependence of IK in physiological concentrations of Ca2+ and Mg2+.
Full text
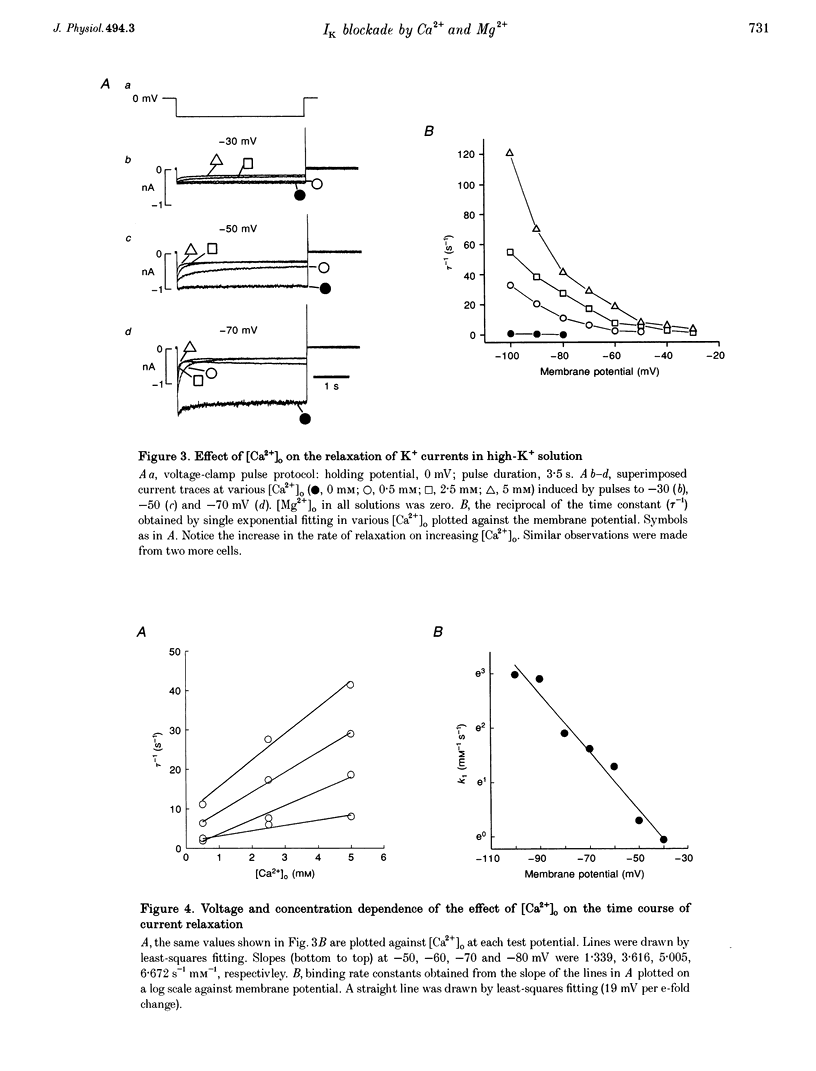
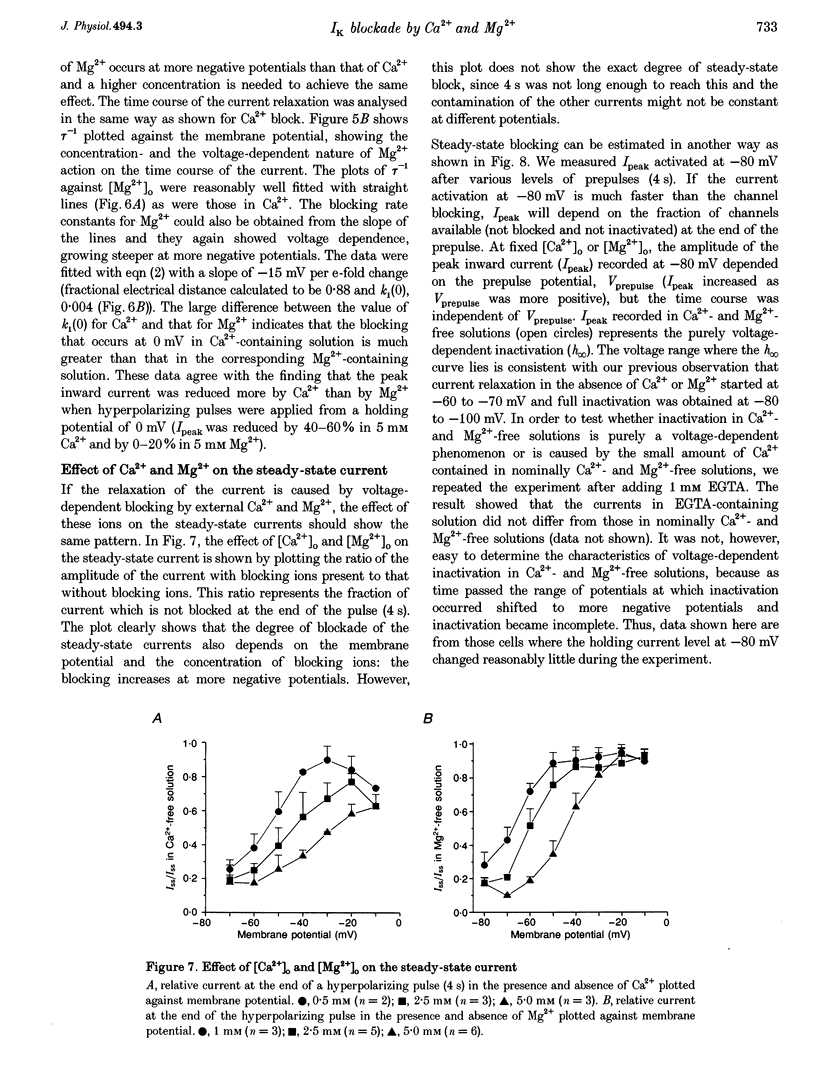
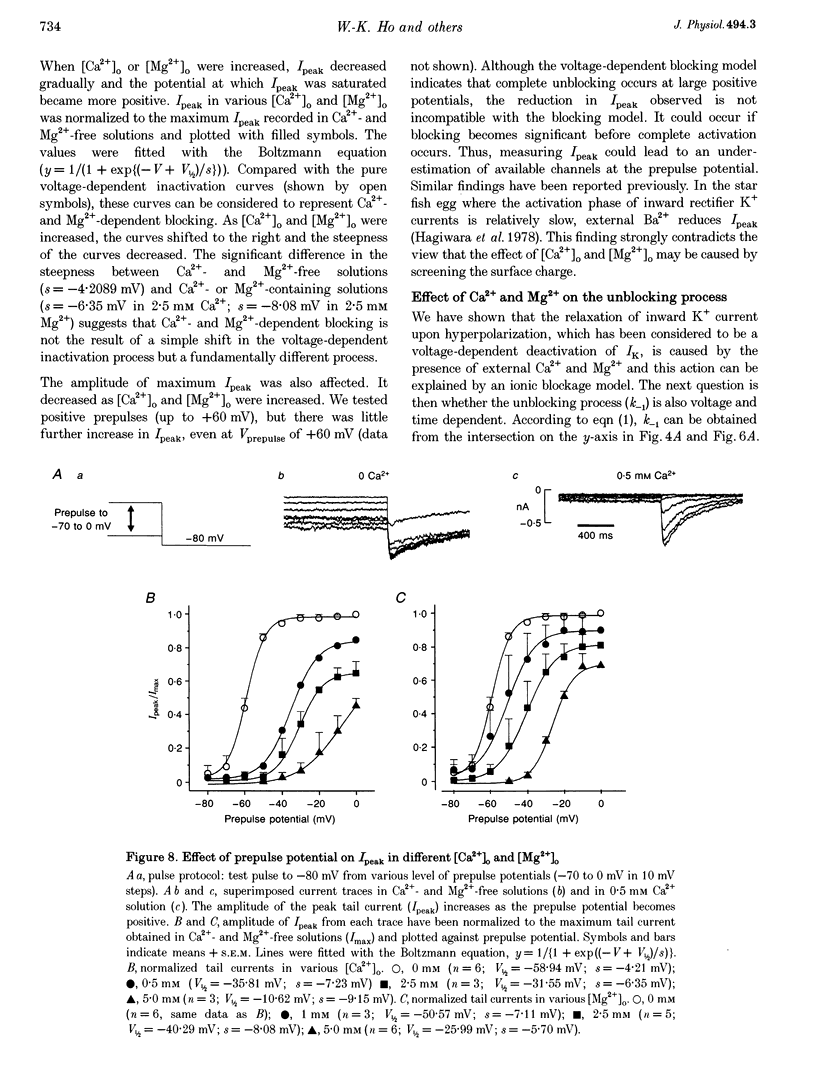
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Dukes I. D., Morad M. Divalent cations modulate the transient outward current in rat ventricular myocytes. Am J Physiol. 1991 Aug;261(2 Pt 1):C310–C318. doi: 10.1152/ajpcell.1991.261.2.C310. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Lopez-Barneo J. External calcium ions are required for potassium channel gating in squid neurons. Science. 1987 May 8;236(4802):712–714. doi: 10.1126/science.2437654. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Matteson D. R. The role of calcium ions in the closing of K channels. J Gen Physiol. 1986 May;87(5):817–832. doi: 10.1085/jgp.87.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Clark A., Noble S. J. Analysis of pace-maker and repolarization currents in frog atrial muscle. J Physiol. 1976 Jul;258(3):547–577. doi: 10.1113/jphysiol.1976.sp011435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H., Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol. 1980 Nov;308:331–351. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Denyer J. C., Brown H. F. Pacemaking in rabbit isolated sino-atrial node cells during Cs+ block of the hyperpolarization-activated current if. J Physiol. 1990 Oct;429:401–409. doi: 10.1113/jphysiol.1990.sp018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Hiraoka M. Depression of delayed outward K+ current by Co2+ in guinea pig ventricular myocytes. Am J Physiol. 1991 Jul;261(1 Pt 1):C23–C31. doi: 10.1152/ajpcell.1991.261.1.C23. [DOI] [PubMed] [Google Scholar]
- Follmer C. H., Lodge N. J., Cullinan C. A., Colatsky T. J. Modulation of the delayed rectifier, IK, by cadmium in cat ventricular myocytes. Am J Physiol. 1992 Jan;262(1 Pt 1):C75–C83. doi: 10.1152/ajpcell.1992.262.1.C75. [DOI] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. Cs(+) causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977 May 12;267(5607):169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- Green W. N., Andersen O. S. Surface charges and ion channel function. Annu Rev Physiol. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H., Brown H. F., Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993 Jan;73(1):197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Ito H., Ono K. A rapidly activating delayed rectifier K+ channel in rabbit sinoatrial node cells. Am J Physiol. 1995 Aug;269(2 Pt 2):H443–H452. doi: 10.1152/ajpheart.1995.269.2.H443. [DOI] [PubMed] [Google Scholar]
- Ito H., Ono K., Noma A. Background conductance attributable to spontaneous opening of muscarinic K+ channels in rabbit sino-atrial node cells. J Physiol. 1994 Apr 1;476(1):55–68. [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):205–231. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Nakayama T., Kurachi Y., Irisawa H. Resting K conductances in pacemaker and non-pacemaker heart cells of the rabbit. Jpn J Physiol. 1984;34(2):245–254. doi: 10.2170/jjphysiol.34.245. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990 Jul;96(1):195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987 Jun;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires S., Begenisich T. Chemical properties of the divalent cation binding site on potassium channels. J Gen Physiol. 1992 Aug;100(2):181–193. doi: 10.1085/jgp.100.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires S., Begenisich T. Modulation of potassium channel gating by external divalent cations. J Gen Physiol. 1994 Oct;104(4):675–692. doi: 10.1085/jgp.104.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisgirda M. E., Dryer S. E. Divalent cations selectively alter the voltage dependence of inactivation of A-currents in chick autonomic neurons. Pflugers Arch. 1993 Jun;423(5-6):418–426. doi: 10.1007/BF00374936. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


