Abstract
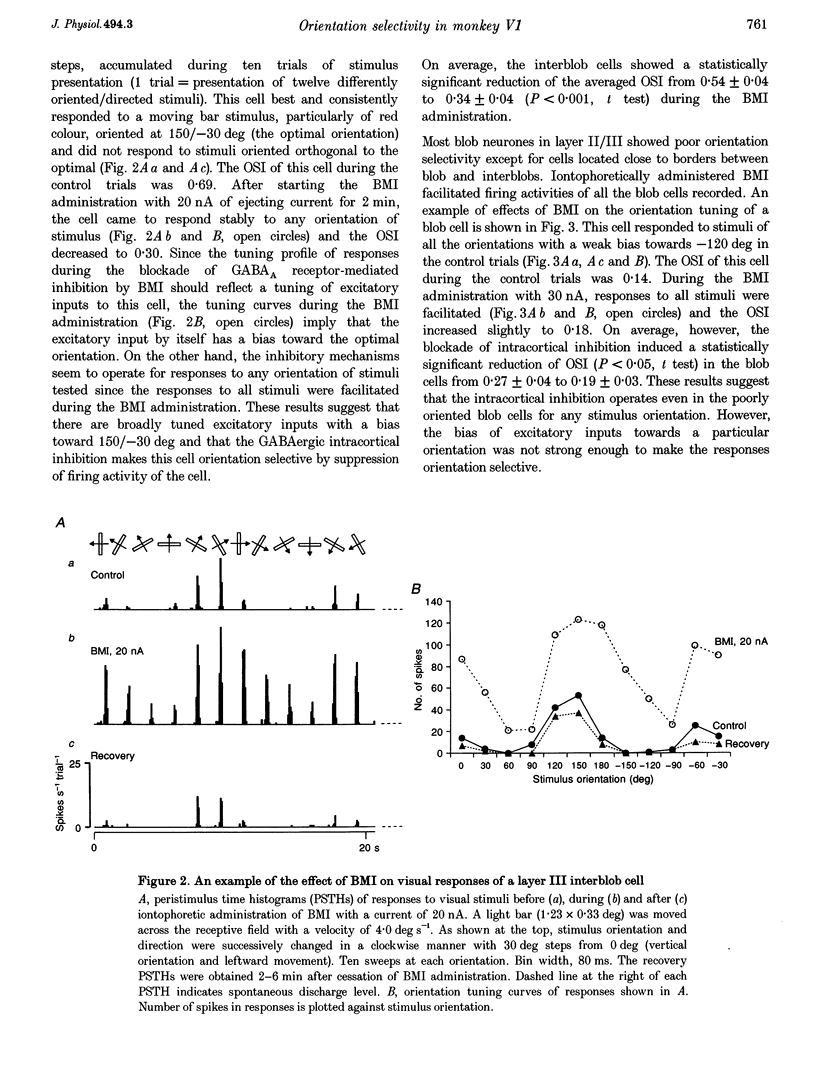
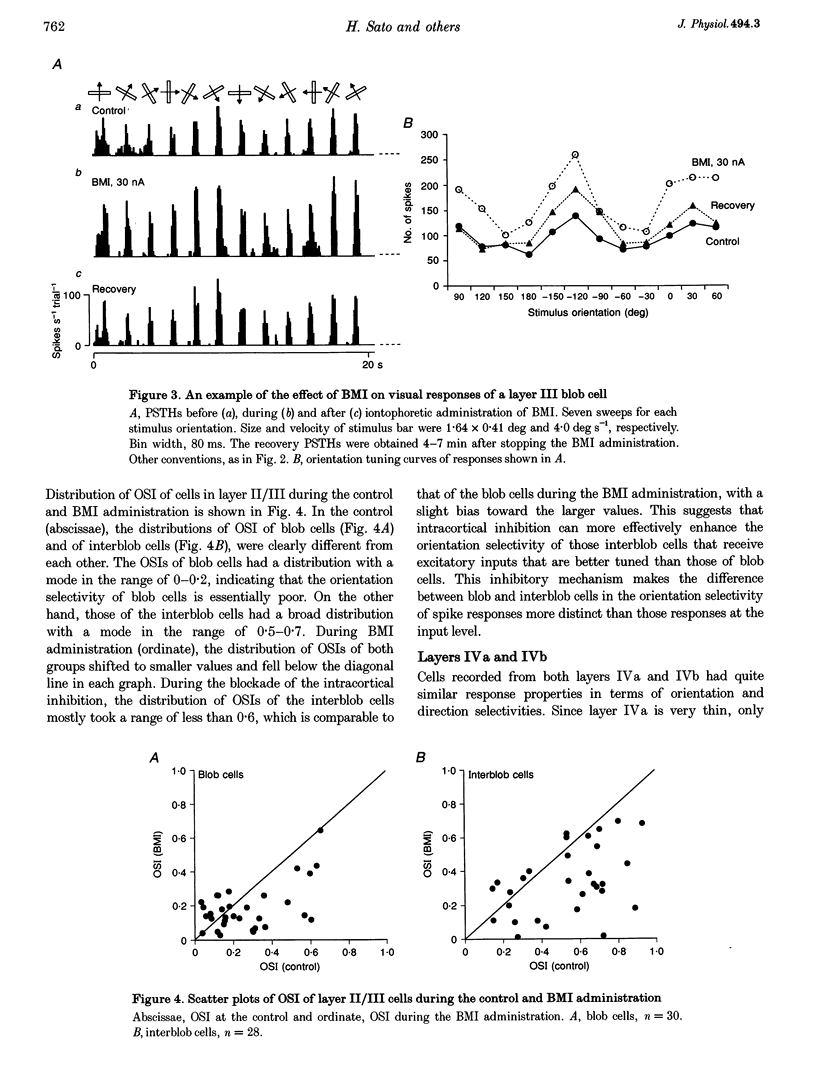
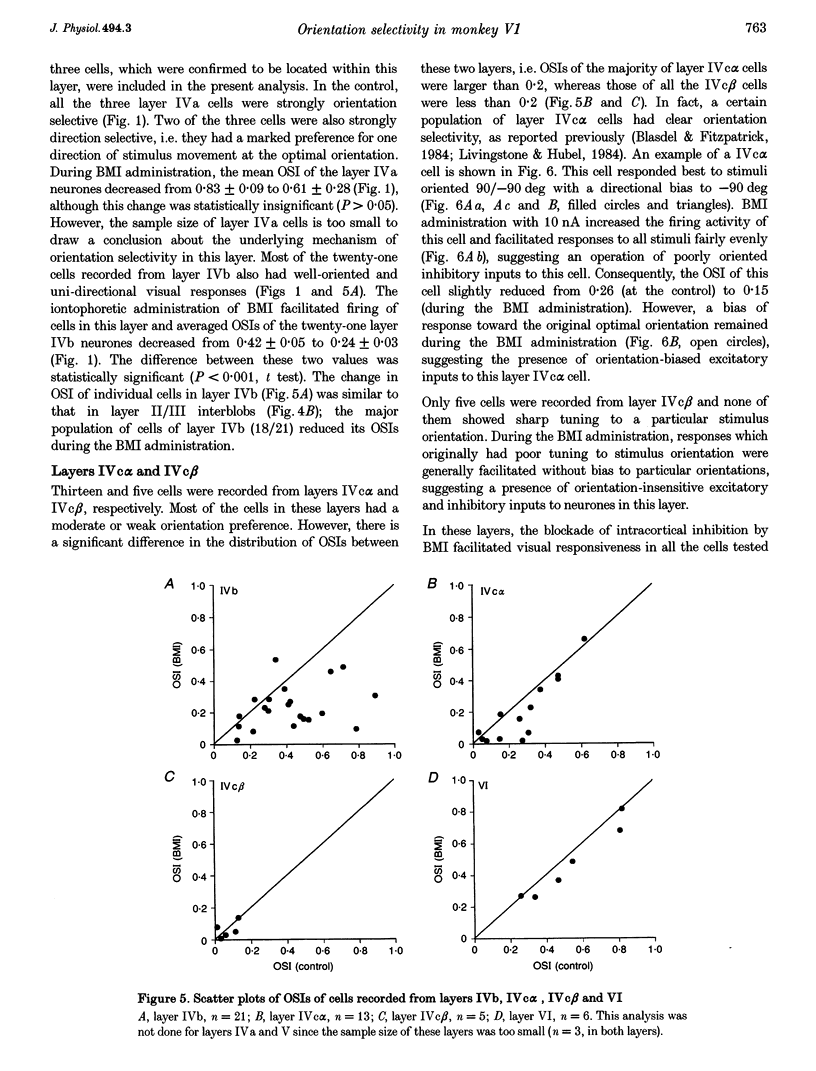
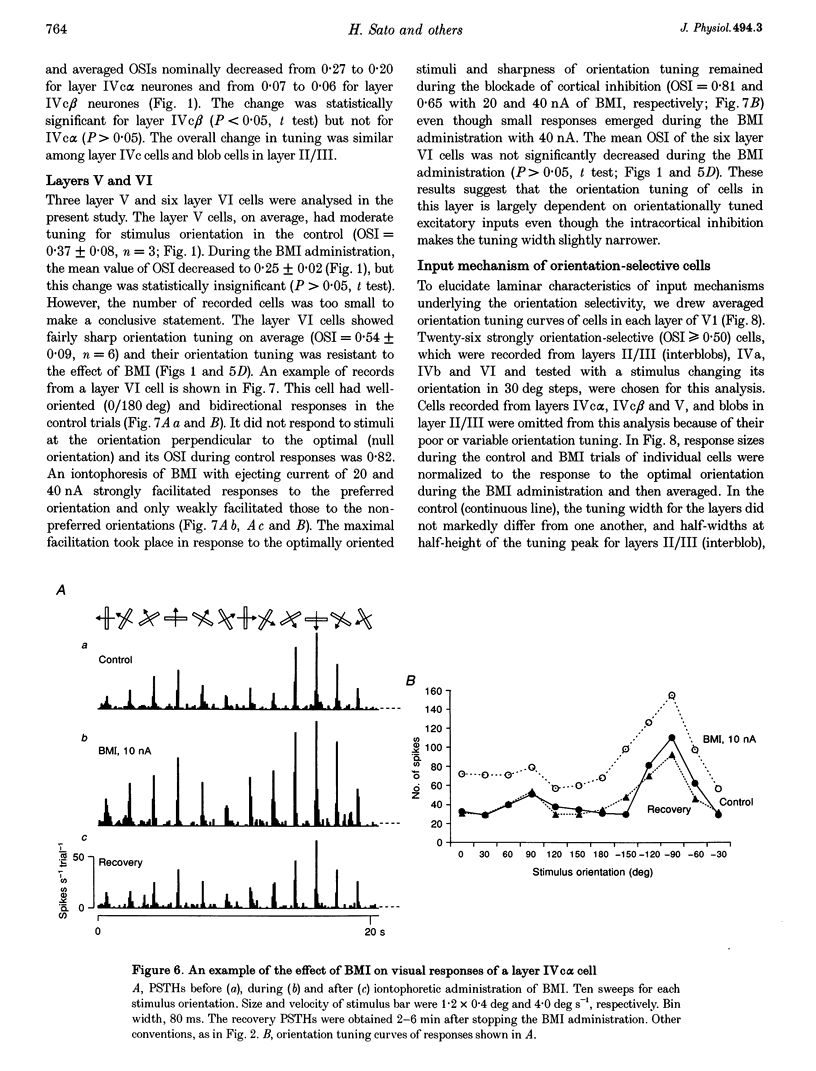
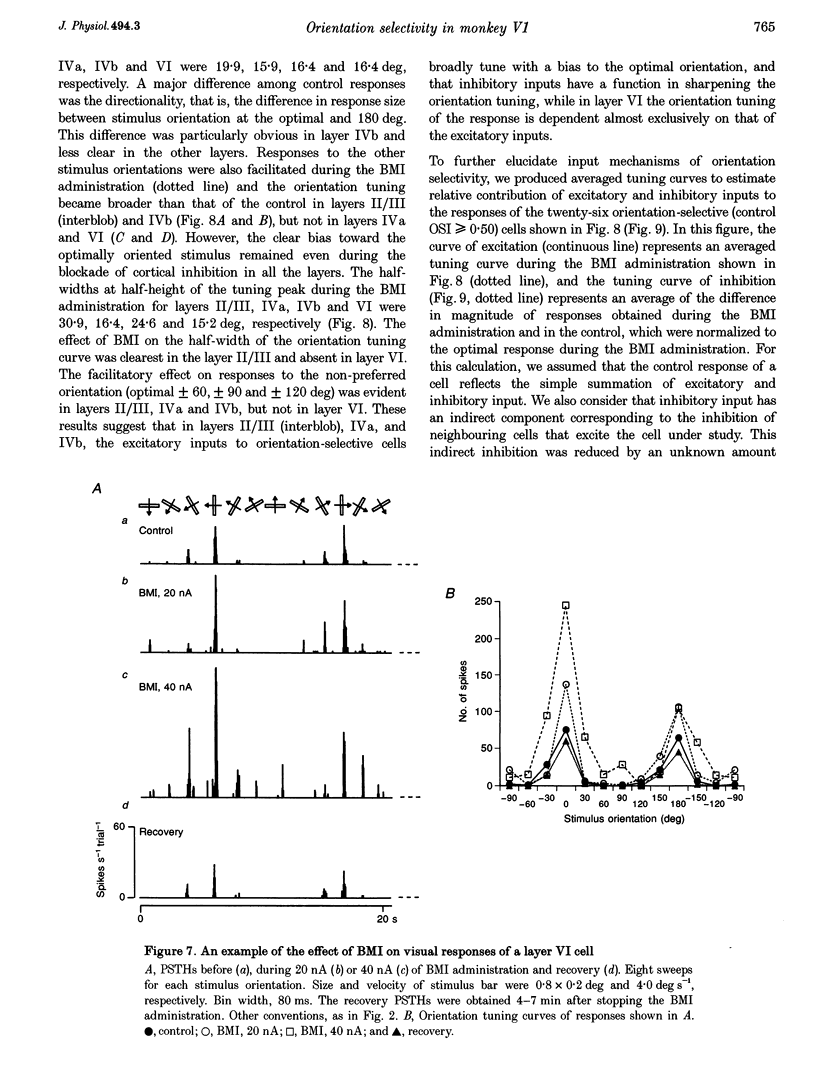
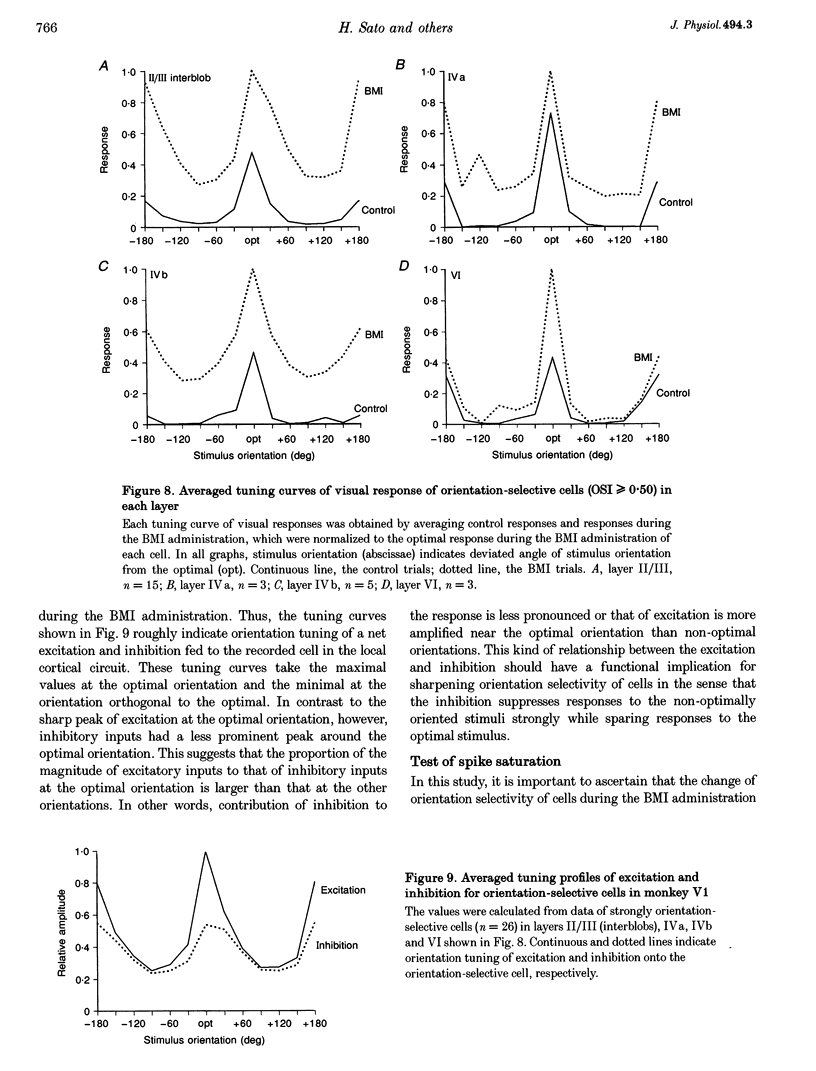
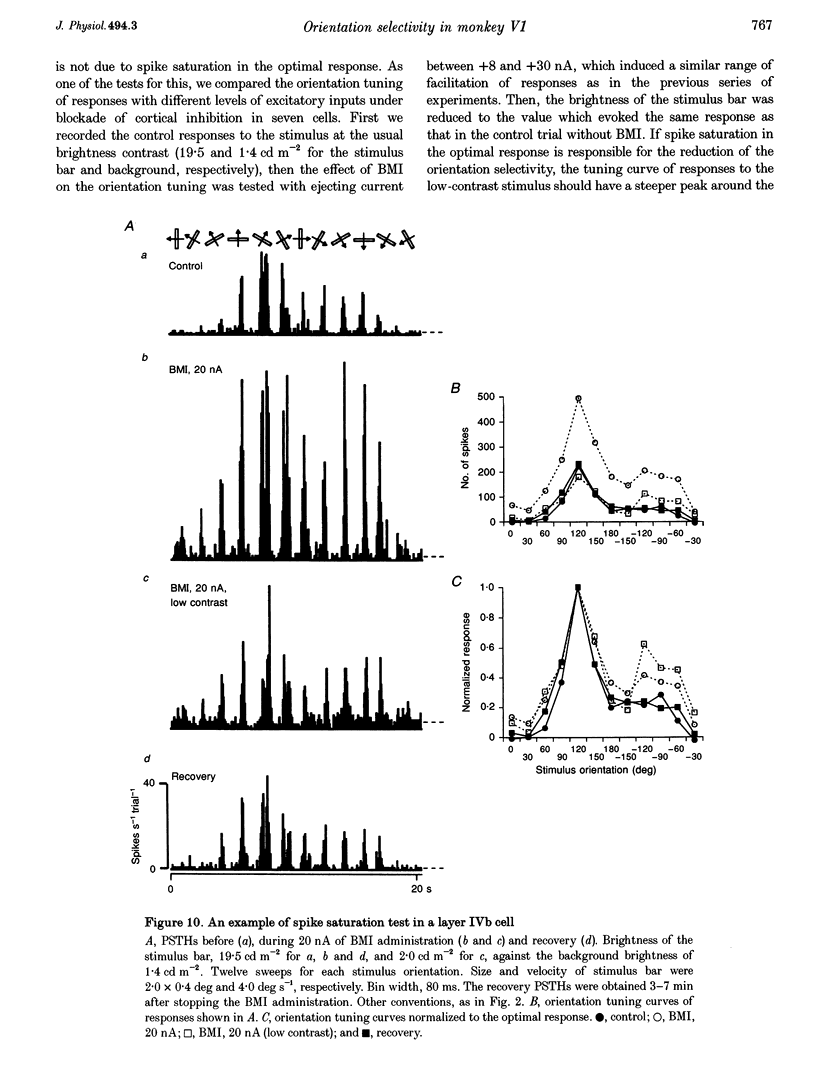
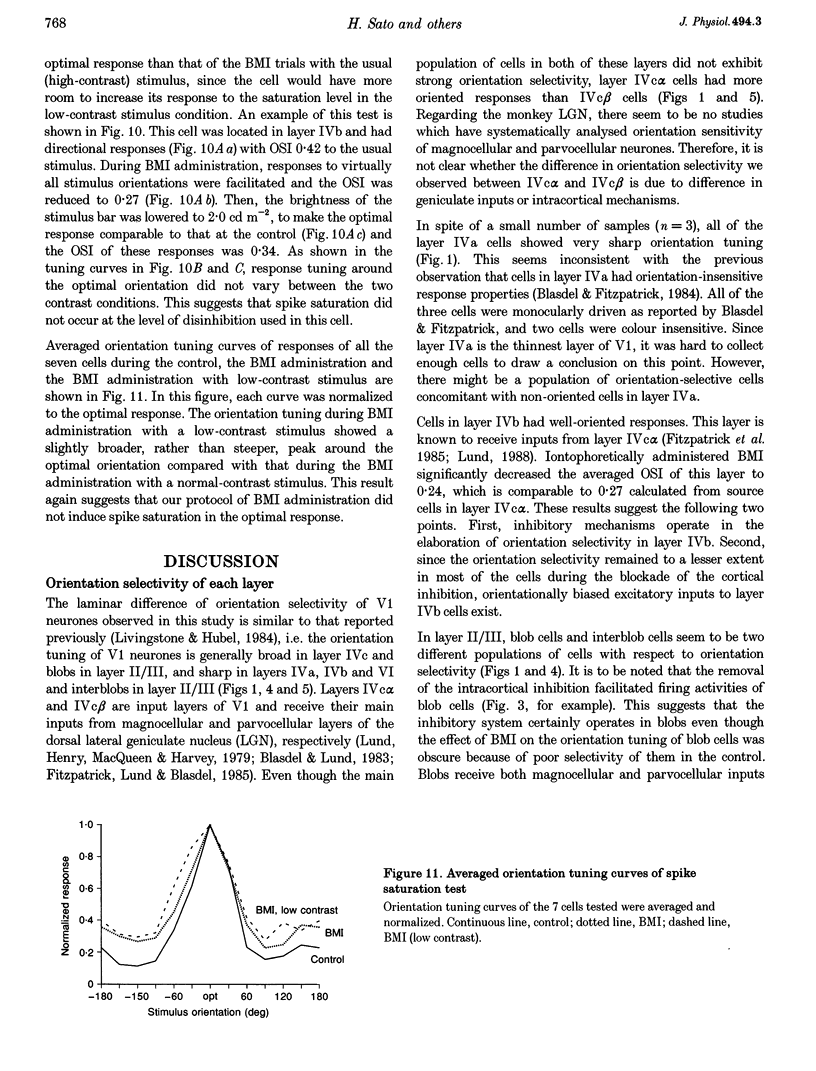
1. Effects of blocking intracortical inhibition by microiontophoretic administration of bicuculline methiodide (BMI), a selective antagonist for GABAA receptors, on orientation selectivity of 109 neurones were studied in the primary visual cortex (V1) of anaesthetized and paralysed monkeys. 2. The averaged orientation tuning of visual responses of cells was poor in cytochrome oxidaserich blobs of layer II/III and in layer IVc beta, moderate in layers IVb, IVc alpha and V, and sharp in the interblob region of layer II/III and in layers IVa and VI. 3. Iontophoretic administration of BMI reduced the sharpness of orientation tuning of cells to a varying extent in each layer. In most cells, furthermore, the originally ineffective stimuli induced visual responses during the BMI administration, suggesting that excitatory inputs evoked by the non-optimally oriented stimuli were masked by GABAergic inhibition. Nevertheless, the maximal facilitation was observed in the response to the optimally or near-optimally oriented stimuli. 4. There was a difference in such an effect of BMI among layers. Orientation selectivity of cells in interblobs in layer II/III and in layer IVb was sensitive to BMI whereas that of cells in layer VI was relatively insensitive to BMI, suggesting a larger contribution of excitatory mechanisms to the orientation selectivity in this layer. 5. In the orientation-selective cells, an analysis of the magnitude of excitation and inhibition evoked by stimuli at various orientations suggests that both inputs tune around the optimal orientation and their magnitudes are almost proportional to each other except at the optimal orientation. This analysis also indicates that the orientation tuning of inhibition had a less prominent peak around the optimal orientation than that of excitation. This dominance of excitation over inhibition around the optimal orientation may function to accentuate the response to the optimally oriented stimulus. 6. These results suggest that, in the monkey V1, the orientation selectivity of cells is largely dependent on the orientation-biased excitatory and inhibitory inputs which have a broader tuning profile, covering from the optimal to null-orientation, than that observed in extracellularly recorded responses at the control level.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumfalk U., Albus K. Phaclofen antagonizes baclofen-induced suppression of visually evoked responses in the cat's striate cortex. Brain Res. 1988 Nov 1;463(2):398–402. doi: 10.1016/0006-8993(88)90418-0. [DOI] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Receptive fields of simple cells in the cat striate cortex. J Physiol. 1973 May;231(1):31–60. doi: 10.1113/jphysiol.1973.sp010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Tobin E. A. Lateral inhibition between orientation detectors in the cat's visual cortex. Exp Brain Res. 1972;15(4):439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- Blasdel G. G., Fitzpatrick D. Physiological organization of layer 4 in macaque striate cortex. J Neurosci. 1984 Mar;4(3):880–895. doi: 10.1523/JNEUROSCI.04-03-00880.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasdel G. G., Lund J. S. Termination of afferent axons in macaque striate cortex. J Neurosci. 1983 Jul;3(7):1389–1413. doi: 10.1523/JNEUROSCI.03-07-01389.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds A. B. Role of inhibition in the specification of orientation selectivity of cells in the cat striate cortex. Vis Neurosci. 1989;2(1):41–55. doi: 10.1017/s0952523800004314. [DOI] [PubMed] [Google Scholar]
- Chapman B., Zahs K. R., Stryker M. P. Relation of cortical cell orientation selectivity to alignment of receptive fields of the geniculocortical afferents that arborize within a single orientation column in ferret visual cortex. J Neurosci. 1991 May;11(5):1347–1358. doi: 10.1523/JNEUROSCI.11-05-01347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. J., Martin K. A., Whitteridge D. An intracellular analysis of the visual responses of neurones in cat visual cortex. J Physiol. 1991;440:659–696. doi: 10.1113/jphysiol.1991.sp018730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. J., Martin K. A., Whitteridge D. Selective responses of visual cortical cells do not depend on shunting inhibition. Nature. 1988 Apr 14;332(6165):642–644. doi: 10.1038/332642a0. [DOI] [PubMed] [Google Scholar]
- Ferster D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J Neurosci. 1986 May;6(5):1284–1301. doi: 10.1523/JNEUROSCI.06-05-01284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D., Lund J. S., Blasdel G. G. Intrinsic connections of macaque striate cortex: afferent and efferent connections of lamina 4C. J Neurosci. 1985 Dec;5(12):3329–3349. doi: 10.1523/JNEUROSCI.05-12-03329.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K., Sato H., Daw N. The location and function of NMDA receptors in cat and kitten visual cortex. J Neurosci. 1989 Jul;9(7):2443–2454. doi: 10.1523/JNEUROSCI.09-07-02443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y., Tsumoto T., Sato H., Hagihara K., Tamura H. Inhibition contributes to orientation selectivity in visual cortex of cat. Nature. 1988 Oct 27;335(6193):815–817. doi: 10.1038/335815a0. [DOI] [PubMed] [Google Scholar]
- Hata Y., Tsumoto T., Sato H., Tamura H. Horizontal interactions between visual cortical neurones studied by cross-correlation analysis in the cat. J Physiol. 1991 Sep;441:593–614. doi: 10.1113/jphysiol.1991.sp018769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggelund P., Moors J. Orientation selectivity and the spatial distribution of enhancement and suppression in receptive fields of cat striate cortex cells. Exp Brain Res. 1983;52(2):235–247. doi: 10.1007/BF00236632. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Yoshioka T. A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science. 1994 Apr 22;264(5158):575–577. doi: 10.1126/science.8160015. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica E. A., Beck P. D., Casagrande V. A. Parallel pathways in macaque monkey striate cortex: anatomically defined columns in layer III. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3566–3570. doi: 10.1073/pnas.89.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984 Jan;4(1):309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M., Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988 May 6;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Lund J. S. Anatomical organization of macaque monkey striate visual cortex. Annu Rev Neurosci. 1988;11:253–288. doi: 10.1146/annurev.ne.11.030188.001345. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Henry G. H., MacQueen C. L., Harvey A. R. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol. 1979 Apr 15;184(4):599–618. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- Lund J. S. Organization of neurons in the visual cortex, area 17, of the monkey (Macaca mulatta). J Comp Neurol. 1973 Feb 15;147(4):455–496. doi: 10.1002/cne.901470404. [DOI] [PubMed] [Google Scholar]
- Merigan W. H., Katz L. M., Maunsell J. H. The effects of parvocellular lateral geniculate lesions on the acuity and contrast sensitivity of macaque monkeys. J Neurosci. 1991 Apr;11(4):994–1001. doi: 10.1523/JNEUROSCI.11-04-00994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa A. S., Shadlen M., Skottun B. C., Freeman R. D. A comparison of inhibition in orientation and spatial frequency selectivity of cat visual cortex. Nature. 1986 May 15;321(6067):237–239. doi: 10.1038/321237a0. [DOI] [PubMed] [Google Scholar]
- Sato H., Daw N. W., Fox K. An intracellular recording study of stimulus-specific response properties in cat area 17. Brain Res. 1991 Mar 22;544(1):156–161. doi: 10.1016/0006-8993(91)90899-7. [DOI] [PubMed] [Google Scholar]
- Sato H., Katsuyama N., Tamura H., Hata Y., Tsumoto T. Broad-tuned chromatic inputs to color-selective neurons in the monkey visual cortex. J Neurophysiol. 1994 Jul;72(1):163–168. doi: 10.1152/jn.1994.72.1.163. [DOI] [PubMed] [Google Scholar]
- Sato H., Katsuyama N., Tamura H., Hata Y., Tsumoto T. Mechanisms underlying direction selectivity of neurons in the primary visual cortex of the macaque. J Neurophysiol. 1995 Oct;74(4):1382–1394. doi: 10.1152/jn.1995.74.4.1382. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Logothetis N. K., Charles E. R. Functions of the colour-opponent and broad-band channels of the visual system. Nature. 1990 Jan 4;343(6253):68–70. doi: 10.1038/343068a0. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A., Milson J. A., Berardi N. A re-evaluation of the mechanisms underlying simple cell orientation selectivity. Brain Res. 1980 Aug 4;194(2):517–520. doi: 10.1016/0006-8993(80)91234-2. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975 Sep;250(2):305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáry G., Vogels R., Kovács G., Orban G. A. Responses of monkey inferior temporal neurons to luminance-, motion-, and texture-defined gratings. J Neurophysiol. 1995 Apr;73(4):1341–1354. doi: 10.1152/jn.1995.73.4.1341. [DOI] [PubMed] [Google Scholar]
- Tsumoto T., Eckart W., Creutzfeldt O. D. Modification of orientation sensitivity of cat visual cortex neurons by removal of GABA-mediated inhibition. Exp Brain Res. 1979 Jan 15;34(2):351–363. doi: 10.1007/BF00235678. [DOI] [PubMed] [Google Scholar]
- Tsumoto T., Masui H., Sato H. Excitatory amino acid transmitters in neuronal circuits of the cat visual cortex. J Neurophysiol. 1986 Mar;55(3):469–483. doi: 10.1152/jn.1986.55.3.469. [DOI] [PubMed] [Google Scholar]
- Volgushev M., Pei X., Vidyasagar T. R., Creutzfeldt O. D. Excitation and inhibition in orientation selectivity of cat visual cortex neurons revealed by whole-cell recordings in vivo. Vis Neurosci. 1993 Nov-Dec;10(6):1151–1155. doi: 10.1017/s0952523800010257. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Zeki S., Shipp S. The functional logic of cortical connections. Nature. 1988 Sep 22;335(6188):311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]



