Abstract
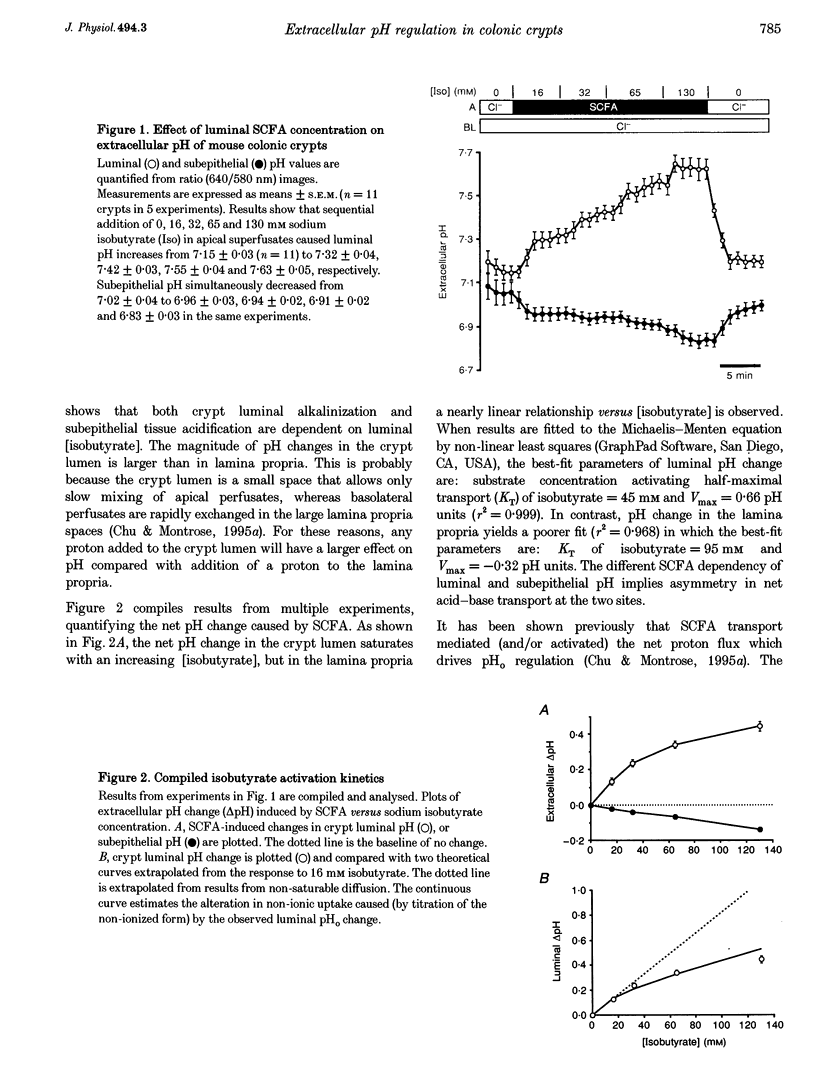
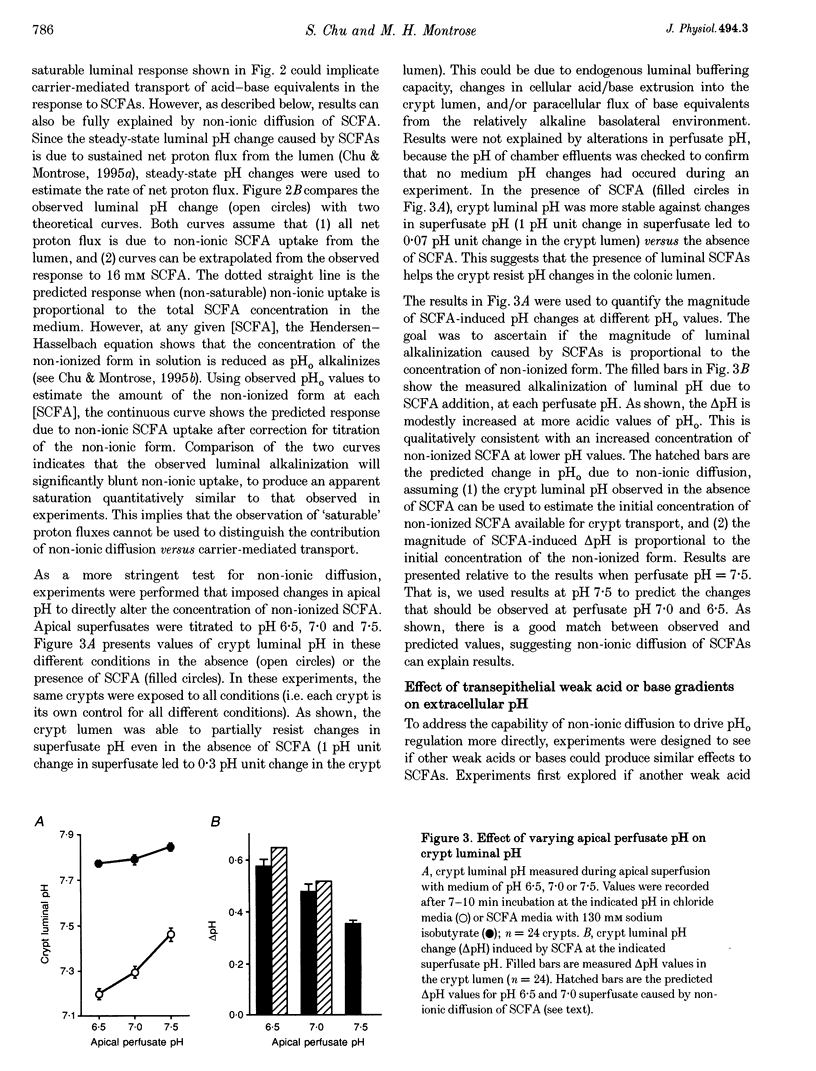
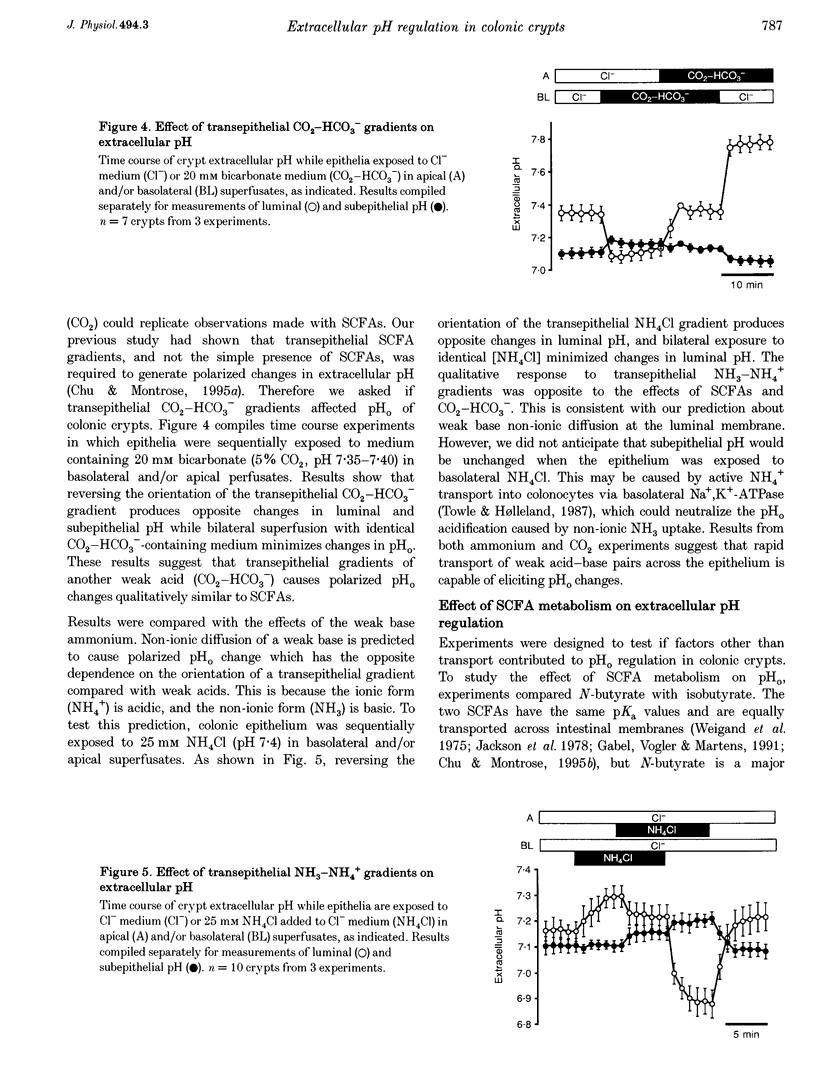
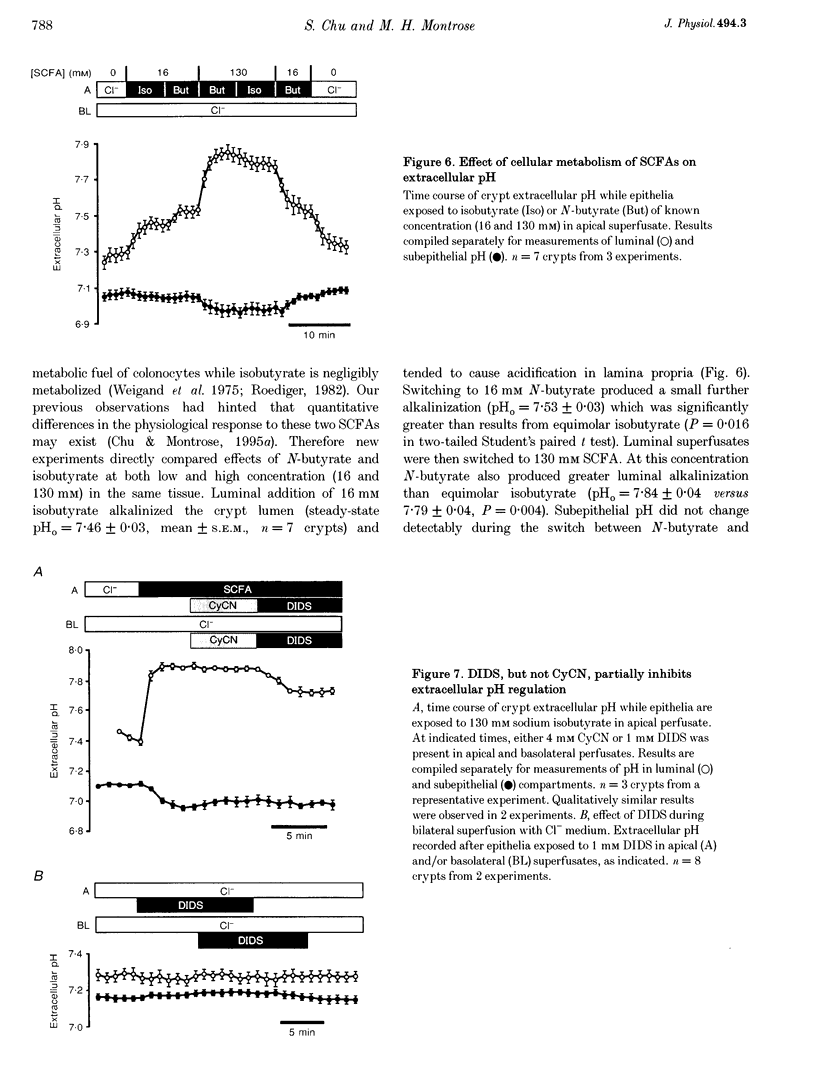
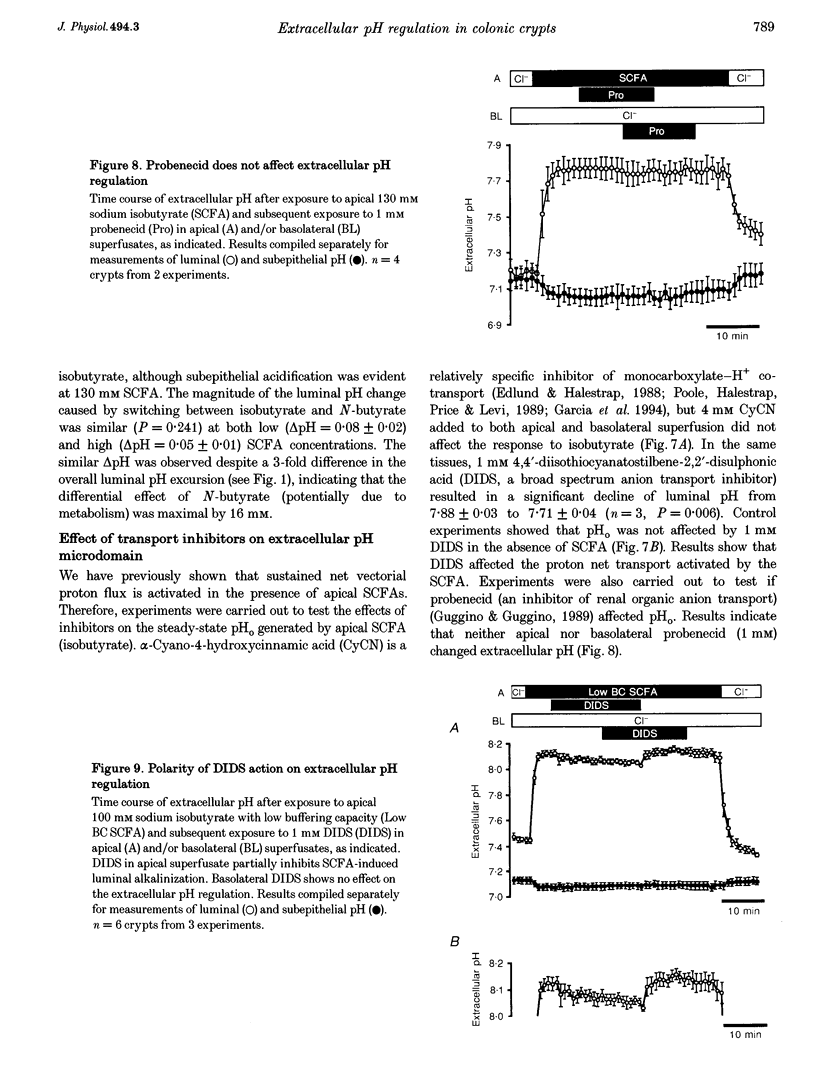
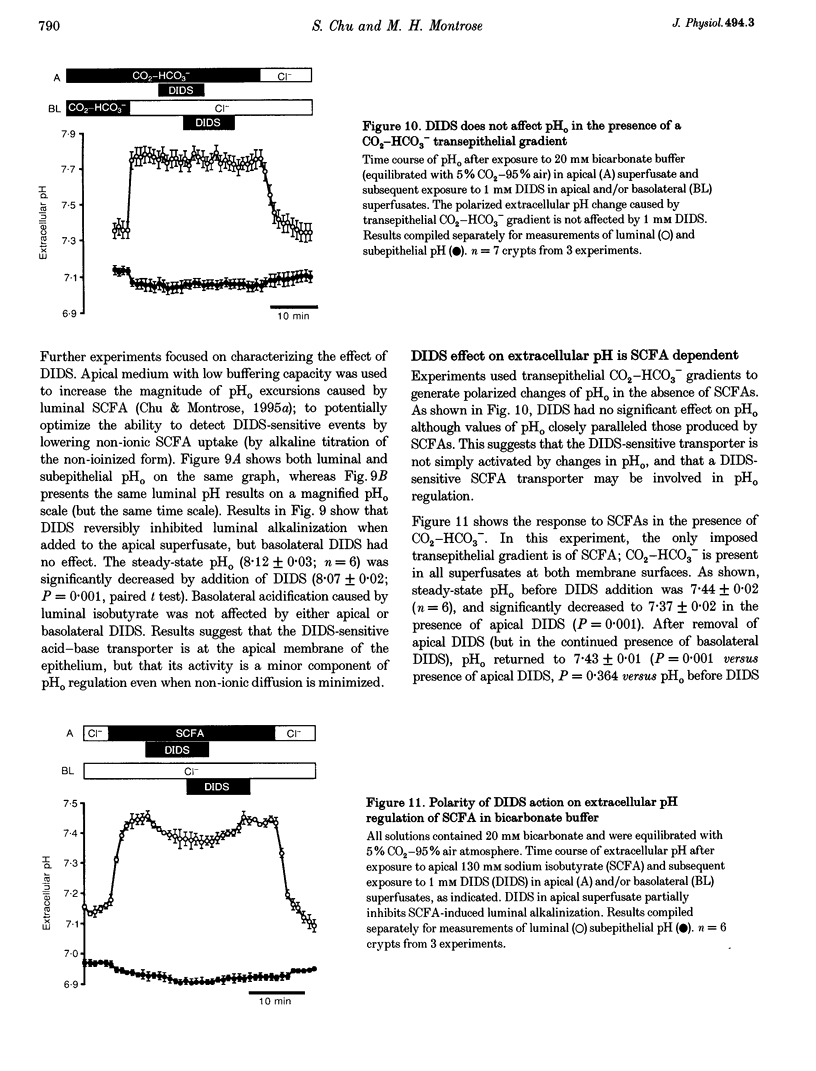
1. Extracellular pH (pHo) regulation within mouse colonic crypt lumens is stimulated by transepithelial gradients of short-chain fatty acids (SCFAs). Current work assesses underlying mechanisms contributing to pHo regulation. 2. Crypt luminal alkalinization was saturable by apical SCFA (substrate concentration activating half-maximal transport (KT) of isobutyrate = 45 mM). However, saturation was consistent with either carrier-mediated SCFA flux or non-ionic diffusion, because the non-ionized form was titrated by luminal alkalinization. Direct acidification of apical perfusates increased the magnitude of SCFA-induced luminal alkalinization, roughly in the same proportion to the increased concentration of non-ionized SCFA in the crypt lumen. 3. Transepithelial gradients of an alternative weak acid (CO2) produce pHo changes similar to SCFA. In contrast, a weak base (NH3) changes pHo with reverse dependence on the orientation of the transepithelial gradient compared with SCFA. Results implicate non-ionic diffusion in pHo regulation, and suggest that pHo changes may underly SCFA-stimulated bicarbonate secretion and ammonium absorption. 4. SCFA metabolism plays a minor role in extracellular pH regulation. An avidly metabolized SCFA (N-butyrate) augments crypt luminal alkalinization only slightly (0.08 pH units) versus a poorly metabolized SCFA (isobutyrate). 5. Apical addition of 1 mM 4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid (DIDS) partially inhibits luminal alkalinization caused by apical SCFA. DIDS has no effect on luminal alkalinization caused by transepithelial CO2 gradients. Probenecid (1 mM), alpha-cyano-4-hydroxycinnamic acid (4 mM) or basolateral DIDS (1 mM) do not affect pHo regulation. Results suggest that DIDS-sensitive, SCFA-dependent transport in the colonocyte apical membrane contributes to pHo regulation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argenzio R. A., Southworth M., Lowe J. E., Stevens C. E. Interrelationship of Na, HCO3, and volatile fatty acid transport by equine large intestine. Am J Physiol. 1977 Dec;233(6):E469–E478. doi: 10.1152/ajpendo.1977.233.6.E469. [DOI] [PubMed] [Google Scholar]
- Bergman E. N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990 Apr;70(2):567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- Binder H. J., Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology. 1989 Apr;96(4):989–996. doi: 10.1016/0016-5085(89)91614-4. [DOI] [PubMed] [Google Scholar]
- Bödeker D., Shen Y., Kemkowski J., Höller H. Influence of short-chain fatty acids on ammonia absorption across the rumen wall in sheep. Exp Physiol. 1992 Mar;77(2):369–376. doi: 10.1113/expphysiol.1992.sp003597. [DOI] [PubMed] [Google Scholar]
- Chu S., Montrose M. H. An Na(+)-independent short-chain fatty acid transporter contributes to intracellular pH regulation in murine colonocytes. J Gen Physiol. 1995 May;105(5):589–615. doi: 10.1085/jgp.105.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S., Montrose M. H. Extracellular pH regulation in microdomains of colonic crypts: effects of short-chain fatty acids. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3303–3307. doi: 10.1073/pnas.92.8.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund G. L., Halestrap A. P. The kinetics of transport of lactate and pyruvate into rat hepatocytes. Evidence for the presence of a specific carrier similar to that in erythrocytes. Biochem J. 1988 Jan 1;249(1):117–126. doi: 10.1042/bj2490117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C. K., Goldstein J. L., Pathak R. K., Anderson R. G., Brown M. S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994 Mar 11;76(5):865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Guggino W. B., Guggino S. E. Renal anion transport. Kidney Int. 1989 Sep;36(3):385–391. doi: 10.1038/ki.1989.207. [DOI] [PubMed] [Google Scholar]
- Gutknecht J., Tosteson D. C. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science. 1973 Dec 21;182(4118):1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- Gäbel G., Vogler S., Martens H. Short-chain fatty acids and CO2 as regulators of Na+ and Cl- absorption in isolated sheep rumen mucosa. J Comp Physiol B. 1991;161(4):419–426. doi: 10.1007/BF00260803. [DOI] [PubMed] [Google Scholar]
- Harig J. M., Soergel K. H., Barry J. A., Ramaswamy K. Transport of propionate by human ileal brush-border membrane vesicles. Am J Physiol. 1991 May;260(5 Pt 1):G776–G782. doi: 10.1152/ajpgi.1991.260.5.G776. [DOI] [PubMed] [Google Scholar]
- Holtug K., McEwan G. T., Skadhauge E. Effects of propionate on the acid microclimate of hen (Gallus domesticus) colonic mucosa. Comp Biochem Physiol Comp Physiol. 1992 Dec;103(4):649–652. doi: 10.1016/0300-9629(92)90160-r. [DOI] [PubMed] [Google Scholar]
- Jackson M. J., Williamson A. M., Dombrowski W. A., Garner D. E. Intestinal transport of weak electrolytes: Determinants of influx at the luminal surface. J Gen Physiol. 1978 Mar;71(3):301–327. doi: 10.1085/jgp.71.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty G., Holtug K., Elbrønd V. S., Ridderstråle Y., Skadhauge E. Mucosal acidification and an acid microclimate in the hen colon in vitro. J Comp Physiol B. 1994;163(8):633–641. doi: 10.1007/BF00369513. [DOI] [PubMed] [Google Scholar]
- Lucas M. L. A contribution to analysis of three-compartment models for intestinal weak electrolyte absorption. Am J Physiol. 1984 Nov;247(5 Pt 1):G463–G467. doi: 10.1152/ajpgi.1984.247.5.G463. [DOI] [PubMed] [Google Scholar]
- Lutz T., Scharrer E. Effect of short-chain fatty acids on calcium absorption by the rat colon. Exp Physiol. 1991 Jul;76(4):615–618. doi: 10.1113/expphysiol.1991.sp003530. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T., Gibson G. R., Cummings J. H. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992 Jan;72(1):57–64. doi: 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- Mascolo N., Rajendran V. M., Binder H. J. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology. 1991 Aug;101(2):331–338. doi: 10.1016/0016-5085(91)90008-9. [DOI] [PubMed] [Google Scholar]
- McNeil N. I., Cummings J. H., James W. P. Short chain fatty acid absorption by the human large intestine. Gut. 1978 Sep;19(9):819–822. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naupert C., Rommel K. Absorption of short and medium chain fatty acids in the jejunum of the rat. Z Klin Chem Klin Biochem. 1975 Dec;13(12):553–562. doi: 10.1515/cclm.1975.13.12.553. [DOI] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P., Price S. J., Levi A. J. The kinetics of transport of lactate and pyruvate into isolated cardiac myocytes from guinea pig. Kinetic evidence for the presence of a carrier distinct from that in erythrocytes and hepatocytes. Biochem J. 1989 Dec 1;264(2):409–418. doi: 10.1042/bj2640409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran V. M., Binder H. J. Apical membrane Cl-butyrate exchange: mechanism of short chain fatty acid stimulation of active chloride absorption in rat distal colon. J Membr Biol. 1994 Jul;141(1):51–58. doi: 10.1007/BF00232873. [DOI] [PubMed] [Google Scholar]
- Rechkemmer G., Wahl M., Kuschinsky W., von Engelhardt W. pH-microclimate at the luminal surface of the intestinal mucosa of guinea pig and rat. Pflugers Arch. 1986 Jul;407(1):33–40. doi: 10.1007/BF00580717. [DOI] [PubMed] [Google Scholar]
- Reynolds D. A., Rajendran V. M., Binder H. J. Bicarbonate-stimulated [14C]butyrate uptake in basolateral membrane vesicles of rat distal colon. Gastroenterology. 1993 Sep;105(3):725–732. doi: 10.1016/0016-5085(93)90889-k. [DOI] [PubMed] [Google Scholar]
- Roediger W. E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982 Aug;83(2):424–429. [PubMed] [Google Scholar]
- Rowe W. A., Blackmon D. L., Montrose M. H. Propionate activates multiple ion transport mechanisms in the HT29-18-C1 human colon cell line. Am J Physiol. 1993 Sep;265(3 Pt 1):G564–G571. doi: 10.1152/ajpgi.1993.265.3.G564. [DOI] [PubMed] [Google Scholar]
- Ruppin H., Bar-Meir S., Soergel K. H., Wood C. M., Schmitt M. G., Jr Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980 Jun;78(6):1500–1507. [PubMed] [Google Scholar]
- Sallee V. L., Dietschy J. M. Determinants of intestinal mucosal uptake of short- and medium-chain fatty acids and alcohols. J Lipid Res. 1973 Jul;14(4):475–484. [PubMed] [Google Scholar]
- Sellin J. H., DeSoignie R., Burlingame S. Segmental differences in short-chain fatty acid transport in rabbit colon: effect of pH and Na. J Membr Biol. 1993 Nov;136(2):147–158. doi: 10.1007/BF02505759. [DOI] [PubMed] [Google Scholar]
- Sellin J. H., DeSoignie R. Short-chain fatty acid absorption in rabbit colon in vitro. Gastroenterology. 1990 Sep;99(3):676–683. doi: 10.1016/0016-5085(90)90954-y. [DOI] [PubMed] [Google Scholar]
- Towle D. W., Hølleland T. Ammonium ion substitutes for K+ in ATP-dependent Na+ transport by basolateral membrane vesicles. Am J Physiol. 1987 Mar;252(3 Pt 2):R479–R489. doi: 10.1152/ajpregu.1987.252.3.R479. [DOI] [PubMed] [Google Scholar]
- Umesaki Y., Yajima T., Yokokura T., Mutai M. Effect of organic acid absorption on bicarbonate transport in rat colon. Pflugers Arch. 1979 Feb 14;379(1):43–47. doi: 10.1007/BF00622903. [DOI] [PubMed] [Google Scholar]
- Weigand E., Young J. W., McGilliard A. D. Volatile fatty acid metabolism by rumen mucosa from cattle fed hay or grain. J Dairy Sci. 1975 Sep;58(9):1294–1300. doi: 10.3168/jds.S0022-0302(75)84709-6. [DOI] [PubMed] [Google Scholar]
- von Engelhardt W., Burmester M., Hansen K., Becker G., Rechkemmer G. Effects of amiloride and ouabain on short-chain fatty acid transport in guinea-pig large intestine. J Physiol. 1993 Jan;460:455–466. doi: 10.1113/jphysiol.1993.sp019481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt W., Gros G., Burmester M., Hansen K., Becker G., Rechkemmer G. Functional role of bicarbonate in propionate transport across guinea-pig isolated caecum and proximal colon. J Physiol. 1994 Jun 1;477(Pt 2):365–371. doi: 10.1113/jphysiol.1994.sp020198. [DOI] [PMC free article] [PubMed] [Google Scholar]