Abstract
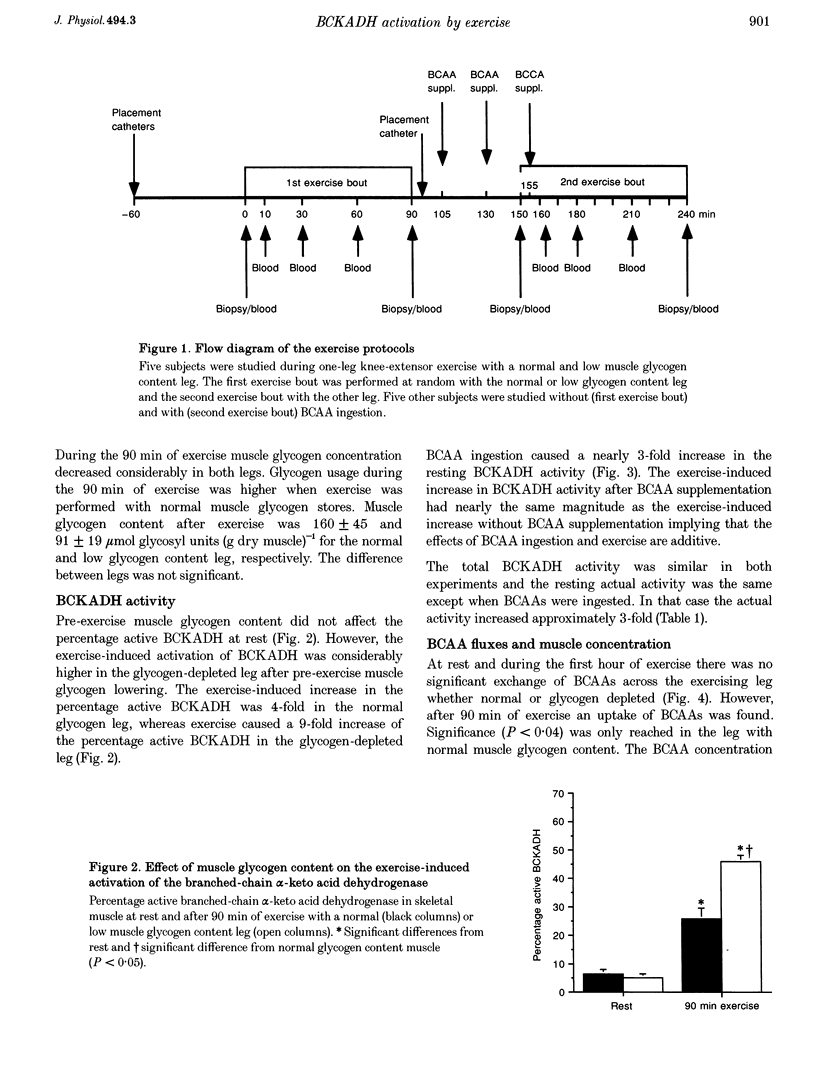
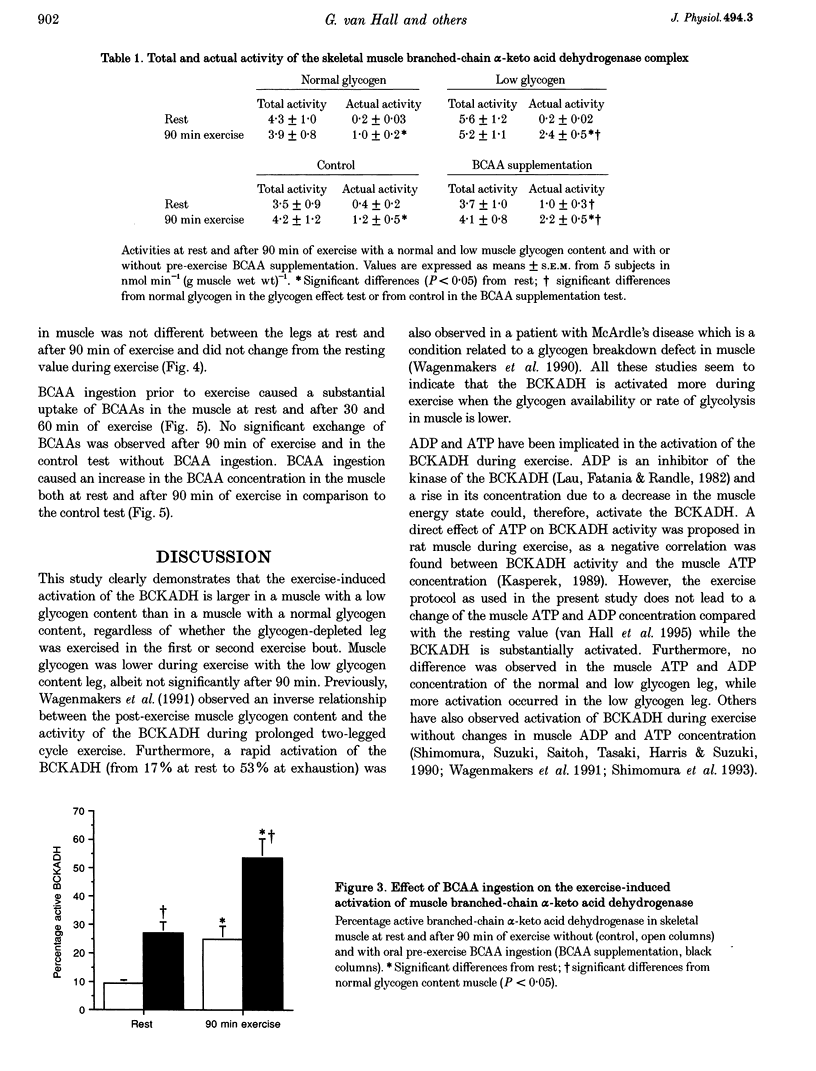
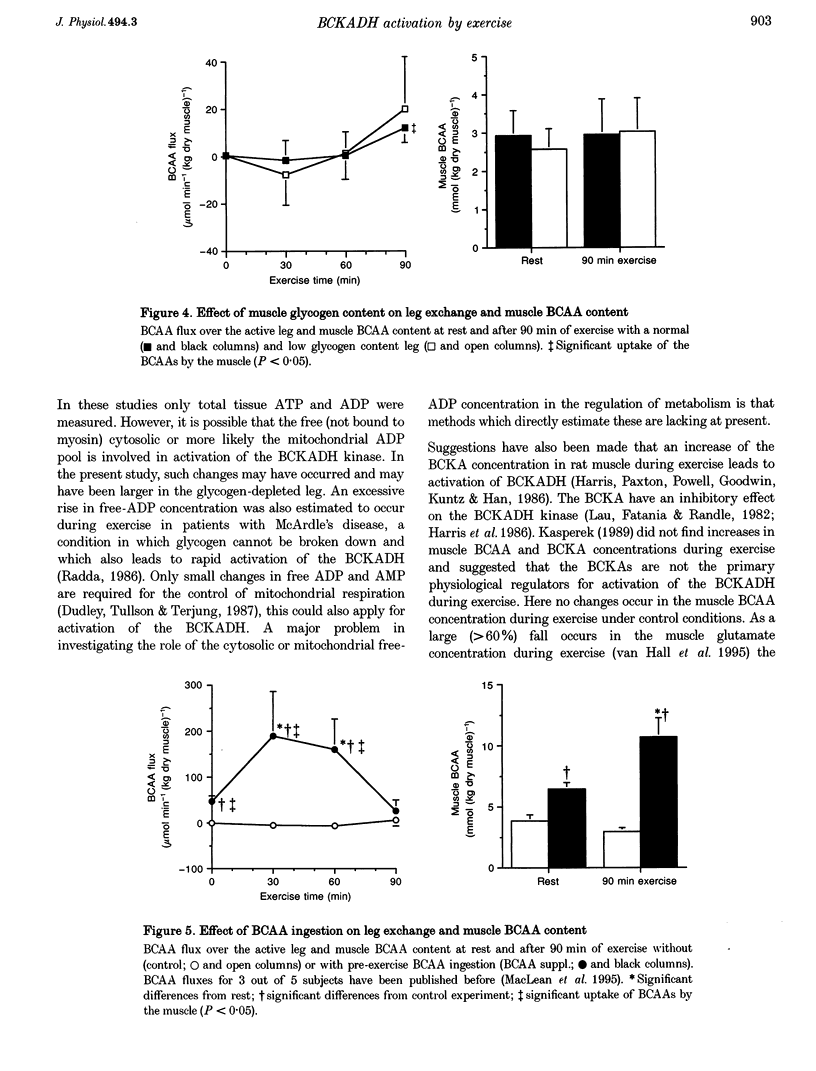
1. Exercise leads to activation (dephosphorylation) of the branched-chain alpha-keto acid dehydrogenase (BCKADH). Here we investigate the effect of low pre-exercise muscle glycogen content and of branched-chain amino acid (BCAA) ingestion on the activity of BCKADH at rest and after 90 min of one-leg knee-extensor exercise at 65% maximal one-leg power output in five subjects. 2. Pre-exercise BCAA ingestion (308 mg BCAAs (kg body wt)-1) caused an increased muscle BCAA uptake, a higher intramuscular BCAA concentration and activation of BCKADH both at rest (9 +/- 1 versus 25 +/- 5% for the control and BCAA test, respectively) and after exercise (27 +/- 4 versus 54 +/- 7%). 3. At rest the percentage active BCKADH was not different, 6 +/- 2% versus 5 +/- 1%, in the normal and low glycogen content leg (392 +/- 21 and 147 +/- 34 mumol glycosyl units (g dry muscle)-1, respectively). The post-exercise BCKADH activity was higher in the low (46 +/- 2%) than in the normal glycogen content leg (26 +/- 2%). 4. It is concluded that: (1) the mechanism of activation by BCAA ingestion probably involves an increase of the muscle BCAA concentration; (2) BCKADH activation caused by exercise and BCAA ingestion are additive; (3) low pre-exercise muscle glycogen content augments the exercise-induced BCKADH activation without an increase in muscle BCAA concentration; and (4) the mechanism of BCKADH activation via BCAA ingestion and low muscle glycogen content are different.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aftring R. P., Block K. P., Buse M. G. Leucine and isoleucine activate skeletal muscle branched-chain alpha-keto acid dehydrogenase in vivo. Am J Physiol. 1986 May;250(5 Pt 1):E599–E604. doi: 10.1152/ajpendo.1986.250.5.E599. [DOI] [PubMed] [Google Scholar]
- Aftring R. P., Miller W. J., Buse M. G. Effects of diabetes and starvation on skeletal muscle branched-chain alpha-keto acid dehydrogenase activity. Am J Physiol. 1988 Mar;254(3 Pt 1):E292–E300. doi: 10.1152/ajpendo.1988.254.3.E292. [DOI] [PubMed] [Google Scholar]
- Andersen P., Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985 Sep;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block K. P., Aftring R. P., Mehard W. B., Buse M. G. Modulation of rat skeletal muscle branched-chain alpha-keto acid dehydrogenase in vivo. Effects of dietary protein and meal consumption. J Clin Invest. 1987 May;79(5):1349–1358. doi: 10.1172/JCI112961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley G. A., Tullson P. C., Terjung R. L. Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem. 1987 Jul 5;262(19):9109–9114. [PubMed] [Google Scholar]
- Fielding R. A., Evans W. J., Hughes V. A., Moldawer L. L., Bistrian B. R. The effects of high intensity exercise on muscle and plasma levels of alpha-ketoisocaproic acid. Eur J Appl Physiol Occup Physiol. 1986;55(5):482–485. doi: 10.1007/BF00421641. [DOI] [PubMed] [Google Scholar]
- Fujii H., Shimomura Y., Tokuyama K., Suzuki M. Modulation of branched-chain 2-oxo acid dehydrogenase complex activity in rat skeletal muscle by endurance training. Biochim Biophys Acta. 1994 Mar 2;1199(2):130–136. doi: 10.1016/0304-4165(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Chang T. W. Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc. 1978 Jul;37(9):2301–2307. [PubMed] [Google Scholar]
- Harris R. A., Paxton R., Powell S. M., Goodwin G. W., Kuntz M. J., Han A. C. Regulation of branched-chain alpha-ketoacid dehydrogenase complex by covalent modification. Adv Enzyme Regul. 1986;25:219–237. doi: 10.1016/0065-2571(86)90016-6. [DOI] [PubMed] [Google Scholar]
- Kasperek G. J. Regulation of branched-chain 2-oxo acid dehydrogenase activity during exercise. Am J Physiol. 1989 Jan;256(1 Pt 1):E186–E190. doi: 10.1152/ajpendo.1989.256.1.E186. [DOI] [PubMed] [Google Scholar]
- Kasperek G. J., Snider R. D. Effect of exercise intensity and starvation on activation of branched-chain keto acid dehydrogenase by exercise. Am J Physiol. 1987 Jan;252(1 Pt 1):E33–E37. doi: 10.1152/ajpendo.1987.252.1.E33. [DOI] [PubMed] [Google Scholar]
- Lau K. S., Fatania H. R., Randle P. J. Regulation of the branched chain 2-oxoacid dehydrogenase kinase reaction. FEBS Lett. 1982 Jul 19;144(1):57–62. doi: 10.1016/0014-5793(82)80568-1. [DOI] [PubMed] [Google Scholar]
- MacLean D. A., Graham T. E., Saltin B. Stimulation of muscle ammonia production during exercise following branched-chain amino acid supplementation in humans. J Physiol. 1996 Jun 15;493(Pt 3):909–922. doi: 10.1113/jphysiol.1996.sp021433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y., Fujii H., Suzuki M., Fujitsuka N., Naoi M., Sugiyama S., Harris R. A. Branched-chain 2-oxo acid dehydrogenase complex activation by tetanic contractions in rat skeletal muscle. Biochim Biophys Acta. 1993 Jul 11;1157(3):290–296. doi: 10.1016/0304-4165(93)90112-l. [DOI] [PubMed] [Google Scholar]
- Shimomura Y., Suzuki T., Saitoh S., Tasaki Y., Harris R. A., Suzuki M. Activation of branched-chain alpha-keto acid dehydrogenase complex by exercise: effect of high-fat diet intake. J Appl Physiol (1985) 1990 Jan;68(1):161–165. doi: 10.1152/jappl.1990.68.1.161. [DOI] [PubMed] [Google Scholar]
- Shinnick F. L., Harper A. E. Branched-chain amino acid oxidation by isolated rat tissue preparations. Biochim Biophys Acta. 1976 Jul 21;437(2):477–486. doi: 10.1016/0304-4165(76)90016-7. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Beckers E. J., Brouns F., Kuipers H., Soeters P. B., van der Vusse G. J., Saris W. H. Carbohydrate supplementation, glycogen depletion, and amino acid metabolism during exercise. Am J Physiol. 1991 Jun;260(6 Pt 1):E883–E890. doi: 10.1152/ajpendo.1991.260.6.E883. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Brookes J. H., Coakley J. H., Reilly T., Edwards R. H. Exercise-induced activation of the branched-chain 2-oxo acid dehydrogenase in human muscle. Eur J Appl Physiol Occup Physiol. 1989;59(3):159–167. doi: 10.1007/BF02386181. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Schepens J. T., Veerkamp J. H. Effect of starvation and exercise on actual and total activity of the branched-chain 2-oxo acid dehydrogenase complex in rat tissues. Biochem J. 1984 Nov 1;223(3):815–821. doi: 10.1042/bj2230815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk H. M., Rooyakkers D. R., Deutz N. E. Rapid routine determination of amino acids in plasma by high-performance liquid chromatography with a 2-3 microns Spherisorb ODS II column. J Chromatogr. 1993 Oct 22;620(1):143–148. doi: 10.1016/0378-4347(93)80062-9. [DOI] [PubMed] [Google Scholar]
- van Hall G., van der Vusse G. J., Söderlund K., Wagenmakers A. J. Deamination of amino acids as a source for ammonia production in human skeletal muscle during prolonged exercise. J Physiol. 1995 Nov 15;489(Pt 1):251–261. doi: 10.1113/jphysiol.1995.sp021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vusse G. J., Janssen G. M., Coumans W. A., Kuipers H., Does R. J., ten Hoor F. Effect of training and 15-, 25-, and 42-km contests on the skeletal muscle content of adenine and guanine nucleotides, creatine phosphate, and glycogen. Int J Sports Med. 1989 Oct;10 (Suppl 3):S146–S152. doi: 10.1055/s-2007-1024963. [DOI] [PubMed] [Google Scholar]


