Abstract
Cobalamin, generally known as vitamin B12, is a crucial component required for humans in several physiological processes. It has been produced from sources that are derived from animals, making it difficult for vegetarians and vegans to consume the recommended amount each day. The importance of vitamin B12 in red blood cell production, DNA synthesis, and brain processes has been highlighted. Recent studies have looked at different methods of producing vitamin B12, such as microbial fermentation. Propionibacterium shermanii and Pseudomonas denitrificans have demonstrated remarkable potential as fermented sources of vitamin B12. Compared to conventional sources, the bioavailability of vitamin B12 produced by P. denitrificans and P. shermanii is more effective in meeting dietary needs. Vitamin B12 can be produced naturally by P. denitrificans. It is equipped with the enzymes and metabolic pathways required to produce this vital vitamin. The fermentation of several dietary substrates by P. shermanii can improve nutrient bioavailability. P. shermanii generates enzymes during fermentation that aid in the breakdown of complex nutrients, facilitating easier absorption and utilization by the body. The motive of the following critical evaluation is to assess the advantages of vitamin B12 for health and the capacity of P. denitrificans and P. shermanii to produce it through fermentation.
Keywords: cobalamin, fermentation, microbial production, vitamin B12
Vitamin B12 is essential to human physiological processes, it might be challenging for vegans and vegetarians to get enough of it in their diets. Propionibacterium shermanii and Pseudomonas denitrificans have been demonstrated as promising fermented sources of vitamin B12. Vitamin B12 is naturally produced by P. denitrificans, but P. shermanii uses enzymes to increase the bioavailability of nutrients.
1. INTRODUCTION
Cobalamin, or vitamin B12, is an essential water‐soluble molecule in the metabolism of numerous organisms (Kokande et al., 2024). Vitamin B12 is a critical micronutrient that is involved in numerous physiological processes, such as the synthesis of DNA, the formation of red blood cells, and the maintenance of neurological health. B12 possesses an intricate composition and a sophisticated biosynthesis process, comprising more than 30 biotransformation stages. The biosynthetic pathway in question is restricted to specific microbes and archaea. Even though the taxa capable of vitamin B12 synthesis are not necessarily interrelated, mammals and thus humans lack the ability to produce it (Calvillo et al., 2022). Vitamin B12 has conventionally been obtained from animal products; however, a viable substitute has arisen in the form of fermentative production, which utilizes microorganisms such as Propionibacterium shermanii, Rhodobacter capsulatus, Sinorhizobium meliloti, Salmonella typhimurium, Bacillus megaterium, Escherichia coli, Pseudomonas denitrificans, and many more (Fang et al., 2017). Exploring the microbial strains responsible for the fermentative production of vitamin B12 presents an opportunity to develop manufacturing processes that are both sustainable and efficient. Sharma et al. (2024) suggested that Propionibacterium shermanii and Pseudomonas denitrificans are examples of microbes that possess the capability of producing vitamin B12 via regulated fermentation procedures. Table 1 represents the list of certain bacterial strains, their pathways, and their mechanism of action involved in the production of vitamin B12. This approach provides an environmentally sustainable, scalable, and cost‐effective substitute for conventional methods of extracting substances from animal products. It has the potential to mitigate vitamin B12 deficiencies in various dietary patterns. Microbial fermentation is a food production method that not only guarantees the bioavailability of vitamin B12 but also adheres to principles of ethics and sustainability (Gharibzahedi et al., 2023).
TABLE 1.
Categories of different bacterial strains following various pathways, intermediate compounds, and the action mechanism.
| Bacterial strain | Pathway followed | Intermediate compound | Mechanism of action | References |
|---|---|---|---|---|
| Rhodospirillum rubrum | Anaerobic pathway | Cobinamide | Anaerobic fermentation of succinate and cobalt ions | Goraya and Kaur (2023) |
| Methanosarcina barkeri | Anaerobic pathway | Cobalamin | Anaerobic fermentation of methanol | Wang et al. (2023) |
| Rhodobacter sphaeroides | Anaerobic pathway | Cobalt‐precorrin | Anaerobic fermentation of propionic acid and cobalt ions | Piwowarek et al. (2018) |
| Lactobacillaceae reuteri | Anaerobic pathway | Cobalt‐precorrin | Anaerobic fermentation of glycerol and cobalt ions | Kristjansdottir et al. (2019) |
| Bacillus megaterium | Anaerobic pathway | Cobalt‐precorrin | Anaerobic fermentation of glucose and cobalt ions | Xie et al. (2021) |
| Pseudomonas denitrificans | De novo pathway | Cobinamide | Aerobic fermentation of 5‐aminolevulinic acid (ALA) | Balabanova et al. (2021) |
| Streptomyces griseus | Anaerobic pathway | Cobinamide | Anaerobic fermentation of propionic acid and cobalt ions | Barbuto Ferraiuolo et al. (2021) |
| Salmonella enterica | De novo pathway | Cobalt‐precorrin | Aerobic fermentation of cobalt ions and glucose | Balabanova et al. (2021) |
| Escherichia coli | Salvage pathway | Cobinamide | Heterologous expression of vitamin B12 synthesis genes | Fang et al. (2018) |
| Propionibacterium freudenreichii | De novo pathway | Cobinamide | Anaerobic fermentation of propionic acid | Kumar et al. (2023) |
| Salmonella typhimurium | De novo pathway | Cobalt‐precorrin | Aerobic fermentation of cobalt ions and glucose | Zheng et al. (2020) |
| Streptomyces avermitilis | Anaerobic pathway | Cobinamide | Anaerobic fermentation of propionic acid and cobalt ions | Ranaei et al. (2020) |
The capacity of the Propionibacterium shermanii and Pseudomonas denitrificans to produce vitamin B12 via a fermentative process has been acknowledged. The bacterium exhibits distinctive metabolic pathways that facilitate its efficient synthesis of cobalamin (Balabanova et al., 2021). Propionibacterium shermanii has the capability to produce vitamin B12 through cultivation on appropriate substrates, including propionic acid and other organic compounds, within a controlled fermentation environment. The utilization of fermentation as a method of vitamin B12 production presents numerous benefits in comparison to conventional extraction techniques, including enhanced scalability, cost‐efficiency, and diminished environmental footprint. Considerable research has been devoted to examining the capacity of this bacterium to produce cobalamin under suitable growth conditions and precursor conditions (Piwowarek et al., 2018). The incorporation of Pseudomonas denitrificans into the fermentative synthesis of vitamin B12 enhances the process's adaptability by permitting the use of distinct microbial strains to fulfill production criteria. The potential of these microorganisms to produce vitamin B12 through fermentation is substantial, as it could be utilized to treat nutritional deficiencies and improve human health (Calvillo et al., 2022). An insufficiency of vitamin B12 can result in neurological disorders, as it is vital for the normal operation of the nervous system. Fermentation of vitamin B12 could provide a sustainable and dependable source of the vitamin, which could substantially aid in the fight against deficiency‐related health problems, particularly in populations that have limited access to animal‐derived foods. Individuals adhering to vegetarian or vegan diets can benefit from the fermentative method as it provides a cruelty‐free substitute for conventional animal‐derived vitamin B12 sources (Serin & Arslan, 2019). The microbial synthesis of vitamin B12 is consistent with the increasing inclination toward sustainable and ethical food production methods. The convergence of scientific advancements and sustainable practices is exemplified by the fermentative synthesis of vitamin B12 by Propionibacterium shermanii and Pseudomonas denitrificans, microorganisms that are integral to the era of precision nutrition (Fang et al., 2017). This novel methodology not only caters to the dietary requirements of individuals with varied preferences but also advances the overarching objective of establishing a food system that is more sustainable and resilient to environmental issues. The prime objective of this study is to clarify the metabolic pathway's effective synthesis of vitamin B12 via controlled fermentation. This study aims to investigate the potential health advantages that may be linked to vitamin B12 obtained through this fermentative method, with a particular focus on its capacity to rectify nutritional insufficiencies and enhance overall human health. The aim of this review is to provide significant contributions to the understanding of the nutritional effectiveness, sustainability, and feasibility of producing vitamin B12 using Propionibacterium shermanii and Pseudomonas denitrificans via microbes.
2. ROLE OF VITAMIN B12 IN REGULATING HEALTH
Meat, poultry, fish, eggs, and dairy foods contain cobalamin, a form of vitamin B12, organically. It could be incorporated into supplements or meals. Hematopoiesis and the formation of DNA require vitamin B12. It plays a significant function in the growth and operation of brain and nerve cells. The production of nucleotides and the metabolism of amino/fatty acids are biological activities that depend on vitamin B12 (Zhao et al., 2023). Lack of vitamin B12 negatively affects the production of phospholipids and the myelin layer in the neurological system by reducing methionine biosynthesis. Rodent peripheral nerve injury that has been demonstrated to be improved by vitamin B12 administration is assumed to be related to the inhibition of superoxide generation in neural tissue (Akbari et al., 2023). When vitamin B12 is used in conjunction with COX‐1 and COX‐2 inhibiting agents, the previously reported neuroprotective effect has been proven to be more powerful (Martínez‐Iglesias et al., 2023; Rauf et al., 2022). Conversely, prior research with individual participants confirms that vitamin B12 intake has a positive impact on intellectual performance (Demirtas & Erdal, 2023; Prajjwal et al., 2023). Vitamin B12 plays a critical role in overall development. Figure 1 is reflecting the significant role of B12 in the human body in various aspects.
FIGURE 1.
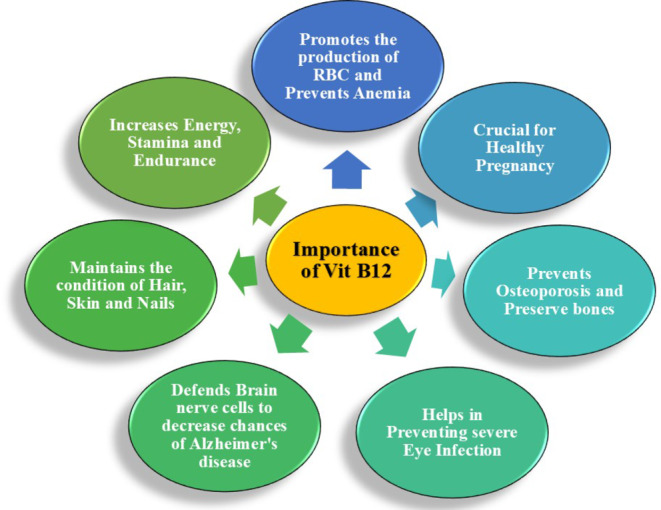
Pharmacological role of vitamin B12 in human body.
Consuming functional foods that are enriched in vitamin B12 is a good approach to reduce human vitamin B12 insufficiency, especially among vegans. Since only bacteria and archaea can produce vitamin B12, its industrial manufacture is predominantly accomplished by microbial fermentation. Recent developments in metabolic engineering have made it possible to create microorganisms that are effective microbial cell factories to produce vitamin B12 by engineering them with powerful expression vectors, gene amplification, and promoters. Improvements to the fermentation process, such as substrate optimization, predecessor subjunction, precise oxygenation, and identification of growth‐limiting variables, have made it possible to implement tactics that will increase production (Kumar et al., 2023). Vitamin B12 contributes to the degradation of the protein homocysteine. Elevated levels of homocysteine have been linked to an increased risk of heart disease and stroke owing to its ability to promote the formation of blood clots, excess free radical cells, and to obstruct proper blood vessel function. If inadequate doses of vitamin B12 are consumed, homocysteine levels may increase. Even epidemiological studies have shown that taking vitamin B12 supplements can reduce homocysteine levels, but there is no conclusive evidence that this reduces the risk of cardiovascular events. Consequently, the American Heart Association opposes the frequent consumption of B complex supplements to reduce the risk of cardiovascular disease. Certain people with genetic variations that cause excessive homocysteine levels may benefit from vitamin B12 supplementation. Methionine synthase is a vitamin B‐dependent enzyme which uses the folate derivative S‐methyltetrahydrofolate (S‐methyl TH) as a methyl donor to catalyze the synthesis of methionine from homocysteine. Betaine is used as a methyl donor in a different route (not illustrated here) that is catalyzed by betaine homocysteine methyltransferase to remethylate homocysteine to methionine. Most biological methylation processes, particularly DNA methylation, call for the amino acid methionine in the form of S‐adenosylmethionine (Figure 2) (Tan et al., 2023).
FIGURE 2.
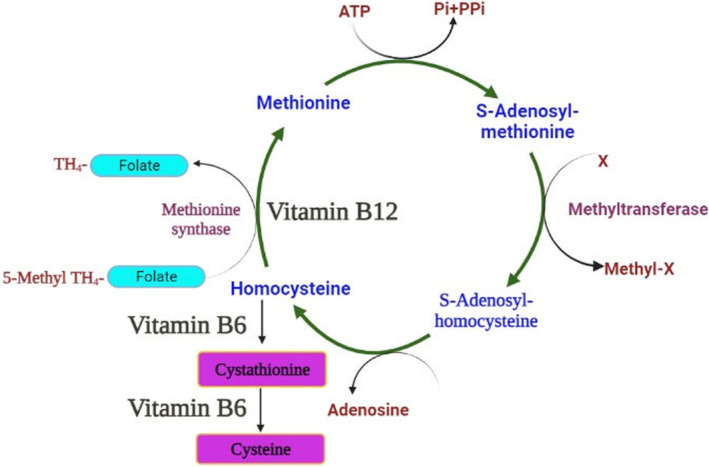
Metabolism of homocysteine with the help of vitamin B12.
Frequent investigations discovered a link between thyroid diseases (TD) and vitamin deficiencies. Antiparietal cell antibodies are a sign of a decreased capacity to absorb vitamin B12. Research published by Benites‐Zapata et al. (2023) investigated differences in vitamin B12 blood levels between sick people with and without tardive dyskinesia (TD), the incidence of vitamin B12 deficiency in TD patients, and the presence of antiparietal cell antibodies in individuals with TD. Vitamin B12 concentrations were decreased in hypothyroid patients than in normal individuals. No appreciable correlations among vitamin B12 levels and hyperthyroidism, autoimmune thyroid disease, or subclinical hypothyroidism were witnessed. B complex has a fundamental role in maintaining the regular operation of the nervous system. Due to their importance for brain function, deficits in the B vitamins B6, B1, B9, and B12 have been associated with depression (Wu et al., 2022). They are preventative toward hypercysteinemia, which is linked to a higher incidence of mental disorders (Cordaro et al., 2021). Additionally, a worse response to antidepressants has been linked to low levels of vitamins B9 and B12 (Oudman, 2020). Contributing to the DNA amalgamation of oligodendrocytes that produce myelin as well as synthesizing of myelin itself are two distinct functions of vitamin B12 (Berkins et al., 2021). An enzyme called methionine synthase uses cobalamin as a cofactor to catalyze the addition of a methyl group to the homocysteine molecule. S‐adenosylmethionine (SAM), a precursor to methionine, has been produced. SAM is vital for the methylation processes necessary for the correct synthesis and/or metabolism of DNA, RNA, neurotransmitters, and membrane phospholipids as well as for the proper function of the myelin sheaths of nerve fibers (Avalos et al., 2023). Few researchers proposed ways through which omega‐3 fatty acids and vitamin B12 impact the way by which brain functions. B12 and omega‐3 fatty acid consumption and metabolism have diverse effects on brain function. Indirectly through heightened oxidative stress and decreased brain omega‐3 fatty acid levels, elevated homocysteine levels and altered epigenetic modification affect brain neurotrophins and neuro‐vascular operation that could increase the likelihood of neurological diseases and dementia (Rathod et al., 2016). B12 is found in meals and supplements that have been fortified, perhaps making them simpler to absorb. There are several vitamin B12 tablets available. Despite claims that various forms, such as sublingual medications, or fluids injected beneath the tongue to be absorbed via the tissues of the mouth, had better absorption than regular tablets, research has not demonstrated a significant difference (Forgie et al., 2023). The recommended dietary limitations for vitamin B12 are far exceeded by the quantities seen in supplements. However, because sufficient intrinsic components are also required for absorption, the absorption of even higher levels is not guaranteed. Doctors may advise muscle B12 injections in situations involving significant vitamin B12 lacking caused by an insufficient intrinsic factor (pernicious anemia) (Park et al., 2023). Various diseases caused by the deficiency of vitamin B12 at different phases of life are represented in Table 2.
TABLE 2.
Various diseases caused by the deficiency of vitamin B12 at different phases of life.
| Different phases of life | Age | Amount of vitamin B12 required (mcg/day) | Rich natural source | Disease caused by deficiency | References |
|---|---|---|---|---|---|
| Newborn | 0–6 months | 0.4 | Breast Milk (Mother should contain vitamin B12‐rich diet) | Breakdown to thrive anemia, hypotonia, developmental delay, microcephaly, and cerebral atrophy, lethargy, failure to thrive, vomiting, hypotonia, and arrest or regression of developmental skills | Dubaj et al. (2020), El Hasbaoui et al. (2021), Demirtas and Erdal (2023), Goraya and Kaur (2023) |
| Infant | 7–12 months | 0.5 | |||
| Toddlers | 1–2 years | 0.9 | Almond milk | Anemia was found in 83%, developmental delay or regression, cerebral atrophy, neuroimaging, Hyperglycinuria | Agnoli et al. (2017), Tangeraas et al. (2023), Goraya and Kaur (2023), Kostecka et al. (2023) |
| Preschoolers | 3–5 years | Unsweetened invigorated soya coconut or almond milk, oat | |||
| Middle Childhood | 6–8 years | 1.2 | Fortified breakfast cereal, Plain fortified soya yogurt | Neurological disorders, megaloblastic anemia and failure to thrive, elevated oxidative stress, hematological abnormalities | Charneca et al. (2023) |
| Children | Ears | 1.8 | Yogurt, plant‐based milk, low‐fat milk, fortified, cheese | Rowicka et al. (2023) | |
| Young Teens | 12–14 years | 2.4 | Shiitake Mushrooms, yeast, Tempeh, Nutritional, Yeast Spreads, Beetroot | Anemia in later life, nervous system damage, weight loss, fragile hair, knuckle pigmentation, hematologic (megaloblastic, macrocytic anemia), gastrointestinal (anorexia, glossitis), neurologic (demyelinization, paresthesia) | Umasanker et al. (2020), Tufan et al. (2012) |
| Adolescents | 14–18 years | Swiss cheese Vitamin water, Butter squash, Whey Powder, Potato, Mushrooms, Noori, Tofu, Chickpea, Soybeans, low‐fat milk, low‐fat yogurt | Developmental delay, irritability, weakness, and failure to thrive | Henjum et al. (2023) | |
| Adults | 18+ | Irritability, negativism, disorientation, confusion, agitation, amnesia, impaired concentration, insomnia, depression, psychosis, bipolar disorder, panic disorder, phobias and dementia | |||
| Pregnancy | All ages | 2.6 | vitamin B12‐fortified breakfast cereals and bland soy drinks are also available. healthy yeast flakes with added vitamin B12 and yeast extracts, such as Marga | Neural tube defects in the baby, Infertility, Stomach cancer | Khosravi et al. (2023), Rooney et al. (2023), Bali and Naik (2023), Goraya and Kaur (2023) |
| Lactation period | All ages | 2.8 | Fortified Soy + Almond Milk, Marmite + Yeast Spreads, Nutritional Yeast, Fortified Cereals, Plant‐Based Meats, Cremini Mushrooms, Chlorella, Tempeh, Nori Seaweed, Seitan (wheat gluten) | Low birth weight, cognitive functions, gestational diabetes, Anemia | Behere et al. (2021), Goraya and Kaur (2023), Bali and Naik (2023) |
3. PRODUCTION OF VITAMIN B12 FROM MICROBIOTA
Due to rising public awareness of health issues and the rising acceptance of nontraditional diets including vegan and vegetarian diets, the requirement of vitamin B12 among the food, nutraceutical, and beverage sectors has dramatically grown in the past few decades. Due to this, several attempts have been made over the years to optimize strain and procedures for synthesizing cyanocobalamin. Many researchers have been exploring the biosynthetic routes of organisms that produce cyanocobalamin because of the structural and organic discoveries of many vitamin B12 molecules in the 1970s. The complex structure of the molecule was subsequently determined to be the result of the “de novo” production of the molecule, which included more than 30 genes and multiple enzyme activities. Since no genetic proof exists that any eukaryotic creature can produce any isoform of cyanocobalamin, this route is limited to some bacteria and archaea. Industrial technologies and bacterial fermentation procedures can be used to produce vitamin B12 on a large scale. Today, the economically significant vitamin B12 is synthesized via P. shermanii and P. denitrificans, two modified strains of bacteria, which are used in the fermentation process. Up to 300 mg/L of vitamin B12 can be produced with this technique. This intricacy is a result of its complicated chemical production, which can take up to 70 steps (Acevedo‐Rocha et al., 2019). These microorganisms' ability to produce cobalamin beneath identifiable culture conditions (supplementation of precursors and metal ions, carbon/nitrogen sources, cultivation time, oxygenic/anoxygenic conditions, etc.) has been thoroughly studied. Their ability to synthesize cobalamin has been improved through spontaneous mutations (chemicals and ultraviolet light), genetic tampering (overexpression, alteration, parameter), and hereditary overexpression, transformation, and regulation (Bryant et al., 2020). The identified naturally occurring or recombinant bacteria that produce cobalamin include the genera Acetobacterium, Methanobacillus, Aerobacter, Alcaligenes, Agrobacterium, Flavobacterium, Arthrobacter, Bacillus, Azotobacter, Escherichia, Corynebacterium, Propionibacterium, Eubacterium, Mycobacterium, Proteus, Methanosarcina, and Clostridium (Rodionov et al., 2019; Shelton et al., 2019). In addition to mutation and overexpression of the biosynthetic genes and/or riboswitch sequences in the selected strains, the catalytic initiator of 5‐aminolevulinic acid (ALA), DMB, and cobalt ions were all introduced to enhance cobalamin production (Nguyen‐Vo et al., 2018).
The family of cobalamin, which also includes the vitamin B12 component cyanocobalamin, is made up of a corrinoid ring, an upper and a lower ligand. The top ligand may be adenosine, methyl, hydroxy, or a cyano group. Vitamin B12, which is produced by prokaryotes, shields mammals from pernicious anemia (Fang et al., 2017). Cobalamin can be synthesized by microorganisms through salvage or de novo (aerobic/anaerobic) pathways, which may each require as many as 30 enzymatic steps (Bryant et al., 2020; Lu et al., 2020). The de novo pathways consist of three major stages: (1) uroporphyrinogen III (Uro III) formation, the initial macrocyclic intermediate in tetrapyrrole synthesis; (2) cobinamide (Cbi) formation from Uro III, which involves the adenylation and formation of the corrin ring; and (3) nucleotide loop assembly, which entails the synthesis of a lower axial ligand, typically 5,6‐dimethylbenzimidazole (DMB), followed by its attachment to the corrin ring (Balabanova et al., 2021). The designations for the genes/enzymes involved in the oxygen‐dependent and oxygen‐independent pathways are, respectively, cob/Cob and cbi/Cbi. Key distinctions between these two pathways include the need for molecular oxygen to aid in ring contraction and cobalt insertion: in the aerobic pathway, this occurs only after the synthesis of hydrogenobyrinic acid a, c‐diamide (late stage), whereas in the anaerobic pathway (as shown in Figure 3), it occurs at the precorrin‐2 stage (early insertion). Additionally, in the anaerobic pathway, cobalt insertion occurs after the biosynthesis of a lower ligand, typically DMB (Danchin & Braham, 2017).
FIGURE 3.
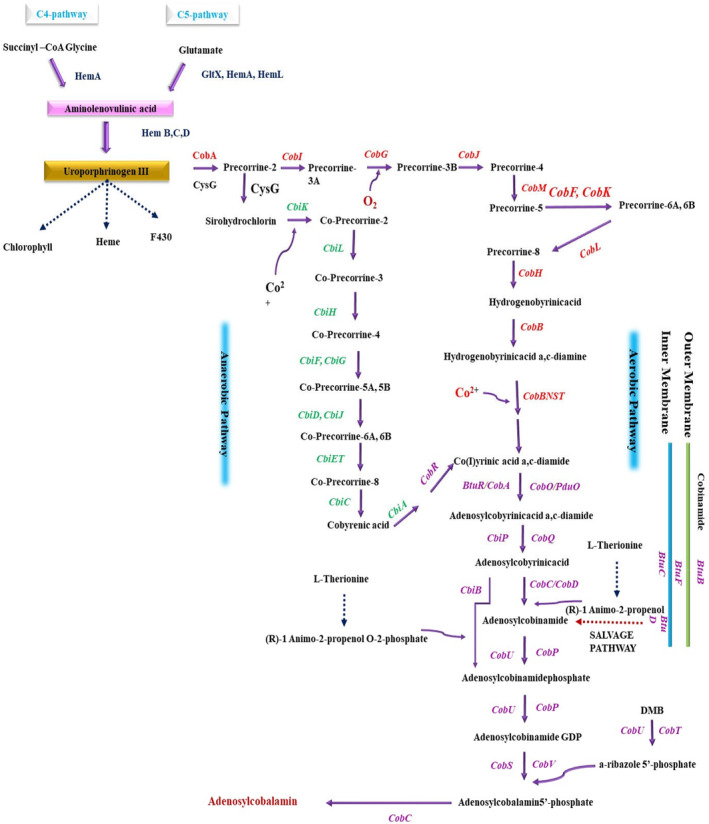
Diagram illustrating the salvage, anaerobic, and aerobic cobalamin biosynthetic pathways: The diagram commences with the aerobic pathway, which is illustrated for P. denitrificans or Sinorhizobium meliloti and the anaerobic pathway for the extensively researched bacteria S. typhimurium and P. shermanii.
From aminolevulinic (ALA), tetrapyrrole compounds such as cobalamin, heme, and bacteriochlorophyll are created. Several bacterial species have complicated reliant and collaborative relationships with these tetrapyrrole compounds (Fang et al., 2017). A cobalamin riboswitch in the 5′ untranslated regions (UTR) of the relevant gene's controls vitamin B12 production and transportation to keep concentrations steady. Through microbial fermentation, vitamin B12 is produced on a large scale in the industrial setting, primarily using Sinorhizobium meliloti. Propionibacterium shermanii, or Pseudomonas denitrificans, these strains do have certain drawbacks, though, including lengthy fermentation cycles, demanding costly medium, and a dearth of acceptable inherent systems for strain building (Li et al., 2020). There have been few studies on metabolic engineering, and most studies carried out by these have concentrated on conventional techniques such as random mutagenesis and improving fermentation processes (Ma et al., 2017). Escherichia coli has recently attracted the curiosity of researchers as a model for the creation of vitamin B12. E. coli has developed into a well‐researched cell industry that is frequently utilized to produce a variety of compounds, including terpenoids, artificial alcohols, and poly‐(lactate‐co‐glycolate) (Fang et al., 2018). To enhance the synthesis of these chemicals, metabolic engineering and techniques from synthetic biology have also been widely used. In addition to producing ALA through the C4 and C5 routes, Escherichia coli is being employed as a microbial cell factory.
Escherichia coli can also produce vitamin B12 by salvage pathway. Salmonella typhimurium, a very similar strain, has the capacity to produce vitamin B12 from scratch. Numerous genes responsible for producing vitamin B12 in S. typhimurium have been demonstrated to activate in E. coli (Choi et al., 2016). The manufacture of vitamin B12 in E. coli was made possible by the introduction of 20 genes of the S. Typhimurium cob locus. These benefits make it easier for E. coli to produce vitamin B12 from scratch. Multiple research projects on vitamin B12 production have been carried out by multiple teams due to the intricacy of the route and its control of metabolism (Fang et al., 2017). List of enzymes involved in the production of vitamin B12 and their action mechanism have been shown in Table 3.
TABLE 3.
List of enzymes involved in the production of vitamin B12 and their action mechanism.
| Enzymes involved in the production of vitamin B12 | Cofactor | Mechanism of action | References |
|---|---|---|---|
| Cobalt chelatase | ATP, Mg2+, S‐adenosylmethionine (SAM) | Catalyzes the insertion of cobalt into the corrin ring structure during vitamin B12 biosynthesis | Osman et al. (2021) |
| Cobalt‐precorrin‐6A reductase | NADPH | Reduces cobalt‐precorrin‐6A to cobalt‐precorrin‐6B, contributing to the formation of the corrin ring | Moore et al. (2013) |
| Cobalt‐precorrin‐5B C (1)‐methyltransferase | S‐adenosylmethionine (SAM) | Catalyzes the methylation of cobalt‐precorrin‐5B to cobalt‐precorrin‐6B, adding a methyl group to the corrin ring structure | Moore et al. (2013) |
| Cobalt‐precorrin‐4 methyltransferase | S‐adenosylmethionine (SAM) | Catalyzes the methylation of cobalt‐precorrin‐4 to cobalt‐precorrin‐5, another important step in vitamin B12 synthesis | Abdelraheem et al. (2022) |
| Adenosylcobalamin synthase | ATP, cob(I)alamin, S‐adenosylmethionine (SAM) | Converts cobinamide to adenosylcobalamin, the biologically active form of vitamin B12, through a series of reactions involving ATP and SAM | Balabanova et al. (2021) |
4. FERMENTATION BY PSEUDOMONAS DENITRIFICANS FOR SYNTHESIS OF CYANOCOBALAMIN
4.1. Characteristics of Pseudomonas denitrificans
Pseudomonas denitrificans are distinguished by their rod‐shaped morphology, polar flagella, Gram‐negative cell wall composition, and aerobic metabolism. This bacterium has the potential to grow heterotrophically and can make vitamin B12 (Calvillo et al., 2022). One of the few bacteria which can synthesize vitamin B12 in an aerobic environment is P. denitrificans. P. denitrificans, as its name implies, is also able to undergo denitrification, a step in the nitrogen cycle when nitrate is converted into nitrogen gas (N2) (Balabanova et al., 2021). Considering the vast amount of information that is already discussed about P. denitrificans, still there is a great deal that is unexplained. Its cytochrome cc’ protein, which is present in the mitochondria and is crucial for the electron transport chain, has not yet undergone extensive research to determine how it relates to proteins that exist in photosynthetic bacteria conditions (Xia, Chen, et al., 2015; Xia, Peng, et al., 2015). Since the route is believed to have evolved to support fermentation activities, although this has not yet been convincingly established, the evolutionary suggestions of preserved genes encoding for vitamin B12 synthesis may give an understanding of the origins of metabolism. Concerning its commercial utility to produce vitamin B12, possible nitrate toxicity, or wastewater treatment applications, P. denitrificans' medicinal and ecological relevance is likewise unknown (Zhou et al., 2014). Since P. denitrificans is thought to be chronologically old, it offers a chance to investigate the evolution of metabolism. Its addition of denitrification to oxidative phosphorylation sheds light on the way it occupies and retains a niche despite changing N and O levels in the surroundings. P. denitrificans could be regulated to create other industrial substances, such as 3‐hydroxypropionic acid. Its capacity for denitrification has significant potential for handling wastewater. P. denitrificans has the potential to opportunistically cause meningitis in people. Fish intestines can additionally be infected by P. denitrificans (Xia, Chen, et al., 2015; Xia, Peng, et al., 2015).
The single circular chromosome of P. denitrificans ATCC 13867 has a genomic size of 5,696,307 bps and a Guanine + Cytosine concentration of 65.2%. Its genome has 2567 operons and 5059 protein‐coding genes, and 59.56% of its proteins are cytoplasmic while 19.41% are not. The remaining percentage is yet undetermined (Ainala et al., 2013). Its genome has 63 transfer RNAs and genes for each of the 20 amino acids. In addition, it has 816 transcription terminators and 1279 ribosome‐binding sites (Kumpangcum et al., 2023). The genome of P. denitrificans additionally contains genes that code for 26 enzymes essential to produce vitamin B12, and these genes are organized into two distinct chromosomal clusters. The first and second clusters contain genes associated with the fabrication of vitamin B12 (Tran et al., 2017). China, which includes the North China Pharmaceutical Company, the Henan Luyuan Pharmaceutical Company, the Hebei Yuxing Bio‐Engineering Company, and the Chinese CSPC Huarong Pharmacy Company, is the world's largest manufacturer of vitamin B12. These four companies are stated to have produced 31.41 tons of vitamin B12 in 2020, with a reported marketplace value of USD 339.8 million. Due to various studies from Huarong Pharmaceutical Company, it is believed that the strains utilized in their commercial productions are aerobic strains, and the most recent trademarks on bioprocess optimization with P. denitrificans exhibited in China claim volumetric manufacturing of up to 281 mg/L (Sun et al., 2023).
Improvements in culture medium optimization and bioprocessing parameters have also been made to increase vitamin B12 productivity in P. denitrificans in addition to genetic alterations. Tests have been conducted, for instance, on the impact of residue elements in media, dissolved oxygen management, pH, and the accumulation of various supplements. In this regard, it has been discovered that the synthesis of ALA and PBG, two of the main precursors of cobalamin, is greatly improved by the presence of Zn2+, whilst the addition of Co2+ and DMBI (dimethylbenzimidazole), the base inserted into the nucleotide loop, favorably impacts synthesis (Wang et al., 2021). Cobalamin synthesis increased by 13% because of the planning of experiments' optimization of the beginning concentrations of these three chemicals. The pH stability of cultures was impacted by the media composition, which also had a big impact on vitamin synthesis. A diet that uses glucose as a carbon source and betaine as a methyl donor was discovered to be beneficial for vitamin production while being employed in 120 m3 bioreactor setups (Wang et al., 2014). Furthermore, even while it is well established that betaine aids in the creation of various significant mediates, including glutamate, ALA, methionine, and glycine, it may also limit cell development at high concentrations. This is because betaine works as a methyl donor for the manufacture of vitamin B12 (Lyon et al., 2020; Pesqueda‐Cendejas et al., 2023). In P. denitrificans, oxygen transfer rate (OTR) optimization has received significant attention. Higher OTRs at the beginning of the culture process promote cell proliferation, but decreased OTRs at subsequent phases are essential for greater productivity (García‐Cabrera et al., 2020). Later research found that the enhanced production seen under poor oxygenation circumstances may be due to changes in cell shape that promote the transition from the cell growing phase to an extension stage which exhibits greater vitamin B12 synthesis (Lyon et al., 2020). Various multistep liquefied oxygen management techniques were created in response to this, where oxygenation and stirring were progressively decreased until thawed oxygen values plunged below 2%, resulting in a rise in output of about 17% (kazadi Mbamba et al., 2019). Despite having a negative effect on the development of cells, the adjunct of respiratory chain inhibitors, like as rotenone, might improve vitamin synthesis (Cheng et al., 2014). Finally, other sources of nitrogen and carbon are being investigated as cheaper replacements for more pricey improved glucose and sucrose including maltose syrup, glucose, steep liquor, beetroot molasses, and maize. A few of these substances may have a detrimental impact on pH stability and thus, the result of vitamin synthesis is also affected (Hernández‐Pérez et al., 2020). However, a viable and less expensive substitute for the conventional media formulations has been described using a mixture of maltose syrup, maize steep liquor, and betaine (Xia, Chen, et al., 2015; Xia, Peng, et al., 2015).
4.2. Synthesis of vitamin B12 by Pseudomonas denitrificans
It is feasible to exclude Pseudomonas denitrificans from surroundings like soil or water. As part of the separation procedure, samples are collected, diluted, and plated on the appropriate medium for development. Conventional microbiological methods, such as nutritional agar or broth enriched with the right nutrients for growth, can be used to cultivate Pseudomonas denitrificans. To encourage bacterial development, the culture is kept at the right temperature and pH. Optimizing nutritive circumstances is necessary for Pseudomonas denitrificans culture for vitamin B12 synthesis. Carbon sources (such as glucose or glycerol), nitrogen sources (such as ammonium salts or amino acids), and trace elements are examples of important nutrients. To produce vitamin B12, cobalt, and other necessary elements is very crucial. The appropriate quantities of these elements for optimum synthesis of vitamin B12 are able to be found through nutrient optimization studies. The procedure of fermentation is the phase that follows the bacteria that has been cultivated. A batch, fed batch, or continuous form of fermentation can be used (Balabanova et al., 2021). The fermentation medium is made to offer the best circumstances for bacterial development and the production of vitamin B12. To increase production, variables including temperature, pH, oxygen availability, and agitation are carefully regulated. To give the bacteria a chance to thrive and produce vitamin B12, the procedure of fermentation usually takes a few weeks. A complicated biochemical process is used by Pseudomonas denitrificans to produce vitamin B12. The route is made up of several successive enzyme processes. Precorrin‐3B synthase, precorrin‐4 methyltransferase, and cobalt chelatase are the main enzymes in this process. These digestive enzymes facilitate chemical processes that result in the production of vitamin B12. For effective vitamin B12 synthesis, the manifestation and operation of these enzymes must be controlled (Binod et al., 2010). Several methods can be used to strengthen the synthesis of vitamin B12. This entails tweaking fermentation settings, modifying Pseudomonas dentifrices strains genetically, and incorporating precursor chemicals or other precursors into the fermentation media. By altering variables such as temperature, pH, and dissolved oxygen levels, fermentation settings may be optimized to produce a setting that promotes the production of vitamin B12. The bacterium's metabolic pathways can be modified using methods involving genetic engineering to increase its capacity to manufacture vitamin B12.
For instance, enhancing the biosynthetic pathway's essential enzyme expression can boost vitamin B12 production. Additionally, the required components for vitamin B12 biosynthesis can be supplemented to the fermentation medium in the form of precursor molecules or predecessors, thereby boosting production (Fang et al., 2017). The broth holding vitamin B12 must go through downstream processing after fermentation to separate and refine the desired component (Figure 4). Cell segregation, filtering, centrifugation, and chromatography methods are frequently used in the procedure. These procedures assist in removing undesirable cellular debris and contaminants, producing a vitamin B12 product that has been refined. The vitamin B12 can then be dried and made into different forms after being cleaned. It is crucial to keep an eye on and manage the vitamin B12 product's integrity throughout the course of production. This entails measuring and determining the existence of vitamin A using methods of analysis such as mass spectrometry and high‐performance liquid chromatography (HPLC) (Żandarek et al., 2023).
FIGURE 4.
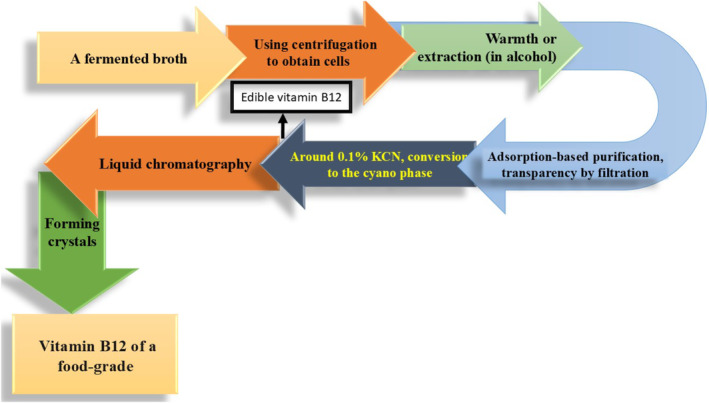
Downstream processing for the production of vitamin B12.
5. FERMENTATION BY PROPIONIBACTERIUM SHERMANII FOR SYNTHESIS OF CYANOCOBALAMIN
5.1. Characteristics of Propionibacterium shermanii
Propionibacterium spp. are Gram‐positive bacilli, which means they are catalase positive, have a length of 1–5 μm, are nonmotile, and do not produce bacterial spores. They are categorized as anaerobic or partially anaerobic bacteria. When present in anaerobic settings, PAB are very small and resemble cocci. These bacteria can exhibit pleomorphism in the presence of oxygen, taking on club‐shaped, V‐shaped, or Y‐shaped shapes. Pseudomonas denitrificans has an accelerated growth rate even in the presence of high salt concentrations, up to 6.5% NaCl, and performs best at a pH of 7.0 (with a range of 4.5–8.0). These bacteria are famous for their capacity to synthesize both propionic acid and vitamin B12. Based on its habitat, the Propionibacterium genus is divided into two separate groups: one group includes skin‐dwelling (acnes) bacteria, while the other group includes classical (dairy) bacteria. The first group includes species like Propionibacterium lymphophilum, Propionibacterium acnes, Propionibacterium avidum, Propionibacterium propionicum, and Propionibacterium granulosum (all of which are pathogenic microorganisms) that are found on human skin as well as in the gastrointestinal mucosa and oral. The second phylogenetic group includes the classical strains of bacteria. The first phylogenetic group consists of bacteria from the Propionibacterium jensenii, Propionibacterium acidipropionici, and Propionibacterium thoenii species. The next group of bacteria is made up of subspecies of Propionibacterium freudenreichii (subsp. Freudenreichii and subsp. shermanii). These subtypes differ in two characteristics: the capacity to decrease nitrates and the capacity to metabolize lactose. Bacterial strains generated from P. freudenreichii subsp. freudenreichii may reduce nitrates but cannot ferment lactose. Although P. freudenreichii subsp. shermanii strains include genes for the enzyme‐D galactosidase (EC 3.2.1.23), they are unable to reduce nitrates. As a gram‐positive bacterium, Propionibacterium shermanii preserves the crystal violet stain during the Gramme staining process. Its cell wall has a thick coating of peptidoglycan that accounts for this property (Dank et al., 2023). The bacteria are pleomorphic, which implies that it may have many sizes and forms. Depending on the growing circumstances, it can take the shape of rods, cocci, or even filamentous forms. The bacterium Propionibacterium shermanii is not mobile. It lacks flagella, which are bacterial motility‐causing extensions. Propionibacterium shermanii is predominantly anaerobic, indicating that it can grow without oxygen. It is also facultatively anaerobic. As a facultatively anaerobic organism, it can withstand and thrive in circumstances of oxygen shortages (Dank et al., 2022). Propionic acid production is one of Propionibacterium shermanii's distinguishing characteristics. A variety of carbohydrates, including lactate, glucose, and lactose, are fermented by this bacterium to create propionic acid, carbon dioxide, and trace quantities of acetic acid. Propionibacterium shermanii is essential to the dairy business. It serves as a beginning culture for the cheeses Emmental and the Swiss kind. The bacteria convert lactate into propionic acid during the cheese‐making process, giving these cheeses their distinctive flavor and texture (Blasco et al., 2011). Propionibacterium shermanii can produce vitamin B12, commonly referred to as cobalamin. The biological activities that take place in both humans and animals depend on this vitamin. The bacteria use a convoluted metabolic route to produce vitamin B12 (Calvillo et al., 2023).
Propionic acid bacteria may create vital molecules like vitamin B12, which can be utilized to create foods for vegan diets that are vitamin B12 enhanced. Trehalose and other vitamins from the B group are abundant in PAB feedstock. All traditional Propionibacterium genus bacteria are capable of fermentation. Cheese (Swiss‐style Dutch cheeses and vaccine components for Swiss cheeses), pickles, probiotics, and silage are all made with propionic acid bacteria (PAB). Preservation agents are made from PAB‐derived metabolites. Propionibacterium spp. may be found on herbaceous plants, in bovine rumens, in herbivore dung, soil, sewage, sludge, milk, pickles, water used to make oil, and fermented fruit fluids (Piwowarek et al., 2018). By encouraging the expansion of Bifidobacterium bacteria and shielding the animal from possible infections via the synthesis of bacteriocins, the species P. freudenreichii controls the intestinal microbiota. Along with an excellent source of trehalose and the vitamins B12, B9, and K, PAB also boosts the immune system and removes mycotoxins from the digestive tract. It has been demonstrated that adding PAB to the diet promotes both the usage of the substance and the physical progression of young animals (Singh & Vyas, 2022). Corresponding to Cousin et al. (2016), P. freudenreichii subsp. shermanii causes colon cancer cells to undergo apoptosis by producing propionate and acetate. In 2017, findings from studies on the use of P. freudenreichii subsp. shermanii as a beneficial component in Feta‐style cheese were published. Up to 7 days into the maturation process, Propionibacterium colony‐forming units (CFUs) developed in the finished product, and after 60 days, a propionic acid concentration of 52.1 mM was attained. In addition to adding flavor, the resulting Feta‐type cheese was distinguished by health‐indorsing qualities and prolonged the expiry day. A good source of vitamin B12, feed (Bioprofit™) is generated as well using PAB strains because they help animals assimilate iron and calcium and guard the finished product against fungus. Certain PAB strains are fed to animals as probiotics (Angelopoulou et al., 2017). By encouraging the evolution of Bifidobacterium bacteria and defending the body against the growth of harmful microorganisms through the production of bacteriocins, P. freudenreichii controls the flora of the intestines. PAB can remove mycotoxins from the gastrointestinal system. They produce trehalose, folic acid, vitamins B12, and H, as well as encourage the immune system and reduce the carcinogenic realizes of fecal enzymes. When PAB is added to the meal, its usage increases, promoting the development of young animals (Piwowarek et al., 2018). T82 strain's genome also included genes that could be related to glycogen metabolism. The P. freudenreichii ssp. shermanii CIRM‐BIA1T strain also possessed this trait (Falentin et al., 2010). The series encoding glycogen synthase (EC 2.4.1.21), glycogen phosphorylase (EC 2.4.1.1), and branching glycogen enzymes (EC 2.4.1.18) are necessary for the T82 strain to be able to synthesize this chemical. P. acnes also possessed several of these genes. These enzymes are anticipated to be responsible for intracellular glycogen buildup and/or hydrolysis because P. freudenreichii and P. acnes cannot ferment extracellular glycogen (Piwowarek et al., 2020).
5.2. Synthesis of vitamin B12 via Propionibacterium shermanii
Cobalamin is produced industrially only by fermentation procedures using microorganisms, mainly P. denitrificans, because of its intricate nature (about 70 steps) and the high expense of its chemical synthesis. Food items are increasingly being enhanced with vitamin B12 by in situ fermentation in recent years. The Wood‐Werkman route is used by P. freudenreichii varieties, which are Gram‐positive rod‐shaped bacteria that can synthesize huge amounts of propionic acid. P. freudenreichii, in comparison to aerobic generators of vitamin B12, has the benefit of having received the FDA's GRAS designation and the EFSA's Qualified Presumption of Safety (QPS) designation. It is important to emphasize in this regard that P. freudenreichii is the only microbe having GRAS certification that can synthesize the physiologically active form of vitamin B12. Due to this distinguishing characteristic, P. freudenreichii plays a special role in the industrial synthesis of vitamin B12 and its application to the microbiological replenishment of food and feed. These bacteria efficiently synthesize therapeutically useful vitamin B12, with just minor amounts of inert analog synthesis (Piwowarek et al., 2018).
There are several known organisms that produce the B12 vitamin and are categorized as GRAS species, for a variety of reasons the PAB is preferred by the food and beverage sector. Lactobacillaceae reuteri bacteria can synthesize vitamin B12 which is ineffective for human consumption because they are incapable of adding ligands beyond the adenine in the bottom half of the ring (Watanabe & Bito, 2018). To strengthen the quantity of vitamin B12 in P. freudenreichii, various genetic engineering techniques were explored. For instance, it has been claimed that improving cobalamin biosynthesis involves using a genome‐shuffle method and overexpressing selected of the key genes implicated in cobalamin amalgamation. To get superior cobalamin growers, the primary commercial strains are frequently generated by accidental mutations utilizing a variety of mutagenic stimuli, such as ultraviolet light or chemical substances. These high‐yielding strains of P. freudenreichii often exhibit greater tolerance and resistance to propionic acid. Propionibacterium may synthesize vitamin B12 in two different methods. The first technique uses microbial cultures to add vitamin B12 to specific fermented food items (Van et al., 2011). To increase the quantity of vitamin B12 in P. freudenreichii, various genetic engineering techniques were explored. It has been claimed that improving cobalamin biosynthesis involves using a genome‐shuffle method and overexpressing specific key genes required in cobalamin production. To get superior cobalamin growers, the primary commercial strains are frequently generated by accidental mutations utilizing a variety of mutagenic stimuli, such as ultraviolet light or chemical substances. These high‐yielding strains of P. freudenreichii often exhibit greater tolerance and resistance to propionic acid. Propionibacterium may synthesize vitamin B12 in two different methods. The primary technique uses microbial cultures to add vitamin B12 to specific fermented food items (Van et al., 2011). P. freudenreichii are autonomous anaerobic strains that produce cobalamin through microbial biosynthesis. Oxygen is mandatory for the production of DMBI and its attachment to the corrin ring, even though they only produce high cobalamin outputs at extremely low oxygen concentrations (Deptula et al., 2017). A typical growing process is divided into two stages: a first stage in which the cells are cultured in complete anaerobic conditions, and a second stage—typically after 72–96 h of cultivation—in which soft oxygenation is generated by agitation to create the microaeration necessary for the formation of DMBI and cobalamin (Mauerhofer et al., 2019). By enabling its frank source in the development of food items, these producers of vitamin B12 were able to broaden the breadth of their market as depicted in Figure 5 (Dudko et al., 2023).
FIGURE 5.
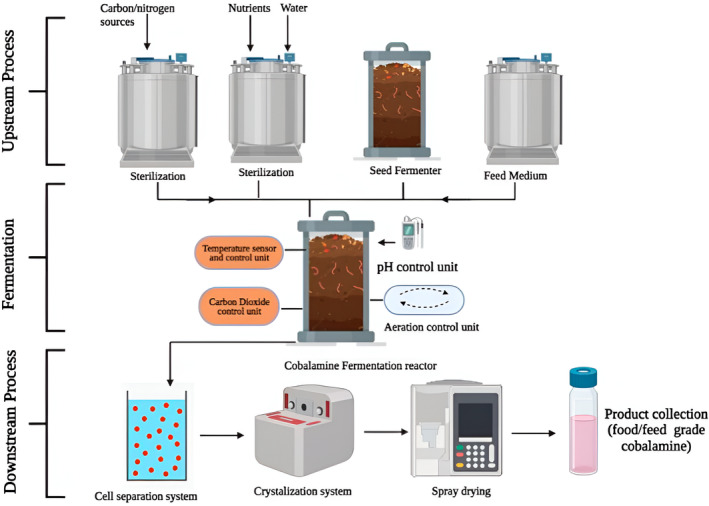
Exemplary commercial cobalamin manufacturing flowchart that applicable for Propionibacterium shermanii.
As a result, Deptula et al. (2017) investigation into the fortification of tempeh revealed that P. freudenreichii has been successfully used in in situ food fortification using food‐like environments such as cheese‐like propionic medium, whey‐based liquid medium, cereal matrices, and even in the fortification of tempeh. This method of fortifying food enables an increase in cobalamin content by utilizing unusual sources and obtaining the necessary daily consumption levels of vitamin B12 through small amounts of fermented goods. This approach works well despite having final cell densities that are considerably lower and reported production levels than with standard medium. Surprisingly, these microorganisms can produce large levels of propionic acid, which, over time, can become harmful and obstruct cell development, as shown in prior investigations. In the bioprocessing field, several optimization strategies have been investigated to address the development of propionic acid (Chamlagain et al., 2018). Results from using in situ product removal (ISPR) techniques to synthesize propionic acid and vitamin B12 together have been positive. It has been demonstrated that volumetric manufacture of vitamin B12 approximately 40 mg/L and 60 mg/L utilizing ISPR technologies is possible using expanded‐bed adsorption bioreactors (EBABs) and high biocompatibility resins, such as ZGA330. While desorption occurs in EBABs, propionic acid is maintained in the resin, allowing the culture to pass down the chromatographic column without clogging (Signorini et al., 2018). The concurrent enhancement of vitamin B12 and propionic acid synthesis has been studied under various culture conditions, carbon and nitrogen sources, and the inclusion of medium dietary supplements, which include DMBI (Wang et al., 2020). Both corn stalk hydroxylates and the mixture of glucose and glycerol were shown to be effective carbon sources in an EBAB system, with reported volumetric CNC cyanocobalamin manufacturing rates of 43.2 mg/L and 47.6 mg/L, correspondingly. Cofermenting P. freudenreichii with other microbes that may metabolize propionic acid is an intriguing method for lowering propionic acid content. For instance, co‐cultivating Ralstonia eutropha and P. freudenreichii increased cobalamin synthesis from 6.73 mg/L to over 19 mg/L. Cofermentation has been effectively used to create multiple products at once or to strengthen different cell cultures in addition to reducing propionic acid (Wang et al., 2020). The cocultivation of Lactobacillaceae plantarum and P. freudenreichii (now known as Lactiplantibacillus plantarum) allowed for the concurrent manufacturing of folate and vitamin B12, and a recent patent for the cofermentation of a Basidiomycota of a Basidiomycota strain with P. freudenreichii allowed for the concurrent fabrication of vitamins D and B12. For microbiological stability as well as security, P. freudenreichii and Lactobacillaceae brevis (now known as Levilactobacillaceae brevis) were cocultivated while producing vitamin B12 in situ in bread dough using the whey‐based medium (Zheng et al., 2020). One typical tactic for raising productivity is fortification with cobalamin precursors. It has frequently been said that the inclusion of common precursors and necessary substances, such as ALA and Co2+, is advantageous for vitamin synthesis. DMBI can be produced independently by all P. freudenreichii strains; however, its biosynthesis is not very high. Furthermore, since oxygen is obliged for the synthesis of DMBI, its creation is not achievable under purely anaerobic circumstances. The cells start to accumulate inactive forms of vitamin B12, such as cobinamide or pseudovitamin B12, if DMBI accessibility is reduced. Instead, the active form of vitamin B12 cannot generated. It has been repeatedly documented that adding DMBI or even DMBI precursors, such as riboflavin or nicotinamide, helps the formation of cobalamin (Chamlagain et al., 2016). The addition of vitamin B12 mimics has also been observed by other research teams to reduce feedback inhibition and boost cobalamin synthesis. P. freudenreichii cultures are intriguing in industrial settings due to their ability to grow in a variety of complex carbon and nitrogen sources as well as waste and spent media, such as molasses, crude glycerol, waste frying sunflower oil, tomato pomace, liquid acid protein residue of soybean, and vegetable juice spent media (Assis et al., 2020).
6. FUTURE PERSPECTIVES
The production of this essential mineral through fermentation by Pseudomonas denitrificans and Propionibacterium shermanii holds great promise as the demand for vitamin B12 supplements keeps rising due to a rise in its prevalence and increased awareness of its health benefits. To improve the effectiveness, sustainability, and applicability of this manufacturing technique, several future views and developments might be investigated. Future genetic engineering and metabolic engineering studies on P. denitrificans and P. shermanii may result in the creation of improved strains with greater vitamin B12 production. Future genetic engineering and metabolic engineering studies on P. denitrificans and P. shermanii may result in the creation of improved strains with greater vitamin B12 production. Researchers can develop robust strains that can produce more vitamin B12 during fermentation by specifically targeting genes involved in cellular processes and vitamin B12 production. The fermentation process will need to continue to be optimized to produce vitamin B12 at its highest potential. Improved fermentation conditions, such as oxygen availability, pH, temperature, and nutrient supplementation, can be the subject of research to help provide an environment that increases productivity and lowers production costs. Cobalt is now the main substrate for vitamin B12 synthesis; however, it can be costly and scarce. Alternative and more environmentally friendly substrate sources to produce vitamin B12 might be explored through research. The total production process may become more economically feasible and ecologically friendly by selecting cost‐efficient and environmentally suitable substrates. Fermentation‐based large‐scale manufacturing of vitamin B12 will require advancements in bioreactor technology and process scalability. In addition to meeting the growing demand for vitamin B12, efficient and economical industrial methods will help increase access to it for underserved populations, particularly in areas with little resources. Even though the potential health advantages of vitamin B12 are widely known, further investigation into its uses in a variety of industries, including medicine, animal husbandry, and agriculture, is possible in the future. Personalized medicine may have novel applications for vitamin B12 if its involvement in the prevention or treatment of illnesses is studied.
7. CONCLUSION
Through a complicated process, microbes produce vitamin B12, which is widely used in the food and pharmaceutical industries. Heme, cobalamin, and siroheme are examples of tetrapyrrole compounds. Rather than being antagonistic, these molecules interact and depend on one another with other substances in different types of bacteria. To maintain constant levels of vitamin B12, riboswitches regulate the transcriptional or translational level of vitamin B12 synthesis and transport. Researchers and numerous sectors of the food, feed, medical, and pharmaceutical industries are becoming interested in PAB due to its great potential. By using strains of bacteria such as P. denitrificans and P. shermanii, vitamin B12 is created by fermentation by microbial means. These bacteria have complex enzyme machinery that allows them to use a wide range of carbon sources. The ability of propionic acid bacteria to produce a range of physiologically valuable compounds from byproducts of industrial processes, such as propionic acid, vitamin B12, trehalose, and bacteriocins, is the most important fact about the usage of these bacteria for commercial reasons. Therefore, the biotechnological use of PAB may help to reduce environmental pollution by transforming the waste into valuable and useful components for other sectors. Further research is needed to increase the biosynthetic efficiency of these metabolites to scale up manufacturing, for example, by employing waste products, which may be less expensive than the current production procedures. It should be highlighted that the production of cyanocobalamin is now subpar and faces several challenges before reaching its full potential as a cost‐effective and successful industrial bioprocess. The underlying issue is that volumetric production levels are often only 200–300 mg/L, even in aerobic strains with higher production rates like Pseudomonas denitrificans. This is significantly less than the values obtained by similar fermentation techniques, such as those for vitamin B12. The long and costly fermentation cycles are also a result of the requirement for costly media components, such as large amounts of complicated nitrogen sources and supplements like betaine. Getting enough cobalt into the soup could potentially be difficult in terms of finances and environmental conditions. Further efforts in bioprocessing, downstream, and media components (with less expensive or recycled components) should be done to increase the ecological sustainability and economic viability of vitamin B12 biotechnological synthesis. The primary reasons for the low productivity of the producing strains that are now available are the inhibition of the cbi operon and cysG by the cobalamin riboswitch and other downregulating mechanisms. Genetic engineering might be required to overcome this constraint, which would not be well received by the public. Fruitarians and vegans are very concerned about the use of genetically modified organisms in their diets (Acevedo‐Rocha et al., 2019). The two main producers of vitamin B12 used in manufacturing operations are P. freudenreichii (206 mg/L) and P. denitrificans (214 mg/L). These bacteria grow slowly (they ferment for 180 h on average), making it challenging to genetically alter them. It is necessary to add more generating hosts to increase the current assembly of vitamin B12. With the aid of thorough knowledge of the steps involved in the biosynthesis of vitamin B12 and their guidelines, the de novo anabolism of the vitamin in well‐researched and commercially acceptable organisms such as B. megaterium, E. coli, and the soil species S. meliloti which can be replaced by strains generated at elevated yields has been engineered over the past 10 years. This has created significant engineering challenges for further study. Scientists are able to look into new concepts for improving B12 biosynthesis because of the usage of inexpensive medium components for fermentation, impacted gene changes, and an efficient explanation of designed strains for vitamin B12 synthesis for short periods of time (24–48 h). Only a few species P. denitrificans, P. freudenreichii, R. capsulatus, S. meliloti, S. typhimurium, B. melitensis, and Rhodopseudomonas palustris are known to use their recombinant advocates and riboswitches, even though the lists of microbiological and genetic resources are continually expanding. Using modern comparative genomics and metabolic restoration tools, it will be possible to identify potential hosts as well as genes, regulatory elements, or metabolic pathways for an additional efficient cobalamin metabolic rate or the relevant also simultaneous trials.
AUTHOR CONTRIBUTIONS
Anjali Tripathi: Writing – original draft (lead). Vinay Kumar Pandey: Project administration (lead). Parmjit S. Panesar: Supervision (lead). Anam Taufeeq: Methodology (equal). Hridyanshi Mishra: Investigation (equal). Sarvesh Rustagi: Resources (equal). Sumira Malik: Data curation (equal). Béla Kovács: Writing – Review and editing (equal); Funding acquisition (equal). Tejas Suthar: Methodology (equal). Ayaz Mukarram Shaikh: Writing – Review and editing (equal); Funding acquisition (equal).
FUNDING INFORMATION
Project No. TKP2021‐NKTA‐32 was implemented with support from the National Research, Development, and Innovation Fund of Hungary, financed under the TKP2021‐NKTA funding scheme, and supported by the University of Debrecen Program for Scientific Publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
Authors would like to thank their affiliated institutions mentioned in the article.
Tripathi, A. , Pandey, V. K. , Panesar, P. S. , Taufeeq, A. , Mishra, H. , Rustagi, S. , Malik, S. , Kovács, B. , Suthar, T. , & Shaikh, A. M. (2024). Fermentative production of vitamin B12 by Propionibacterium shermanii and Pseudomonas denitrificans and its promising health benefits: A review. Food Science & Nutrition, 12, 8675–8691. 10.1002/fsn3.4428
Contributor Information
Vinay Kumar Pandey, Email: v.k.pandey30@gmail.com.
Béla Kovács, Email: kovacsb@agr.unideb.hu.
DATA AVAILABILITY STATEMENT
No data have been used in this article.
REFERENCES
- Abdelraheem, E. , Thair, B. , Varela, R. F. , Jockmann, E. , Popadić, D. , Hailes, H. C. , Ward, J. M. , Iribarren, A. M. , Lewkowicz, E. S. , Andexer, J. N. , Hagedoorn, P. L. , & Hanefeld, U. (2022). Methyltransferases: Functions and applications. Chembiochem: A European Journal of Chemical Biology, 23(18), e202200212. 10.1002/cbic.202200212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo‐Rocha, C. G. , Gronenberg, L. S. , Mack, M. , Commichau, F. M. , & Genee, H. J. (2019). Microbial cell factories for the sustainable manufacturing of B vitamins. Current Opinion in Biotechnology, 56, 18–29. 10.1016/j.copbio.2018.07.006 [DOI] [PubMed] [Google Scholar]
- Agnoli, C. , Baroni, L. , Bertini, I. , Ciappellano, S. , Fabbri, A. , Papa, M. , Pellegrini, N. , Sbarbati, R. , Scarino, M. L. , Siani, V. , & Sieri, S. (2017). Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutrition, Metabolism, and Cardiovascular Diseases, 27(12), 1037–1052. 10.1016/j.numecd.2017.10.020 [DOI] [PubMed] [Google Scholar]
- Ainala, S. K. , Somasundar, A. , & Park, S. (2013). Complete genome sequence of Pseudomonas denitrificans ATCC 13867. Genome Announcements, 1(3), e00257‐13. 10.1128/genomeA.00257-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari, E. , Hossaini, D. , Amiry, G. Y. , Ansari, M. , Haidary, M. , Beheshti, F. , & Ahmadi‐Soleimani, S. M. (2023). Vitamin B12 administration prevents ethanol‐induced learning and memory impairment through re‐establishment of the brain oxidant/antioxidant balance, enhancement of BDNF and suppression of GFAP. Behavioural Brain Research, 438, 114156. 10.1016/j.bbr.2022.114156 [DOI] [PubMed] [Google Scholar]
- Angelopoulou, A. , Alexandraki, V. , Georgalaki, M. , Anastasiou, R. , Manolopoulou, E. , Tsakalidou, E. , & Papadimitriou, K. (2017). Production of probiotic feta cheese using Propionibacterium freudenreichii subsp. shermanii as adjunct. International Dairy Journal, 66, 135–139. 10.1016/j.idairyj.2016.11.011 [DOI] [Google Scholar]
- Assis, D. A. D. , Matte, C. , Aschidamini, B. , Rodrigues, E. , & Záchia Ayub, M. A. (2020). Biosynthesis of vitamin B12 by Propionibacterium freudenreichii subsp. shermanii ATCC 13673 using liquid acid protein residue of soybean as culture medium. Biotechnology Progress, 36(5), e3011. 10.1002/btpr.3011 [DOI] [PubMed] [Google Scholar]
- Avalos, L. A. , Nance, N. , Caan, B. , Sujan, A. C. , Uriu‐Adams, J. Y. , Li, D. K. , Quesenberry, C. P. , & Hedderson, M. M. (2023). Association of serum folate levels during pregnancy and prenatal depression. Journal of Maternal‐Fetal and Neonatal Medicine, 36(1), 1–4. 10.1080/14767058.2022.2145878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanova, L. , Averianova, L. , Marchenok, M. , Son, O. , & Tekutyeva, L. (2021). Microbial and genetic resources for cobalamin (vitamin B12) biosynthesis: From ecosystems to industrial biotechnology. International Journal of Molecular Sciences, 22(9), 4522. 10.3390/ijms22094522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali, A. , & Naik, R. (2023). The impact of a vegan diet on many aspects of health: The overlooked side of veganism. Cureus, 15(2), e35148. 10.7759/cureus.35148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbuto Ferraiuolo, S. , Cammarota, M. , Schiraldi, C. , & Restaino, O. F. (2021). Streptomycetes as platform for biotechnological production processes of drugs. Applied Microbiology and Biotechnology, 105(2), 551–568. 10.1007/s00253-020-11064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behere, R. V. , Deshmukh, A. S. , Otiv, S. , Gupte, M. D. , & Yajnik, C. S. (2021). Maternal vitamin B12 status during pregnancy and its association with outcomes of pregnancy and health of the offspring: A systematic review and implications for policy in India. Frontiers in Endocrinology, 12, 619176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benites‐Zapata, V. A. , Ignacio‐Cconchoy, F. L. , Ulloque‐Badaracco, J. R. , Hernandez‐Bustamante, E. A. , Alarcón‐Braga, E. A. , Al‐Kassab‐Córdova, A. , & Herrera‐Añazco, P. (2023). Vitamin B12 levels in thyroid disorders: A systematic review and meta‐analysis. Frontiers in Endocrinology, 14, 1070592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkins, S. , Schiöth, H. B. , & Rukh, G. (2021). Depression and vegetarians: Association between dietary vitamin B6, B12 and folate intake and global and subcortical brain volumes. Nutrients, 13(6), 1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binod, P. , Sindhu, R. , & Pandey, A. (2010). Production of vitamins. In Comprehensive food fermentation and biotechnology (pp. 959–980). [Google Scholar]
- Blasco, L. , Kahala, M. , Tupasela, T. , & Joutsjoki, V. (2011). Determination of aspartase activity in dairy Propionibacterium strains. FEMS Microbiology Letters, 321(1), 10–13. 10.1111/j.1574-6968.2011.02299.x [DOI] [PubMed] [Google Scholar]
- Bryant, D. A. , Hunter, C. N. , & Warren, M. J. (2020). Biosynthesis of the modified tetrapyrroles—The pigments of life. Journal of Biological Chemistry, 295(20), 6888–6925. 10.1074/jbc.REV120.006194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvillo, Á. , Pellicer, T. , Carnicer, M. , & Planas, A. (2022). Bioprocess strategies for vitamin B12 production by microbial fermentation and its market applications. Bioengineering, 9(8), 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvillo, Á. , Pellicer, T. , Carnicer, M. , & Planas, A. (2023). Developing a single‐stage continuous process strategy for vitamin B12 production with Propionibacterium freudenreichii . Microbial Cell Factories, 22(1), 26. 10.1186/s12934-023-02029-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamlagain, B. , Deptula, P. , Edelmann, M. , Kariluoto, S. , Grattepanche, F. , Lacroix, C. , Varmanen, P. , & Piironen, V. (2016). Effect of the lower ligand precursors on vitamin B12 production by food‐grade Propionibacteria . LWT‐ Food Science and Technology, 72, 117–124. 10.1016/j.lwt.2016.04.023 [DOI] [Google Scholar]
- Chamlagain, B. , Sugito, T. A. , Deptula, P. , Edelmann, M. , Kariluoto, S. , Varmanen, P. , & Piironen, V. (2018). In situ production of active vitamin B12 in cereal matrices using Propionibacterium freudenreichii . Food Science & Nutrition, 6(1), 67–76. 10.1002/fsn3.528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charneca, S. , Gomes, A. I. , Branco, D. , Guerreiro, T. , Barros, L. , & Sousa, J. (2023). Intake of added sugar, fruits, vegetables, and legumes of Portuguese preschool children: Baseline data from SmartFeeding4Kids randomized controlled trial participants. Frontiers in Nutrition, 10, 1150627. 10.3389/fnut.2023.1150627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X. , Chen, W. , Peng, W. F. , & Li, K. T. (2014). Improved vitamin B12 fermentation process by adding rotenone to regulate the metabolism of Pseudomonas denitrificans . Applied Biochemistry and Biotechnology, 173(3), 673–681. 10.1007/s12010-014-0878-2 [DOI] [PubMed] [Google Scholar]
- Choi, S. Y. , Park, S. J. , Kim, W. J. , Yang, J. E. , Lee, H. , Shin, J. , & Lee, S. Y. (2016). One‐step fermentative production of poly (lactate‐co‐glycolate) from carbohydrates in Escherichia coli . Nature Biotechnology, 34(4), 435–440. [DOI] [PubMed] [Google Scholar]
- Cordaro, M. , Siracusa, R. , Fusco, R. , Cuzzocrea, S. , Di Paola, R. , & Impellizzeri, D. (2021). Involvements of hyperhomocysteinemia in neurological disorders. Metabolites, 11(1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin, F. J. , Jouan‐Lanhouet, S. , Théret, N. , Brenner, C. , Jouan, E. , Le Moigne‐Muller, G. , Dimanche‐Boitrel, M. T. , & Jan, G. (2016). The probiotic Propionibacterium freudenreichii as a new adjuvant for TRAIL‐based therapy in colorectal cancer. Oncotarget, 7(6), 7161–7178. 10.18632/oncotarget.6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin, A. , & Braham, S. (2017). Coenzyme B12 synthesis as a baseline to study metabolite contribution of animal microbiota. Microbial Biotechnology, 10(4), 688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dank, A. , Abee, T. , & Smid, E. J. (2023). Expanded metabolic diversity of Propionibacterium freudenreichii potentiates novel applications in food biotechnology. Current Opinion in Food Science, 52, 101048. 10.1016/j.cofs.2023.101048 [DOI] [Google Scholar]
- Dank, A. , Biel, G. , Abee, T. , & Smid, E. J. (2022). Microaerobic metabolism of lactate and propionate enhances vitamin B12 production in Propionibacterium freudenreichii . Microbial Cell Factories, 21(1), 225. 10.1186/s12934-022-01945-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas, M. S. , & Erdal, H. (2023). Evaluation of thiol disulfide balance in adolescents with vitamin B12 deficiency. Italian Journal of Pediatrics, 49(1), 3. 10.1186/s13052-022-01396-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deptula, P. , Chamlagain, B. , Edelmann, M. , Sangsuwan, P. , Nyman, T. A. , Savijoki, K. , Piironen, V. , & Varmanen, P. (2017). Food‐like growth conditions support production of active vitamin B12 by Propionibacterium freudenreichii 2067 without DMBI, the lower ligand base, or cobalt supplementation. Frontiers in Microbiology, 8, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubaj, C. , Czyż, K. , & Furmaga‐Jabłońska, W. (2020). Vitamin B12 deficiency as a cause of severe neurological symptoms in breastfed infant–a case report. Italian Journal of Pediatrics, 46(1), 40. 10.1186/s13052-020-0804-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudko, D. , Holtmann, D. , & Buchhaupt, M. (2023). Methylotrophic bacteria with cobalamin‐dependent mutases in primary metabolism as potential strains for vitamin B12 production. Antonie Van Leeuwenhoek, 116(3), 207–220. 10.1007/s10482-022-01795-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hasbaoui, B. , Mebrouk, N. , Saghir, S. , El Yajouri, A. , Abilkassem, R. , & Agadr, A. (2021). Vitamin B12 deficiency: Case report and review of literature. Pan African Medical Journal, 38(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falentin, H. , Deutsch, S. M. , Jan, G. , Loux, V. , Thierry, A. , Parayre, S. , Maillard, M. B. , Dherbécourt, J. , Cousin, F. J. , Jardin, J. , Siguier, P. , Couloux, A. , Barbe, V. , Vacherie, B. , Wincker, P. , Gibrat, J. F. , Gaillardin, C. , & Lortal, S. (2010). The complete genome of Propionibacterium freudenreichii CIRM‐BIA1T, a hardy actinobacterium with food and probiotic applications. PLoS One, 5(7), e11748. 10.1371/journal.pone.0011748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, H. , Kang, J. , & Zhang, D. (2017). Microbial production of vitamin B12: A review and future perspectives. Microbial Cell Factories, 16(1), 15. 10.1186/s12934-017-0631-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, H. , Li, D. , Kang, J. , Jiang, P. , Sun, J. , & Zhang, D. (2018). Metabolic engineering of Escherichia coli for de novo biosynthesis of vitamin B12 . Nature Communications, 9(1), 4917. 10.1038/s41467-018-07412-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgie, A. J. , Pepin, D. M. , Ju, T. , Tollenaar, S. , Sergi, C. M. , Gruenheid, S. , & Willing, B. P. (2023). Over supplementation with vitamin B12 alters microbe‐host interactions in the gut leading to accelerated Citrobacter rodentium colonization and pathogenesis in mice. Microbiome, 11(1), 21. 10.1186/s40168-023-01461-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Cabrera, R. I. , Valdez‐Cruz, N. A. , Blancas‐Cabrera, A. , & Trujillo‐Roldán, M. A. (2020). Oxygen transfer rate affect polyhydroxybutyrate production and oxidative stress response in submerged cultures of Rhizobium phaseoli . Biochemical Engineering Journal, 162, 107721. 10.1016/j.bej.2020.107721 [DOI] [Google Scholar]
- Gharibzahedi, S. M. T. , Moghadam, M. , Amft, J. , Tolun, A. , Hasabnis, G. , & Altintas, Z. (2023). Recent advances in dietary sources, health benefits, emerging encapsulation methods, food fortification, and new sensor‐based monitoring of vitamin B12: A critical review. Molecules, 28(22), 7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goraya, J. S. , & Kaur, S. (2023). Vitamin B12 deficiency in mothers and children: Risk of neuro‐regression. Paediatrics and International Child Health, 1–7, 50–56. 10.1080/20469047.2023.2171767 [DOI] [PubMed] [Google Scholar]
- Henjum, S. , Groufh‐Jacobsen, S. , Lindsay, A. , Raael, E. , Israelsson, A. M. , Shahab‐Ferdows, S. , & Hampel, D. (2023). Adequate vitamin B12 and folate status of Norwegian vegans and vegetarians. British Journal of Nutrition, 129(12), 2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Pérez, A. F. , Jofre, F. M. , de Souza Queiroz, S. , de Arruda, P. V. , Chandel, A. K. , & de Almeida Felipe, M. D. G. (2020). Biotechnological production of sweeteners. In Biotechnological production of bioactive compounds (pp. 261–292). Elsevier. [Google Scholar]
- Kazadi Mbamba, C. K. , Lindblom, E. , Flores‐Alsina, X. , Tait, S. , Anderson, S. , Saagi, R. , Batstone, D. J. , Gernaey, K. V. , & Jeppsson, U. (2019). Plant‐wide model‐based analysis of iron dosage strategies for chemical phosphorus removal in wastewater treatment systems. Water Research, 155, 12–25. 10.1016/j.watres.2019.01.048 [DOI] [PubMed] [Google Scholar]
- Khosravi, F. , Mirzaei, S. , Hojati, V. , Hashemi, M. , & Entezari, M. (2023). Co‐administration of vitamins B12 and D during pregnancy have strong neuroprotective effects in Parkinson disease. Molecular Neurobiology, 60(4), 1986–1996. 10.1007/s12035-022-03186-7 [DOI] [PubMed] [Google Scholar]
- Kokande, A. M. , Surana, K. R. , Jain, V. N. , Mahajan, S. K. , Patil, D. M. , & Sonawane, D. D. (2024). Overview and recent advances of vitamins. In Preventive and therapeutic role of vitamins as nutraceuticals (pp. 253–279). [Google Scholar]
- Kostecka, M. , Kostecka, J. , Jackowska, I. , & Iłowiecka, K. (2023). Parental nutritional knowledge and type of diet as the key factors influencing the safety of vegetarian diets for children aged 12–36 months. Nutrients, 15(10), 2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansdottir, T. , Bosma, E. F. , Branco Dos Santos, F. , Özdemir, E. , Herrgård, M. J. , França, L. , Ferreira, B. , Nielsen, A. T. , & Gudmundsson, S. (2019). A metabolic reconstruction of Lactobacillus reuteri JCM1112 and analysis of its potential as a cell factory. Microbial Cell Factories, 18(1), 186. 10.1186/s12934-019-1229-3A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, R. , Singh, U. , Tiwari, A. , Tiwari, P. , Sahu, J. K. , & Sharma, S. (2023). Vitamin B12: Strategies for enhanced production, fortified functional food products and health benefits. Process Biochemistry, 127, 44–55. 10.1016/j.procbio.2023.02.002 [DOI] [Google Scholar]
- Kumpangcum, S. , Anek, P. , Khamnoi, P. , Prommeenate, P. , & Phannachet, K. (2023). Complete genome sequence of Pseudomonas aeruginosa PA99 clinical isolate from Thailand carrying two novel class 1 integrons, in 2083 and in 2084. Journal of Global Antimicrobial Resistance, 33, 97–100. 10.1016/j.jgar.2023.02.027 [DOI] [PubMed] [Google Scholar]
- Li, J. , Ge, Y. , Zadeh, M. , Curtiss, R., 3rd , & Mohamadzadeh, M. (2020). Regulating vitamin B12 biosynthesis via the cbiMCbl riboswitch in Propionibacterium strain UF1. Proceedings of the National Academy of Sciences of the United States of America, 117(1), 602–609. 10.1073/pnas.1916576116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, X. , Heal, K. R. , Ingalls, A. E. , Doxey, A. C. , & Neufeld, J. D. (2020). Metagenomic and chemical characterization of soil cobalamin production. The ISME Journal, 14(1), 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon, P. , Strippoli, V. , Fang, B. , & Cimmino, L. (2020). B vitamins and one‐carbon metabolism: Implications in human health and disease. Nutrients, 12(9), 2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Q. , Zhang, Q. , Xu, Q. , Zhang, C. , Li, Y. , Fan, X. , Xie, X. , & Chen, N. (2017). Systems metabolic engineering strategies for the production of amino acids. Synthetic and Systems Biotechnology, 2(2), 87–96. 10.1016/j.synbio.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Iglesias, O. , Naidoo, V. , Carrera, I. , Corzo, L. , & Cacabelos, R. (2023). Natural bioactive products as epigenetic modulators for treating neurodegenerative disorders. Pharmaceuticals, 16(2), 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauerhofer, L. M. , Pappenreiter, P. , Paulik, C. , Seifert, A. H. , Bernacchi, S. , & Rittmann, S. K. R. (2019). Methods for quantification of growth and productivity in anaerobic microbiology and biotechnology. Folia Microbiologica, 64(3), 321–360. 10.1007/s12223-018-0658-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, S. J. , Lawrence, A. D. , Biedendieck, R. , Deery, E. , Frank, S. , Howard, M. J. , Rigby, S. E. , & Warren, M. J. (2013). Elucidation of the anaerobic pathway for the corrin component of cobalamin (vitamin B12). Proceedings of the National Academy of Sciences of the United States of America, 110(37), 14906–14911. 10.1073/pnas.1308098110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen‐Vo, T. P. , Ainala, S. K. , Kim, J. R. , & Park, S. (2018). Analysis and characterization of coenzyme B12 biosynthetic gene clusters and improvement of B12 biosynthesis in Pseudomonas denitrificans ATCC 13867. FEMS Microbiology Letters, 365(21), fny211. [DOI] [PubMed] [Google Scholar]
- Osman, D. , Cooke, A. , Young, T. R. , Deery, E. , Robinson, N. J. , & Warren, M. J. (2021). The requirement for cobalt in vitamin B12: A paradigm for protein metalation. Biochimica et Biophysica Acta. Molecular Cell Research, 1868(1), 118896. 10.1016/j.bbamcr.2020.118896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudman, E. (2020). Wernicke encephalopathy in patients with depression: A systematic review. Psychiatry and Clinical Neurosciences, 74(10), 569–572. 10.1111/pcn.13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, D. J. , Han, S. U. , Hyung, W. J. , Hwang, S. H. , Hur, H. , Yang, H. K. , Lee, H. J. , Kim, H. I. , Kong, S. H. , Kim, Y. W. , Lee, H. H. , Kim, B. S. , Park, Y. K. , Lee, Y. J. , Ahn, S. H. , Lee, I. , Suh, Y. S. , Park, J. H. , Ahn, S. , … Kim, H. H. (2023). Effect of laparoscopic proximal gastrectomy with double‐tract reconstruction vs total gastrectomy on hemoglobin level and vitamin B12 supplementation in upper‐third early gastric cancer: A randomized clinical trial. JAMA Network Open, 6(2), e2256004. 10.1001/jamanetworkopen.2022.56004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesqueda‐Cendejas, K. , Campos‐López, B. , Mora‐García, P. E. , Moreno‐Ortiz, J. M. , & De la Cruz‐Mosso, U. (2023). Methyl donor micronutrients: A potential dietary epigenetic target in systemic lupus erythematosus patients. International Journal of Molecular Sciences, 24(4), 3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwowarek, K. , Lipińska, E. , Hać‐Szymańczuk, E. , Kieliszek, M. , & Kot, A. M. (2020). Sequencing and analysis of the genome of Propionibacterium freudenreichii T82 strain: Importance for industry. Biomolecules, 10(2), 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwowarek, K. , Lipińska, E. , Hać‐Szymańczuk, E. , Kieliszek, M. , & Ścibisz, I. (2018). Propionibacterium spp.—Source of propionic acid, vitamin B12, and other metabolites important for the industry. Applied Microbiology and Biotechnology, 102(2), 515–538. 10.1007/s00253-017-8616-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajjwal, P. , Asharaf, S. , Makhanasa, D. , Yamparala, A. , Tariq, H. , Aleti, S. , Gadam, S. , & Vora, N. (2023). Association of Alzheimer's dementia with oral bacteria, vitamin B12, folate, homocysteine levels, and insulin resistance along with its pathophysiology, genetics, imaging, and biomarkers. Disease‐a‐Month, 69(5), 101546. 10.1016/j.disamonth.2023.101546 [DOI] [PubMed] [Google Scholar]
- Ranaei, V. , Pilevar, Z. , Khaneghah, A. M. , & Hosseini, H. (2020). Propionic acid: Method of production, current state and perspectives. Food Technology and Biotechnology, 58(2), 115–127. 10.17113/ftb.58.02.20.6356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod, R. , Kale, A. , & Joshi, S. (2016). Novel insights into the effect of vitamin B 12 and omega‐3 fatty acids on brain function. Journal of Biomedical Science, 23, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf, A. , Badoni, H. , Abu‐Izneid, T. , Olatunde, A. , Rahman, M. M. , Painuli, S. , Semwal, P. , Wilairatana, P. , & Mubarak, M. S. (2022). Neuroinflammatory markers: Key indicators in the pathology of neurodegenerative diseases. Molecules, 27(10), 3194. 10.3390/molecules27103194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov, D. A. , Arzamasov, A. A. , Khoroshkin, M. S. , Iablokov, S. N. , Leyn, S. A. , Peterson, S. N. , Novichkov, P. S. , & Osterman, A. L. (2019). Micronutrient requirements and sharing capabilities of the human gut microbiome. Frontiers in Microbiology, 10, 1316. 10.3389/fmicb.2019.01316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney, D. J. , Conway, M. , O'Keeffe, L. M. , McDonnell, C. M. , Bartels, H. C. , Yelverton, C. , Segurado, R. , Mehegan, J. , & McAuliffe, F. M. (2023). Dietary intakes of iron, folate, and vitamin B12 during pregnancy and correlation with maternal hemoglobin and fetal growth: Findings from the ROLO longitudinal birth cohort study. Archives of Gynecology and Obstetrics, 1–11, 183–193. 10.1007/s00404-023-06916-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowicka, G. , Klemarczyk, W. , Ambroszkiewicz, J. , Strucińska, M. , Kawiak‐Jawor, E. , Weker, H. , & Chełchowska, M. (2023). Assessment of oxidant and antioxidant status in Prepubertal children following vegetarian and omnivorous diets. Antioxidants, 12(3), 682. 10.3390/antiox12030682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serin, H. M. , & Arslan, E. A. (2019). Neurological symptoms of vitamin B12 deficiency: Analysis of pediatric patients. Acta Clinica Croatica, 58(2), 295–302. 10.20471/acc.2019.58.02.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, R. , Ojha, A. , & Bhunia, B. (2024). Microbial production of vitamin B12 using food matrices. In Food process engineering and technology: Safety, packaging, nanotechnologies and human health (pp. 471–492). Springer Nature Singapore. [Google Scholar]
- Shelton, A. N. , Seth, E. C. , Mok, K. C. , Han, A. W. , Jackson, S. N. , Haft, D. R. , & Taga, M. E. (2019). Uneven distribution of cobamide biosynthesis and dependence in bacteria predicted by comparative genomics. ISME Journal, 13(3), 789–804. 10.1038/s41396-018-0304-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorini, C. , Carpen, A. , Coletto, L. , Borgonovo, G. , Galanti, E. , Capraro, J. , Magni, C. , Abate, A. , Johnson, S. K. , Duranti, M. , & Scarafoni, A. (2018). Enhanced vitamin B12 production in an innovative lupin tempeh is due to synergic effects of Rhizopus and Propionibacterium in cofermentation. International Journal of Food Sciences and Nutrition, 69(4), 451–457. 10.1080/09637486.2017.1386627 [DOI] [PubMed] [Google Scholar]
- Singh, J. , & Vyas, A. (Eds.). (2022). Advances in dairy microbial products. Woodhead Publishing. [Google Scholar]
- Sun, W. L. , Hua, S. , Li, X. Y. , Shen, L. , Wu, H. , & Ji, H. F. (2023). Microbially produced vitamin B12 contributes to the lipid‐lowering effect of silymarin. Nature Communications, 14(1), 477. 10.1038/s41467-023-36079-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Y. , Zhou, L. , Huang, J. , Chen, X. , Wu, Y. , Song, X. , Wang, J. , Hu, H. , & Yang, Q. (2023). Vitamin B12, folate, homocysteine, inflammatory mediators (interleukin‐6, tumor necrosis factor‐α and C‐reactive protein) levels in adolescents with anxiety or depressive symptoms. Neuropsychiatric Disease and Treatment, 19, 785–800. 10.2147/NDT.S399378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangeraas, T. , Ljungblad, U. W. , Lutvica, E. , Kristensen, E. , Rowe, A. D. , Bjørke‐Monsen, A. L. , Rootwelt‐Revheim, T. , Sæves, I. , & Pettersen, R. D. (2023). Vitamin B12 deficiency (un‐)detected using newborn screening in Norway. International Journal of Neonatal Screening, 9(1), 3. 10.3390/ijns9010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, P. N. , Savka, M. A. , & Gan, H. M. (2017). In‐silico taxonomic classification of 373 genomes reveals species misidentification and new genospecies within the genus pseudomonas. Frontiers in Microbiology, 8, 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufan, A. E. , Bilici, R. , Usta, G. , & Erdoğan, A. (2012). Mood disorder with mixed, psychotic features due to vitamin B12 deficiency in an adolescent: Case report. Child and Adolescent Psychiatry and Mental Health, 6(1), 25. 10.1186/1753-2000-6-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umasanker, S. , Bhakat, R. , Mehta, S. , Rathaur, V. K. , Verma, P. K. , Bhat, N. K. , Naithani, M. , & Chacham, S. (2020). Vitamin B12 deficiency in children from northern India: Time to reconsider nutritional handicaps. Journal of Family Medicine and Primary Care, 9(9), 4985–4991. 10.4103/jfmpc.jfmpc_712_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Qin, R. , Guo, Y. , Ma, C. , Wang, X. , Chen, K. , & Ouyang, P. (2023). Engineering the native methylotrophs for the bioconversion of methanol to value‐added chemicals: Current status and future perspectives. Green Chemical Engineering, 4(2), 199–211. [Google Scholar]
- Wang, P. , Shen, C. , Li, L. , Guo, J. , Cong, Q. , & Lu, J. (2020). Simultaneous production of propionic acid and vitamin B12 from corn stalk hydrolysates by Propionibacterium freudenreichii in an expanded bed adsorption bioreactor. Preparative Biochemistry and Biotechnology, 50(8), 763–767. 10.1080/10826068.2020.1734942 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Liu, L. , Jin, Z. , & Zhang, D. (2021). Microbial cell factories for green production of vitamins. Frontiers in Bioengineering and Biotechnology, 9, 661562. 10.3389/fbioe.2021.661562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. J. , Wang, H. Y. , Wang, P. , Zhang, Y. M. , Chu, J. , Zhuang, Y. P. , & Zhang, S. L. (2014). Enhance vitamin B12 production by online CO2 concentration control optimization in 120‐m3 fermentation. Journal of Bioprocessing and Biotechniques, 4(4). [Google Scholar]
- Watanabe, F. , & Bito, T. (2018). Vitamin B12 sources and microbial interaction. Experimental Biology and Medicine, 243(2), 148–158. 10.1177/1535370217746612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Zhang, L. , Li, S. , & Zhang, D. (2022). Associations of dietary vitamin B1, vitamin B2, vitamin B6, and vitamin B12 with the risk of depression: A systematic review and meta‐analysis. Nutrition Reviews, 80(3), 351–366. 10.1093/nutrit/nuab014 [DOI] [PubMed] [Google Scholar]
- Xia, W. , Chen, W. , Peng, W. F. , & Li, K. T. (2015). Industrial vitamin B12 production by Pseudomonas denitrificans using maltose syrup and corn steep liquor as the cost‐effective fermentation substrates. Bioprocess and Biosystems Engineering, 38(6), 1065–1073. 10.1007/s00449-014-1348-5 [DOI] [PubMed] [Google Scholar]
- Xia, W. , Peng, W. F. , Chen, W. , & Li, K. T. (2015). Interactive performances of betaine on the metabolic processes of Pseudomonas denitrificans . Journal of Industrial Microbiology and Biotechnology, 42(2), 273–278. 10.1007/s10295-014-1562-9 [DOI] [PubMed] [Google Scholar]
- Xie, Y. , Peng, Q. , Ji, Y. , Xie, A. , Yang, L. , Mu, S. , Li, Z. , He, T. , Xiao, Y. , Zhao, J. , & Zhang, Q. (2021). Isolation and identification of antibacterial bioactive compounds from Bacillus megaterium L2. Frontiers in Microbiology, 12, 645484. 10.3389/fmicb.2021.645484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. C. , Wang, H. Y. , Li, Y. F. , Yang, X. Y. , Li, Y. , & Wang, T. J. (2023). The action of topical application of vitamin B12 ointment on radiodermatitis in a porcine model. International Wound Journal, 20(2), 516–528. 10.1111/iwj.13899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. , Wittouck, S. , Salvetti, E. , Franz, C. M. A. P. , Harris, H. M. B. , Mattarelli, P. , O'Toole, P. W. , Pot, B. , Vandamme, P. , Walter, J. , Watanabe, K. , Wuyts, S. , Felis, G. E. , Gänzle, M. G. , & Lebeer, S. (2020). A taxonomic note on the genus Lactobacillaceae: Description of 23 novel genera, emended description of the genus Lactobacillaceae Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. International Journal of Systematic and Evolutionary Microbiology, 70(4), 2782–2858. 10.1099/ijsem.0.004107 [DOI] [PubMed] [Google Scholar]
- Zhou, S. , Ashok, S. , Ko, Y. , Kim, D. M. , & Park, S. (2014). Development of a deletion mutant of Pseudomonas denitrificans that does not degrade 3‐hydroxypropionic acid. Applied Microbiology and Biotechnology, 98(10), 4389–4398. 10.1007/s00253-014-5562-5 [DOI] [PubMed] [Google Scholar]
- Żandarek, J. , Binert‐Kusztal, Ż. , Starek, M. , & Dąbrowska, M. (2023). Development and optimization of chromatographic conditions for the determination of selected B vitamins in pharmaceutical products. Processes, 11(3), 937. 10.3390/pr11030937 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data have been used in this article.


