Abstract
Currently, there is limited evidence regarding the association between prenatal exposure to environmental fine particulate matter (PM2.5) and the occurrence of cryptorchidism. The objective of this study was to evaluate the potential correlation between prenatal exposure to PM2.5 and the likelihood of cryptorchidism developing in offspring. We performed a 1:1 case–control study, defining the cases as children diagnosed with cryptorchidism at the Children’s Hospital Affiliated to Chongqing Medical University from 2013 to 2017, while the control group comprised children born in the corresponding years who did not have any birth defects, chromosomal abnormalities, and had only trauma-related treatments. Between 2012 and 2017, monthly averages of PM2.5, other pollutants (O3, PM10) and temperature were gathered based on the geographical coordinates of patients’ residences. The study assessed the correlation between the two using multivariate logistic regression model, and sensitivity analysis was conducted to assess the stability of the model. We included a total of 2137 cases and 2137 matched controls from 2013 to 2017. Our findings revealed that there was a positive association between exposure to PM2.5 during the first 2 months of pregnancy and the occurrence of cryptorchidism. According to this study, the development of cryptorchidism appears to be associated with maternal exposure to PM2.5 during early pregnancy.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81024-7.
Keywords: PM2.5, Fine particular matter, Cryptorchidism, Birth defect, Clinical study
Subject terms: Environmental sciences, Paediatric urology, Urogenital diseases
Introduction
Cryptorchidism, defined as the failure of testicles to descend into the scrotum or the presence of testicles outside the scrotum, is the most common malformation of the urogenital system in children1. The prevalence of cryptorchidism among full-term male newborns ranges from 1.0 to 4.6%, while the prevalence among preterm or low-birth-weight male newborns ranges from 1.1 to 45.3%2. Some scholars in China have also reported that from 2007 to 2021, the overall prevalence of cryptorchidism has increased from 3.86 cases per 10,000 full-term male infants to 11.20 cases per 10,000 full-term male infants3. Cryptorchidism represents a risk factor for future infertility and testicular malignancy and has a considerable impact on individuals and families4,5. Cryptorchidism is thought to be multifactorial, with genes, endocrine and environmental factors all playing a role6,7.
The problem of environmental pollution has become increasingly acute with rapid urbanization in recent years. According to the World Health Organization’s 2016 Global Air Quality Report, nearly 2 million people in China die each year from exposure to particulate matter pollution8. Fine particulate matter, defined as particles in ambient air with an aerodynamic diameter of 2.5 µm or less, is one of the main components of air pollution. In recent years, several scholars have directed their attention to the effects of PM2.5 exposure on urogenital malformations. For example, research conducted by Sheng Ren et al. suggested that PM2.5 exposure during the first month of pregnancy has the greatest impact on abdominal wall defects and hypospadias9. In contrast, there are fewer studies on the relationship between ambient PM2.5 and the risk of cryptorchidism, and no studies point to a critical window of exposure. Some studies have reported that PM2.5 has a role in regulating endocrine hormones10,11. Exposure to PM2.5 during pregnancy may influence fetal hormone levels, subsequently affecting testicular descent. Additionally, exposure to PM2.5 during pregnancy can increase the risk of low birth weight, preterm birth12,13. And several studies have now confirmed low birth weight and preterm birth as risk factors for cryptorchidism14–16.
Therefore, the aim of this study was to assess the correlation between prenatal exposure to PM2.5 and the risk of cryptorchidism and to indicate the exposure window period.
Materials and methods
Study design
This study used a 1:1 case‒control design. During the period from 1 January 2013 to 31 December 2017, we gathered data on children who were diagnosed with cryptorchidism at the Children’s Hospital of Chongqing Medical University. The diagnostic criteria for cryptorchidism were in accordance with international diagnostic criteria17. After excluding children with familial histories of urinary tract malformations, genetic disorders, or abnormalities in sexual development (including chromosomal abnormalities or Müller’s persistent duct syndrome), as well as those with incomplete data or missing diagnoses, the study ultimately comprised a case group of 2137 children. Upon age matching, a control group was composed of children admitted to the same hospital for trauma during the same period, excluding those with birth defects. This study was approved by the Institutional Review Board of Children’s Hospital of Chongqing Medical University.
Cases and controls
Children who were reported to have cryptorchidism (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] code Q53.9) and children who presented with trauma (code T10-12, T30) but had no chromosomal abnormalities or birth defects were included in the case group. The diagnosis of cryptorchidism was made by a specialist pediatrician. Children without any birth defects or chromosomal abnormalities who presented due to trauma were defined as controls. For each case of cryptorchidism, one control was matched by age. The information and characteristics of the patients were collected. Maternal information included: residence address, parity, conception season, whether it was multiple pregnancy, history of medication use during pregnancy, complications during pregnancy. History of medication use during pregnancy was defined as the use of birth control drugs or hormone-containing drugs during pregnancy. Complications during pregnancy were defined as a history of diabetes, hypertension, or thyroid disease during pregnancy. Information of the child included: date of birth, gestational weeks, and birth weight. In our study, all methods were carried out in accordance with the relevant guidelines and regulations. Specifically, we confirm that informed consent was obtained from all subjects and/or their legal guardians, as applicable.
Air pollution data
The concentration data of PM2.5, O3, PM10 (with a spatial resolution of 1 km × 1 km) utilized in this study were obtained from China’s near-real-time tracking dataset of atmospheric composition (http://tapdata.org.cn)18,19. Temperature data were obtained from the ERA5 (ECMWF Reanalysis v5) dataset20. Utilizing the latitudinal and longitudinal coordinates of the patients’ residential addresses, monthly average concentration data of pollutants and temperature were procured from 2012 to 2017. Additionally, for each child included in the study, the concentration of environmental pollutants and temperature was matched to the period spanning from 3 months prior to conception up to 3 months post-conception.
Statistical analysis
The study’s binary outcome variable was the incidence of cryptorchidism, while the key predictor variables encompassed estimated exposure levels to PM2.5. Categorical data are expressed as percentages [n (%)], while continuous data are expressed as medians (Q1, Q3) or mean ± standard deviation. The baseline data for the case and control groups were analyzed using the t-test or Mann–Whitney-U test for continuous variables and the chi-square test for categorical variables, respectively. Bonferroni’s method was used to correct for multiple comparisons. In this study, we treated the exposure level of PM2.5 as a continuous variable and reported the ratio of ratios (OR) for each 10 μg/m3 increase in its concentration. Using a conditional logistic regression model, the relationship between exposure to PM2.5 during pregnancy (as a continuous variable) and the risk of cryptorchidism was evaluated on a monthly basis. Additionally, the relationship between quartiles of PM2.5 (as a categorical variable) during specific periods and the risk of cryptorchidism was further explored. To address potential biases, adjustments were made for low birth weight, prematurity, medication history during pregnancy, pregnancy complications, birth year, conception season and temperature. Finally, the remaining pollutants were included for sensitivity analyses to assess the robustness of the model21.The statistical analyses were conducted utilizing SPSS version 27 (IBM, China) and R version 4.2.3 (The R Foundation for Statistical Computing), with P values below 0.05 deemed significant.
Results
Baseline characteristics
A total of 2137 children were reported to have cryptorchidism between 2013 and 2017, so we randomly selected 2137 children without birth defects who presented for trauma as a control group. Figure 1 displays the monthly average concentrations of PM2.5 and the geographical distribution of the registered cities. Table 1 presents the demographic characteristics and monthly temperatures of the children included in this study. The infants in the case group exhibited lower median birth weights compared to the control group, leading to a notably higher percentage of low-birth-weight infants (9.5% versus 3.1%) among the cases. The proportion of preterm babies was higher in the case group than in the control group (11.5% vs. 4.6%). The case and control groups exhibited notable disparities in terms of drug use history, pregnancy complications, conception season, and birth year. For the other variables, including multiple pregnancies and city of birth, no significant differences were observed. A statistically significant temperature difference was observed between the case group and the control group during the period from three months prior to conception to one month post-conception. During the remaining periods, no statistically significant difference was observed.
Fig. 1.
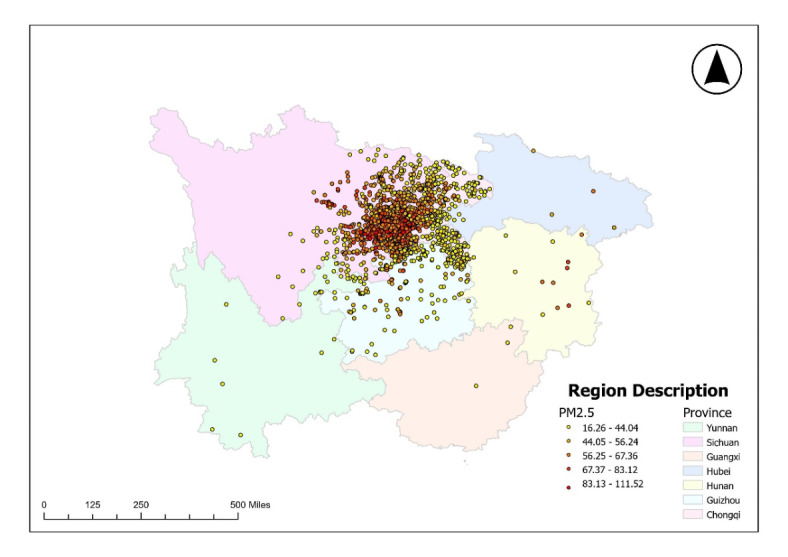
Geographical distribution of registered communities and monthly average PM2.5 concentrations.
Table 1.
Demographic characteristics of enrolled male children
| Cases | Controls | |
|---|---|---|
| M (Q₁, Q₃), N (%) | M (Q₁, Q₃), N (%) | |
| Neonatal characteristics | ||
| Birth Weight, g | 3200.00(2900.00,3505.00) | 3300.00(3000.00,3600.00)* |
| Gestational age, week | 38.14(38.00,39.14) | 38.00(38.00,39.00)* |
| Premature | 245(11.46) | 99(4.63)* |
| Low birth weight (< 2500 g) | 204(9.54) | 66(3.08)* |
| Maternal characteristics | ||
| Multiple pregnancy | 18(0.84) | 25(1.17) |
| Drug use | 118(5.52) | 16(0.75)* |
| Complication | 71(3.32) | 34(1.59)* |
| Current address | ||
| Within Chongqing | 1554(72.72) | 1527(71.46) |
| Outside Chongqing | 583(27.28) | 610(28.54) |
| Birth year | ||
| 2013 | 447(20.92) | 479(22.41)* |
| 2014 | 307(14.36) | 485(22.70) |
| 2015 | 435(20.36) | 422(19.75) |
| 2016 | 499(23.35) | 421(19.70) |
| 2017 | 449(21.01) | 330(15.44) |
| Season of conception | ||
| Spring | 422(19.74) | 520(24.33) * |
| Summer | 550(25.74) | 555(25.97) |
| Autumn | 600(28.08) | 535(25.04) |
| Winter | 565(26.44) | 527(24.66) |
| Temperature | ||
| Pre-conception, | ||
| 0–1 month | 17.96(10.60, 22.84) | 19.19(11.14, 23.91) * |
| 1–2 month | 17.96(10.54, 23.22) | 19.02(11.23, 23.86) * |
| 2–3 month | 18.29(10.48, 23.46) | 19.28(11.14, 23.91) * |
| 0–3 month | 16.97(11.38, 23.90) | 18.42(11.69, 23.90) * |
| Post-conception, | ||
| 0–1 month | 18.10(10.70, 23.23) | 19.42(10.91, 23.89) * |
| 1–2 month | 19.15(10.85, 23.64) | 19.41(11.23, 24.02) |
| 2–3 month | 19.75(11.49, 24.04) | 19.53(11.44, 24.04) |
| 0–3 month | 18.34(11.54, 23.79) | 18.61(11.77, 24.17) |
M, median; N, number; Q1, 1st quartile; Q3, 3rd quartile; Drug use: whether the mother used tocolytic drugs or other hormone-containing drugs during pregnancy; Complication: whether there were complications including diabetes, hypertension and thyroid disorders during pregnancy. The Mann–Whitney U test was utilized for analyzing continuous variables, while the chi-square test was employed for analyzing categorical variables. Bonferroni’s method was used to correct for multiple comparisons.
*P < 0.05 was considered significant.
Air pollution characteristics
Table 2 lists the distribution of PM2.5 exposure in all study children during the 3 months before and after conception. During the 3 months before pregnancy, the median concentration (Q1, Q3) of PM2.5 was 60.52 (45.36, 80.60) μg/m3. During the 3 months after conception, the median concentration (Q1, Q3) of PM2.5 was 58.90 (44.67, 79.70) μg/m3. By comparison, it can be found that the concentration of PM2.5 in the 3 months after conception was lower than that in the 3 months before conception.
Table 2.
Exposure levels to air pollutants during the first three pre and post-conceptional months for the enrolled infants.
| PM2.5(μg/m3) | Mean (SD) | Median | 25tile | IQR | 75tile | Range |
|---|---|---|---|---|---|---|
| Pre-conception 0–3 month | 65.02(26.36) | 60.52 | 45.36 | 35.24 | 80.60 | 159.83 |
| Post-conception 0–3 month | 63.74(26.08) | 58.90 | 44.67 | 35.03 | 79.70 | 180.93 |
SD, standard deviation; IQR, interquartile range.
Associations between PM2.5 and the incidence of cryptorchidism
Table 3 showcases the outcomes of simple logistic regression models, analyzing the monthly associations between prenatal exposure to air pollutants and the risk of cryptorchidism. Compared with other exposure periods, the risk of cryptorchidism patients exposed to PM2.5 in the first and second months after conception increased (OR 1.024, 95% CI 1.002–1.047; OR 1.027, 95% CI 1.004–1.050).
Table 3.
Simple logistic regression models of association between pre and post-conceptional exposure to PM2.5 and risk of cryptorchidism among male children.
| PM2.5(10 μg/m3) | ||
|---|---|---|
| Crude OR (95% CI) | P | |
| Pre-conception, | ||
| 0–1 month | 1.02(0.99,1.04) | 0.06 |
| 1–2 month | 1.01(0.99,1.03) | 0.41 |
| 2–3 month | 1.00(0.98,1.02) | 0.75 |
| 0–3 month | 1.01(0.99,1.04) | 0.37 |
| Post-conception, | ||
| 0–1 month | 1.02(1.00,1.05) | 0.03 |
| 1–2 month | 1.03(1.00,1.05) | 0.02 |
| 2–3 month | 1.09(0.99,1.03) | 0.47 |
| 0–3 month | 1.03(1.00,1.05) | < 0.05 |
The results of the multivariable logistic regression model are presented in Table 4. After adjusting for confounding factors, which include low birth weight, preterm, drug use during pregnancy, complications during pregnancy, birth year, season of conception and temperature, there remained a strong positive correlation between PM2.5 and cryptorchidism, specifically in the first and second months after conception. The correlation coefficients for PM2.5 were 1.11 (95% CI 1.06, 1.15) and 1.07 (95% CI 1.03, 1.12), respectively. Compared to the results of univariate regression, both the OR value and 95% CI fluctuated. The model coefficients have been provided in Appendix 1.
Table 4.
Multivariable logistic regression models of association between post-conceptional exposure to air pollutants and risk of cryptorchidism among male infants.
| PM2.5(10 μg/m3) | ||
|---|---|---|
| Adjusted OR (95% CI) a | P | |
| Post-conception, | ||
| 0–1 month | 1.11(1.06,1.15) | < 0.01 |
| 1–2 month | 1.07(1.03,1.12) | < 0.01 |
aAdjusted for low birth weight, preterm delivery, drug use during pregnancy, complications during pregnancy, birth year, season of conception and temperature.
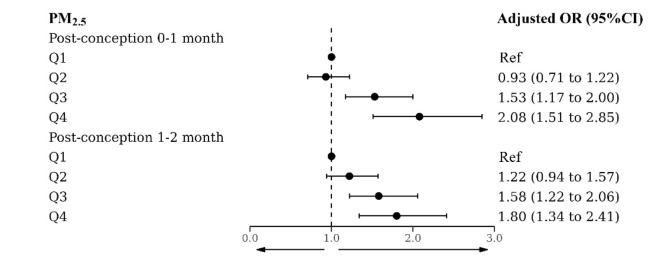
We further investigated the relationship between the quartiles of PM2.5 exposure during specific periods and the risk of cryptorchidism, as shown in Fig. 2. The risk of cryptorchidism corresponding to the third and fourth quartiles of PM2.5 was significantly elevated compared to the lowest value during the first and second months after conception, suggesting that the association between PM2.5 and the risk of cryptorchidism may be non-linear.
Fig. 2.
The regression coefficients associated with the risk of cryptorchidism and the quartiles of PM2.5 exposure. aAdjusted for low birth weight, preterm, use of drugs during pregnancy, complications during pregnancy, birth year, season of conception and temperature.
Results of sensitivity analyses
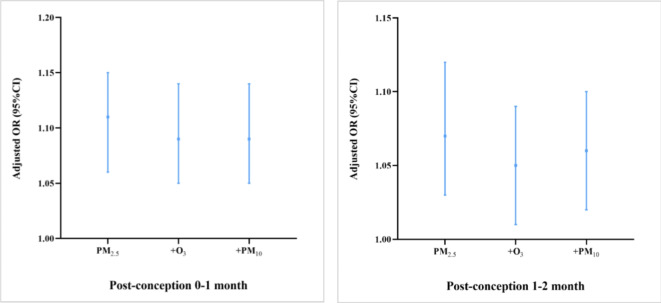
PM2.5 is negatively correlated with O3 (ρ = − 0.349) and positively correlated with PM10 (ρ = 0.774). Figure 3 demonstrates the extent of changes in the odds ratios and 95% confidence intervals corresponding to PM2.5 after incorporating other pollutants. The results indicate that the association between PM2.5 and cryptorchidism remains statistically significant even when O3 and PM10 are included in the model, albeit with fluctuations in OR values and 95% CIs. Therefore, the model is robust.
Fig. 3.
Odds Ratio and 95% CI for PM2.5 in two-pollutant models. aAdjusted for low birth weight, preterm, use of drugs during pregnancy, complications during pregnancy, birth year, season of conception and temperature.
Discussion
There are few previous studies on the relationship between PM2.5 and cryptorchidism. Our case–control investigation represents the first attempt to understand the relationship between PM2.5 exposure and cryptorchidism. Here, we enrolled 4274 children over a period of five years. Our findings revealed that there was a positive association between exposure to PM2.5 and the development of cryptorchidism during the first 2 months of pregnancy. Similar results were not found for the rest of the exposure period.
The descent of the testicles occurs in two hormonally controlled stages: the first stage involves descent from the abdominal cavity to the internal inguinal ring (between the 8th and 15th weeks of gestation), and the second stage involves descent from the inguinal canal to the scrotum (between the 25th and 35th weeks of gestation)22–24. Any form of interference during these stages can lead to incomplete descent of the testicles. Many scholars have reported on the mechanisms by which air pollution affects fetal growth and development. This may occur through the following mechanisms. First, the descent of the testicles is primarily regulated by insulin-like peptide 3 (INSL3) and testosterone25,26. INSL3, produced by Leydig cells, is involved in the first stage of testicular descent by acting on the testicular band to thicken it and is thought to be the first promoter of testicular descent27. Testosterone plays a crucial role in normal urogenital development by activating INSL3 expression in the Leydig cell lineage. By interacting with INSL3, testosterone participates in the actual development and functioning of the testis, as well as regulates growth, metabolism, and reproductive processes28. Animal experiments have shown that PM2.5 can induce iron death in mouse Leydig cells by upregulating FDX1, leading to increased cellular ROS levels and iron overload29 or causing autophagy dysfunction in Leydig cells, resulting in decreased testosterone levels30. Some scholars have also reported that long-term exposure to PM2.5 significantly reduces the levels of testosterone biosynthesis-related genes, such as P450scc, P450arom, StAR, ER, and FSHR, as well as the serum testosterone level31,32. Additionally, inorganic nitrate (NO3−), a component of PM2.5, on the other hand, can be converted to NO, which inhibits steroid synthesis and thus affects androgen production33. Second, exposure to estrogen has been suggested to downregulate the expression of INSL3 in mice34. Particulate matter can adsorb various organic matter (OM), such as polycyclic aromatic hydrocarbons and heavy metals, to form endocrine disruptors with anti-androgen activity35,36, thereby affecting the descent of the testicles. Third, PM2.5 can lead to preterm birth or low birth weight by causing inflammation, oxidative stress, hypercoagulability (resulting in reduced uterine blood flow), decreased DNA methylation, and reduced thyroid hormone levels6,37–42. The relationships between cryptorchidism and both preterm birth and low birth weight have been confirmed14–16. Alternatively, due to shared underlying risk factors, preterm birth and low birth weight should be considered surrogate indicators related to the risk of cryptorchidism24. In addition, PM2.5 can also interfere with placenta function, which is critical for harmonizing the hormonal climate, especially in the first trimester6,43.
We assessed the time period during which exposure to air pollutants has an impact on fetal development by discussing the risk of monthly exposure. The male programming window (MPW) represents a crucial phase for male development, occurring primarily during the 7th to 14th weeks of gestation44. Sharpe’s research revealed that reducing androgen exposure in rats during the MPW resulted in the emergence of a spectrum of reproductive disorders resembling testicular dysgenesis syndrome, either at birth or in adulthood45. Given that PM2.5 possesses anti-androgenic properties, exposure to PM2.5 during the MPW may increase the risk of birth defects. However, our study revealed no significant association between PM2.5 exposure in the third month of pregnancy and the risk of cryptorchidism after adjusting for confounders. Taken together, our analysis hints that increased exposure to pollutants during the two months following conception could be potentially harmful.
This study has several limitations. First, meteorological factors and geographic features were not fully taken into account owing to data limitations. Second, due to the large amount of data and the enormous amount of work involved if telephone follow-ups were to be conducted, information on the mother’s gestational age and smoking history during pregnancy, which would need to be obtained through telephone follow-ups, was not included in this paper. In addition, the calculation of pollutant concentrations was not corrected. For instance, due to geographical differences, the same concentration of air pollutants may have different compositions, and factors such as the presence of indoor air purifiers or the wearing of masks when going out may affect the concentration of PM2.5 inhaled by the human body, leading to significant differences in exposure levels21. Furthermore, this study was designed to ensure an adequate number of cases, so any child whose hospitalized diagnosis included cryptorchidism was included in the case group. Finally, the statistical model applied in this study fails to account for time lags.
In summary, our study provides further evidence of the possible connection between prenatal PM2.5 exposure and the likelihood of specific birth anomalies. Despite the significant statistical correlation between air pollutants and cryptorchidism revealed by regression analysis, it is still necessary to conduct large-scale epidemiological studies and design better research programs to further verify the causal relationship between PM2.5 and cryptorchidism, given the variations in exposure estimation, duration of exposure, geographical location, and case diagnosis methods. Additionally, future research should delve deeper into the potential biological mechanisms and comprehensively consider other potential confounding factors such as meteorological factors, in order to gain a more accurate understanding of this association.
Conclusions
Our research supports the hypothesis that the occurrence of cryptorchidism may be linked to maternal exposure to PM2.5 during pregnancy. The theoretical underpinnings for preventing cryptorchidism and understanding its disease process are provided by this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Our sincere gratitude goes to Tracking Air Pollution in China for providing the necessary data. The patients involved in this study were recruited from the Children’s Hospital in Chongqing, China. We thank American Journal Experts (AJE) for their assistance in revising our paper for language, as well as the Extreme Smart Analysis platform (https://www.xsmartanalysis.com/) for its help in the statistical analysis. Additionally, the authors sincerely acknowledge the anonymous reviewers for their insights and comments, which helped improve the quality of the manuscript.
Author contributions
YL: Conceptualization, Methodology, Data curation, Interpretation of the results, Writing—original draft, Writing—review & editing. Yin-lin Chen: Provision of pollutant data. CJY: Writing—review & editing. RH, LC, MLL, MS and ZYZ: Data collection and cleaning. QW and XMX: Guidelines on Statistical Methods. SDW: Conceptualization, Methodology, Supervision, Writing—review & editing.
Funding
The National Natural Science Foundation of China (Grant No. 82071632), Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau) (2022ZDXM033), the Senior Medical Talents for Young and Middle-Aged, the Youth Innovation in Future Medicine Program of Chongqing Medical University (W0069) have all contributed financial support to this study.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participants
The Institutional Review Board of the Children’s Hospital of Chongqing Medical University granted approval for the study procedure.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leslie, S. W., Sajjad, H. & Villanueva, C. A. Cryptorchidism. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Hussain Sajjad declares no relevant financial relationships with ineligible companies. Disclosure: Carlos Villanueva declares no relevant financial relationships with ineligible companies, (StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC. 2024).
- 2.Chedrawe, E. R., Keefe, D. T. & Romao, R. L. P. Diagnosis, classification, and contemporary management of undescended testicles. Urol. Clinics N. Am.50(3), 477–490. 10.1016/j.ucl.2023.04.011 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Li, W. et al. Prevalence of congenital cryptorchidism in China: A nationwide population-based surveillance study, 2007–2021. Andrology10.1111/andr.13686 (2024). [DOI] [PubMed] [Google Scholar]
- 4.Pathak, A. et al. Prospectively identified incident testicular cancer risk in a familial testicular cancer cohort. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol.24(10), 1614–1621. 10.1158/1055-9965.Epi-14-1240 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, R. et al. Higher prevalence of benign tumors in men with testicular tumors and history of treated cryptorchidism. Urol. Oncol.42(2), 33.e1-33.e6. 10.1016/j.urolonc.2023.11.016 (2024). [DOI] [PubMed] [Google Scholar]
- 6.Liu, C. et al. The association between maternal exposure to ambient particulate matter of 2.5 μm or less during pregnancy and fetal congenital anomalies in Yinchuan, China: A population-based cohort study. Environ. Int.122, 316–321. 10.1016/j.envint.2018.11.030 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Ahn, T. G. et al. Association between individual air pollution (PM(10), PM(2.5)) exposure and adverse pregnancy outcomes in Korea: A multicenter prospective cohort, air pollution on pregnancy outcome (APPO) study. J. Korean Med. Sci.39(13), e131. 10.3346/jkms.2024.39.e131 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qingqing, L. et al. The Spatial-temporal Characteristics of PM2.5 Cencentrations in Chinese Cities anad the Influencing Factors. J. Resour. Ecol.14(03), 433–44 (2021). [Google Scholar]
- 9.Ren, S. et al. Periconception exposure to air pollution and risk of congenital malformations. J. Pediatr.193, 76–84. 10.1016/j.jpeds.2017.09.076 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian, Y. et al. Acute effects of exposure to fine particulate matter and its constituents on sex hormone among postmenopausal women—Beijing, Tianjin, and Hebei PLADs China, 2018–2019. China CDC Weekly6(13), 249–53. 10.46234/ccdcw2024.049 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radwan, M. et al. Exposure to ambient air pollution–does it affect semen quality and the level of reproductive hormones?. Ann. Hum. Biol.43(1), 50–56. 10.3109/03014460.2015.1013986 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Hao, H. et al. Air pollution and preterm birth in the U.S. State of Georgia (2002–2006): Associations with concentrations of 11 ambient air pollutants estimated by combining community multiscale air quality model (CMAQ) simulations with stationary monitor measurements. Environ. Health Perspect.124(6), 875–80. 10.1289/ehp.1409651 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh, R. et al. Ambient and household PM2.5 pollution and adverse perinatal outcomes: A meta-regression and analysis of attributable global burden for 204 countries and territories. PLoS Med.18(9), e1003718. 10.1371/journal.pmed.1003718 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igata, Y. et al. Low placental weight may be involved in the etiology of congenital cryptorchidism in neonatal boys. European J. Obstet. Gynecol. Reprod. Biol.289, 136–139. 10.1016/j.ejogrb.2023.08.378 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Kübarsepp, V. et al. Prevalence of congenital cryptorchidism in Estonia. Andrology10(2), 303–309. 10.1111/andr.13121 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Sijstermans, K. et al. The frequency of undescended testis from birth to adulthood: A review. Int. J. Androl.31(1), 1–11. 10.1111/j.1365-2605.2007.00770.x (2008). [DOI] [PubMed] [Google Scholar]
- 17.Correa, C. et al. Congenital malformations of pediatric surgical interest: prevalence, risk factors, and prenatal diagnosis between 2005 and 2012 in the capital city of a developing country Bogotá, Colombia. J. Pediatric Surg.49(7), 1099–103. 10.1016/j.jpedsurg.2014.03.001 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Geng, G. et al. Tracking air pollution in China: near real-time PM(2.5) retrievals from multisource data fusion. Environ. Sci. Technol.55(17), 12106–15. 10.1021/acs.est.1c01863 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Geng, G. et al. Chemical composition of ambient PM2.5 over China and relationship to precursor emissions during 2005–2012. Atmos. Chem. Phys.17(14), 9187–203. 10.5194/acp-17-9187-2017 (2017). [Google Scholar]
- 20.Hersbach, H., Bell, B., Berrisford, P., et al. ERA5 hourly data on single levels from 1940 to present. Copernicus Climate Change Service (C3S) Climate Data Store (CDS). Accessed on 07 Sept, 2024; 10.24381/cds.adbb2d47 (2023).
- 21.He, J. et al. Association of long-term exposure to PM(2.5) in workplace with fasting plasma glucose among asymptomatic adults: A multicenter study in North China. Environ. Int.166, 107353. 10.1016/j.envint.2022.107353 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Gurney, J. K. et al. Risk factors for cryptorchidism. Nat. Rev. Urol.14(9), 534–548. 10.1038/nrurol.2017.90 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niedzielski, J. K., Oszukowska, E. & Słowikowska-Hilczer, J. Undescended testis—Current trends and guidelines: A review of the literature. Arch. Med. Sci. AMS12(3), 667–677. 10.5114/aoms.2016.59940 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holmboe, S. A. et al. The epidemiology of cryptorchidism and potential risk factors, including endocrine disrupting chemicals. Front. Endocrinol.15, 1343887. 10.3389/fendo.2024.1343887 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skakkebaek, N. E. et al. Male reproductive disorders and fertility trends: Influences of environment and genetic susceptibility. Physiol. Rev.96(1), 55–97. 10.1152/physrev.00017.2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rey, R. A. & Grinspon, R. P. Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract. Res. Clin. Endocrinol. Metab.25(2), 221–238. 10.1016/j.beem.2010.08.013 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Bay, K. et al. Testicular descent: INSL3, testosterone, genes and the intrauterine milieu. Nat. Rev. Urol.8(4), 187–196. 10.1038/nrurol.2011.23 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Lăptoiu, A. R. et al. New insights into the role of INSL-3 in the development of cryptorchidism. Children10.3390/children10040737 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, L. et al. FDX1 regulates leydig cell ferroptosis mediates PM(25)-induced testicular dysfunction of mice. Ecotoxicol. Environ. Saf.263, 115309. 10.1016/j.ecoenv.2023.115309 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Jiang, L. et al. METTL3-m6A-SIRT1 axis affects autophagic flux contributing to PM(2.5)-induced inhibition of testosterone production in Leydig cells. Sci. Total Environ.918, 170701. 10.1016/j.scitotenv.2024.170701 (2024). [DOI] [PubMed] [Google Scholar]
- 31.Yang, Y. et al. Concentrated ambient PM(2.5) exposure affects mice sperm quality and testosterone biosynthesis. PeerJ7, e8109. 10.7717/peerj.8109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fengquan, Z. et al. Effects of PM2.5 on reproductive hormone levels and pregnancy outcome in female rats. Acta Lab. Anim. Sci. Sinica25(4), 455–60 (2017). [Google Scholar]
- 33.Panesar, N. S. Role of chloride and inhibitory action of inorganic nitrate on gonadotropin-stimulated steroidogenesis in mouse Leydig tumor cells. Metab. Clin. Exp.48(6), 693–700. 10.1016/s0026-0495(99)90167-1 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Toppari, J. et al. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res. Part A Clin. Mol. Teratol.88(10), 910–919. 10.1002/bdra.20707 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Gea, M. et al. Can oestrogenic activity in air contribute to the overall body burden of endocrine disruptors?. Environ. Toxicol. Pharmacol.102, 104232. 10.1016/j.etap.2023.104232 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Zhou, Q. et al. Toxicity and endocrine-disrupting potential of PM(2.5): Association with particulate polycyclic aromatic hydrocarbons, phthalate esters, and heavy metals. Environ. Pollut.292(1), 118349. 10.1016/j.envpol.2021.118349 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Fleischer, N. L. et al. Outdoor air pollution, preterm birth, and low birth weight: Analysis of the world health organization global survey on maternal and perinatal health. Environ. Health Perspect.122(4), 425–430. 10.1289/ehp.1306837 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritz, B. et al. Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. Am. J. Epidemiol.166(9), 1045–1052. 10.1093/aje/kwm181 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Zhou, L. et al. PM2.5 exposure impairs sperm quality through testicular damage dependent on NALP3 inflammasome and miR-183/96/182 cluster targeting FOXO1 in mouse. Ecotoxicol. Environ. Saf.169, 551–63. 10.1016/j.ecoenv.2018.10.108 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Liu, Y. et al. Effect of fine particulate matter (PM2.5) on rat placenta pathology and perinatal outcomes. Med. Sci. Monit. Int. Med. J. Exp. Clini. Res.22, 3274–3280. 10.12659/msm.897808 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omolaoye, T. S. et al. Implications of exposure to air pollution on male reproduction: The role of oxidative stress. Antioxidants10.3390/antiox13010064 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinle, S. et al. In utero exposure to particulate air pollution during pregnancy: Impact on birth weight and health through the life course. Int. J. Environ. Res. Public Health10.3390/ijerph17238948 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalfa, N. et al. Hypospadias: Interactions between environment and genetics. Mol. Cell. Endocrinol.335(2), 89–95. 10.1016/j.mce.2011.01.006 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Lundgaard Riis, M. et al. Identification of a window of androgen sensitivity for somatic cell function in human fetal testis cultured ex vivo. BMC Med.20(1), 399. 10.1186/s12916-022-02602-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharpe, R. M. Androgens and the masculinization programming window: Human-rodent differences. Biochem. Soc. Trans.48(4), 1725–1735. 10.1042/bst20200200 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.