Abstract
Purpose
To investigate the timing of enucleation, treatment course, and outcome for retinoblastoma (RB) with optic nerve (ON) invasion on imaging.
Study design
Retrospective clinical study.
Methods
Of the 160 patients with RB who presented to the National Center for Child Health and Development in Japan between 2005 and 2022, ON invasion on imaging at the initial presentation was seen in five patients. The clinical, computed tomography (CT), and magnetic resonance imaging (MRI) findings, and treatment courses were reviewed retrospectively.
Results
MRI showed ON invasion in all five patients (three with unilateral RB, 2 with bilateral RB); in two patients CT detected no invasion. Enucleation was performed in four patients, three of whom underwent neoadjuvant therapy and one had a positive ON resection margin following the enucleation as initial treatment. One patient did not undergo enucleation due to cerebrospinal fluid dissemination. All enucleated patients underwent adjuvant chemotherapy. Four patients underwent radiotherapy. During follow-up (mean, 89.4 months), four patients survived and one died.
Conclusion
MRI is recommended to evaluate ON invasion and determine the timing of enucleation for RB. The appropriate choice of neoadjuvant or adjuvant therapy would be helpful to avoid radiotherapy for RB with ON invasion on imaging.
Keywords: Retinoblastoma, Optic nerve invasion, Enucleation, Magnetic resonance imaging, Neoadjuvant chemotherapy
Introduction
Retinoblastoma (RB) is the most common ocular malignant tumor in children [1]. Extraocular extension, especially optic nerve (ON) invasion is the worst factor affecting survival [2]. Although due to alternative therapy for less advanced RB enucleation is now performed less frequently [3], it remains an important option for advanced RB. The additions of neoadjuvant chemotherapy and radiotherapy to the standard treatment for RB with extraocular invasion is not determined [4]. The survival rates for RB with extraocular invasion ranges from 40 to 80% [5–9]. We investigated the timing of enucleation and course of treatment for RB with ON invasion in five patients.
Materials and methods
The ethics committee of the National Center for Child Health and Development (NCCHD) approved this study (permit no. 2022-017). The legal guardians of the five patients and one patient over 18 years old provided informed consent. The medical records of patients diagnosed at the NCCHD between 2005 and 2022 for RB with ON invasion were reviewed retrospectively. The inclusion criteria were the presence of ON invasion based on computed tomography (CT) and/or magnetic resonance imaging (MRI). The data collected included age, sex, B-mode ultrasonography, CT and MRI findings, pathological findings, and treatment courses. Between 2005 and 2022, 160 patients with RB were diagnosed at the NCCHD. Five patients (three boys, two girls) met the inclusion criteria. The mean age at diagnosis was 15.8 months (range, 3–22) and the mean follow-up was 89.4 months (range, 11–209).
Results
Clinical ophthalmologic and radiologic features
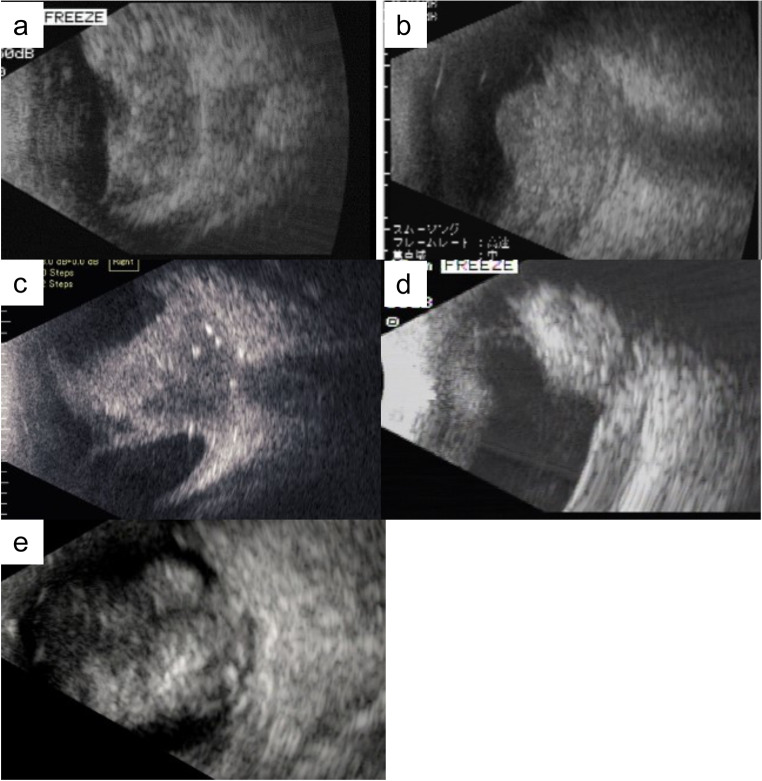
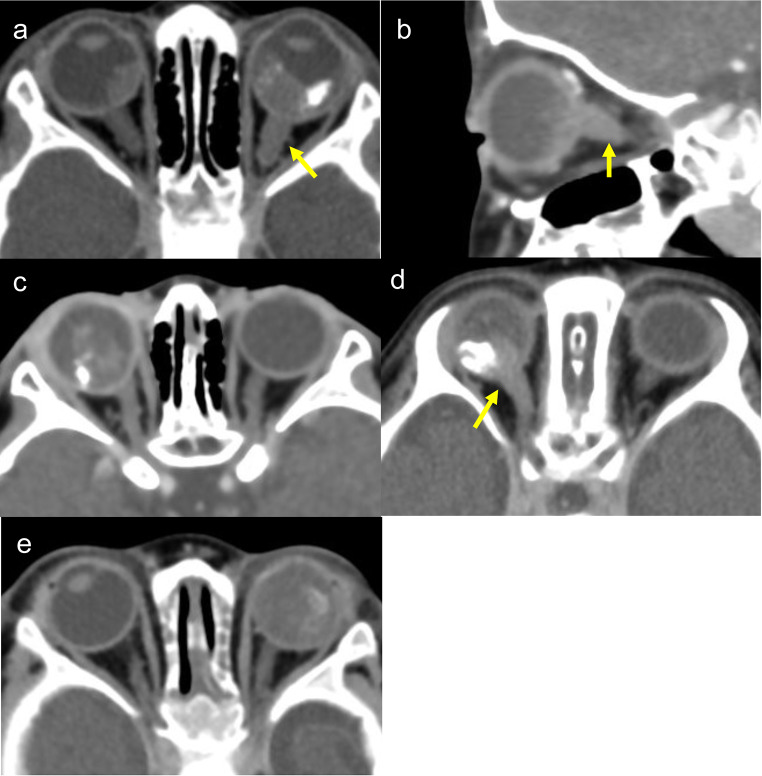
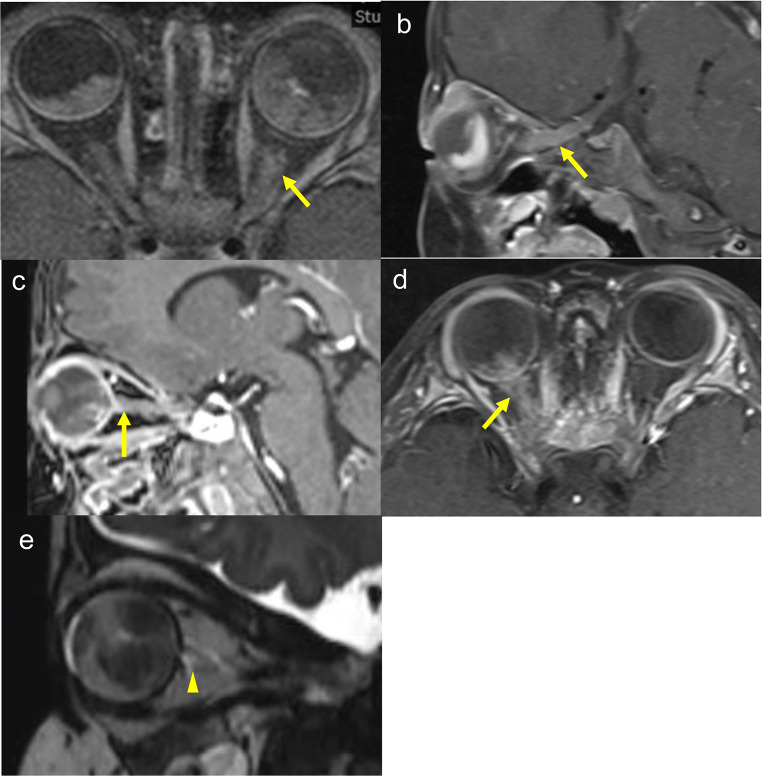
Three patients presented with the chief complaint of leukocoria and two patients with eyelid swelling. Two patients had bilateral RB and three patients had unilateral RB. B-mode ultrasonography of all patients showed solid tumor masses, but there was no obvious invasion of the ON (Fig. 1). All five patients underwent whole-body CT and head MRI imaging as the initial radiologic investigation at the NCCHD before treatment. CT did not detect ON invasion in two patients, whereas MRI showed ON invasion in all patients (Figs. 2 and 3). Cerebrospinal fluid (CSF) cytology was performed for all patients before or during treatment. Bone marrow biopsy was done for four patients (cases 2–5) before treatment. Case 2 showed CSF positivity before treatment, and case 4 showed CSF positivity after enucleation. The bone marrow biopsies were all negative.
Fig. 1.
B-mode ultrasonograms of cases 1–5 (a-e). A solid tumor mass with highly reflective foci is seen in each case, but no obvious invasion of the optic nerve (ON) is detected in any case
Fig. 2.
Contrast-enhanced computed tomography (CT) images of Cases 1–5 (a-e). ON swelling and contrast enhancement indicating ON invasion (arrows) are seen. There is no evidence of ON invasion (c, e)
Fig. 3.
Contrast magnetic resonance imaging (MRI) images of case 1–4 (a-d) and MRI SPACE image of case 5 (e). ON contrast enhancement shows ON invasion (arrows). An enlarged origin of the left ON with disruption of the surrounding subarachnoid space indicates ON invasion (arrowhead)
The pre-treatment findings in the five cases are summarized in Table 1. The specific clinical courses and radiologic features of the five cases are provided below.
Table 1.
Pre-treatment findings
| Case | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age at diagnosis (months) | 18 | 15 | 21 | 22 | 3 |
| Affected side | R/L | L | R | R | R/L |
| ICRB group (R/L) | D/E | E | E | E | A/E |
| Eyelid swelling (R/L) | −/− | −/+ | +/− | −/− | −/− |
| ON invasion (CT) (R/L) | −/+ | −/+ | −/− | +/− | −/− |
| ON invasion (MRI) (R/L) | −/+ | −/+ | +/− | +/− | −/+ |
| Length and place of invasion on MRI |
13 mm, in front of optic canal |
31 mm, in front of optic chiasm |
3 mm, anterior end of ON |
19 mm, in front of optic canal |
5 mm, anterior end of ON |
| Metastasis (bone marrow) | NA | − | − | − | − |
| Metastasis (CSF) | − | + | − | − | − |
| TNM classification | T4aN0M0 | T4aN0M1b | T4aN0M0 | T4aN0M0 | T4aN0M0 |
R right eye; L left eye; TNM tumor-node-metastasis; ON optic nerve; ICRB International Classification for Retinoblastoma; NA not applicable
Case 1
An 18-month-old female infant with leukocoria OS was referred to our center with suspected bilateral RB. At the initial examination, anisocoria was observed and the left eye showed mydriasis and leukocoria. Ophthalmoscopy showed that the RB extended from the inferior to nasal retina, but the macula was not involved in the right eye. In the left eye, the RB extended throughout the fundus. B-mode ultrasonography showed a large solid mass with highly reflective foci OS (Fig. 1a) and a small mass OD. There was no obvious ON invasion on ultrasonography. The right eye was classified in Group D and the left eye in Group E of the International Classification of Retinoblastoma (ICRB) [10]. CSF cytology was negative before treatment. A bone marrow biopsy was not performed in this case.
Brain CT showed an intraocular tumor with bilateral calcification. The left ON was thicker than the right and showed contrast enhancement (Fig. 2a). On MRI, the left ON showed contrast enhancement and swelling, and the RB infiltrated the ON by 13 mm, which is in front of the optic canal on imaging. There was no obvious invasion in the right ON (Fig. 3a).
Case 2
A 15-month-old male infant with swelling of the left upper eyelid was referred to the NCCHD with suspected RB OS. At the initial examination, the left eye showed leukocoria. The fundus of the left eye was not visible. The right eye was normal. B-mode ultrasonography showed a large solid mass OS (Fig. 1b). The ON invasion was not obvious on ultrasonography.
CT showed an intraocular tumor OS. The left ON was obviously thicker than the right and showed contrast enhancement that continued intracranially (Fig. 2b). MRI showed the contrast effect in the left ON with 31 mm of infiltration to just in front of the intracranial optic chiasm (Fig. 3b). A biopsy performed to rule out an ON glioma and hamartoma identified RB. We performed lensectomy and vitrectomy using the limbal approach. Specimens were obtained from the tumor mass. Considering the possibility of surgical dissemination, the patient underwent transcorneal surgery without vascular structures. The left eye was classified in Group E of the ICRB. CSF cytology was positive before treatment and a bone marrow biopsy was negative.
Case 3
A 21-month-old female infant with eyelid swelling of her right upper eyelid was referred to our center. The anterior segment OD showed inflammation, iris rubeosis, and synechia. During the fundus examination, the right eye could not be evaluated because of vitreous opacity and the left eye was normal. B-mode ultrasonography showed a solid mass with highly reflective foci OD (Fig. 1c). No ON invasion was obvious on B-mode ultrasonography. The right eye was classified in Group E of the ICRB. CSF cytology and bone marrow biopsy were negative before treatment.
On CT, an intraocular tumor with contrast enhancement and calcification was seen OD. The right ON has no contrast enhancement or swelling (Fig. 2c). MRI showed contrast enhancement of the right ON and posterior wall of the right eye, with infiltration to 3 mm of the anterior end of the ON (Fig. 3c).
Case 4
A 22-month-old male infant with leukocoria OD was referred to our center. The examination showed corneal edema and iris rubeosis OD. The fundus of the right eye could not be visualized. The fundus of the left eye was normal. B-mode ultrasonography showed a mass with a highly reflective area OD (Fig. 1d). No ON invasion was obvious on B-mode ultrasonography. The right eye was classified in Group E of the ICRB. CSF cytology was negative initially and became positive after enucleation. A bone marrow biopsy was negative.
CT showed an intraocular tumor with contrast enhancement and calcification OD. The right ON was thicker than the left and showed a contrast effect (Fig. 2d). MRI showed contrast enhancement of the right ON with infiltration of 19 mm in front of the optic canal in the orbit (Fig. 3d).
Case 5
A 3-month-old male infant with leukocoria OS was referred to our center. At the examination, the tumor touched the posterior lens capsule. Fundus examination showed RBs on both fundi. Small tumors were scattered through the right fundus, but the macula was intact. The left fundus was covered by tumor. Fluorescein angiography showed five small tumors on the right fundus. B-mode ultrasonography showed a large mass with a highly reflective area OS (Fig. 1e), but it could not detect a tumor mass OD. The right eye was classified in Group A and the left eye in Group E of the ICRB. CSF cytology and bone marrow biopsy were negative before treatment.
CT showed an intraocular tumor with calcification OS, but no obvious tumor was detected OD. A contrast effect and swelling were not detected in either ON (Fig. 2e). An MRI sampling perfection with application-optimized contrasts using different flip angle evolutions (SPACE) image showed that the origin of the left ON was enlarged with disruption of the surrounding subarachnoid space. RB infiltration of 5 mm into the anterior end of the left ON was observed (Fig. 3e).
Primary treatment
Basically, we try to perform neoadjuvant chemotherapy in cases with ON invasion and then perform secondary enucleation when MRI no longer shows ON invasion. However, case 1 had presented before this strategy was established and was preceded by enucleation. Four patients (cases 2–5) underwent chemotherapy as the primary treatment. Three of four patients (cases 3–5), who primarily underwent chemotherapy ultimately underwent enucleation, while one patient (case 2) could not undergo enucleation due to consistent ON invasion seen on MRI, positive results of CSF, and progression of liver metastasis. Case 5 with bilateral RB underwent photocoagulation for small tumors of the right fundus; enucleation was not planned for this eye.
The chemotherapy regimens differed in each case. At NCCHD, the regimens for RB with ON invasion are basically the same as those for medulloblastoma [11–13]. Intrathecal injection (IT) also was basically used according to these regimens, but in some cases, IT was performed at the same time as the CSF examination to determine the efficacy of treatment for positive CSF status. The treatments of the five cases are summarized in Tables 2 and 3.
Table 2.
Treatment of 5 cases
| Case | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Primary treatment | Enucleation | Chemotherapy | Chemotherapy | Chemotherapy | Chemotherapy |
| Timing of enucleation | 1 week after diagnosis | NA | After 2 courses of chemotherapy | After 4 courses of chemotherapy | After 3 courses of chemotherapy |
| Method of enucleation | Anterior approach | NA | Anterior approach | Anterior approach | Double approach |
| Surgical margin | Positive | NA | Negative | Negative | Negative |
| Pathological findings | Tumor cells in epidural tissue | Small cluster formed by small round cells | Tumor cells in ON and choroid |
No viable tumor cells in ON, CSF (+) |
No viable tumor cells in ON |
| Transplant | Auto-BMT | Auto-PBSCT | No | auto PBSCT | No |
| Radiotherapy |
EBRT Right eye Left orbit 40 Gy |
EBRT Brain Spinal cord Liver Left eye 45 Gy |
EBRT Right orbit Optic chiasm 45 Gy |
EBRT Brain Spinal cord Right orbit 45 Gy |
No |
| Follow-up (months) | 209 | 11 (death) | 116 | 98 | 13 |
Auto-BMT autologous bone marrow transplant; ON optic nerve; auto-PBSCT autologous peripheral blood stem cell transplantation; EBRT external beam radiotherapy; CSF cerebrospinal fluid; NA not applicable
Table 3.
Chemotherapy
| Case | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Neoadjuvant chemotherapy | NA |
ICE + VCR + IT*5 VCR + IT*6 VCR + CY + CDDP + ETP + IT*7 VEC + IT*8 2×VCR*9 VCR + CY + CDDP + ETP*10 2×IT*11 VEC*12 IT*11 IT*13 3×Topo + CBDCA*14 |
2×JPBTC MB/PNET regimen*15 |
4×VEC*16 3×IT*17 |
3×VEC*16 VCR*9 IT*22 2×IT*23 |
| Adjuvant chemotherapy regimen |
VCR + CY + CDDP + ETP + IT*1 VCR + CY + CDDP*2 VCR*3 VCR + CY + CDDP + ETP + IT*1 VCR + CY + CDDP*2 |
NA | 4×VEC*16 |
VEC*16 6×IT*18 Packer regimen *19 4×JPBTC MB/PNET regimen*20 |
3× ICE*24 3×IT*23 |
| High-dose chemotherapy | TEPA + MEL*4 | No | No | TEPA + MEL*21 | No |
*1. VCR 1.5 mg/m2 D1 + CY 1 g/m2 D1, 3, 5 + CDDP 90 mg/m2 D2 + ETP 100 mg/m2 D1-5 + IT (MTX 8 mg + DEX 5 mg) [11]
*2. VCR 1.5 mg/m2 D1 + CY 1 g/m2 D1, 3, 5 + CDDP 90 mg/m2D2 [11]
*3. VCR 1.5 mg/m2
*4. TEPA 8 mg/kg D1, 2, 8 ,9 + MEL 1.5 mg/m2 D1, 2, 8, 9
*5. IFO 1.8 mg/m2 D2-5 + CBDCA 18.6 mg/kg D1 + ETP 5 mg/kg D1-5 + VCR 0.05 mg/kg D1,8 + IT (MTX 8 mg + DEX 5 mg) D1,8
*6. VCR 1.5 mg/m2 + IT-MD (MTX 8 mg + DEX 5 mg)
*7. VCR 0.05 mg/kg D1,8 + CY 33 mg/kg D1, 3, 5 + CDDP 3 mg/kg D2 + ETP 3.3 mg/kg D1-5 + IT (MTX 8 mg + DEX 5 mg) D1, 8 [11]
*8. VCR 0.05 mg/kg D1 + ETP 3.3 mg/kg D1,2 + CDDP 3 mg/kg D1 + IT-MD (MTX 8 mg + DEX 5 mg)
*9. VCR 0.05 mg/kg
*10. VCR 0.05 mg/kg D1 + CY 33 mg/kg D1, 3, 5 + CDDP 3 mg/kg D2 + ETP 3.3 mg/kg D1-5
*11. IT (Ara-C1 5 mg + MTX 7.5 mg + HDC 15 mg)
*12. VCR 1.5 mg/m2 D1 + ETP 150 mg/m2 D1, 2 + CDDP 560 mg/m2 D1
*13. IT (MTX 8 mg + DEX 5 mg)
*14. Topo 2 mg/30 kg D1-5 + CBDCA 400 mg/30 kg D7-8
*15. VCR 0.05 mg/kg D1, 8, 15 + CY 33 mg/kg D1, 3, 5 + CDDP3 mg/kg D2 + ETP 3.3 mg/kg D1-5 [11]
*16. VCR 0.05 mg/kg D1 + ETP 5 mg/kg D1,2 + CBDCA 18.6 mg/kg D1
*17. IT (MTX 8 mg + PSL 6 mg)
*18. IT (Topo 0.32 mg + Ara-C 24 mg)
*19. VCR 1.5 mg/m2 D1, 8, 15 + CY 1 g/m2 D22,23 + CDDP 75 mg/m2 D1 [12]
*20. VCR 1.5 mg/m2 D1 + CY 1 g/m2 D1,3,5 + CDDP 90 mg/m2 D2 [11]
*21. TEPA800 mg/m2 D1, 2, 8, 9 + MEL 210 mg/m2 D1, 2, 8, 9
*22. IT (MTX 6 mg + HDC 15 mg)
*23. IT (MTX 15 mg/m2 + AraC 60 mg/m2 + HDC 30 mg/m2)
*24. IFO 60 mg/kg D1-5 + CBDCA 13.3 mg/kg D1,2 + ETP 3.3 mg/kg D1-5 [13]
VCR vincristine; CY cyclophosphamide; CDDP cisplatin; ETP etoposide; IT intrathecal injection; MTX methotrexate; DEX dexamethasone; IFO ifosfamide; CBDCA carboplatin; HDC hydrocortisone; Topo topotecan; Ara-C Cytarabine; VEC vincristine, etoposide, carboplatin; JPBTC MB/PNET regimen Japanese Pediatric Brain Tumor Consortium medulloblastoma/primitive neuroectodermal tumors regimen; vincristine, cisplatin, cyclophosphamide; ICE ifosfamide, carboplatin, etoposide; TEPA thiotepa; MEL melphalan; auto BMT autologous bone marrow transplant; auto PBSCT autologous peripheral blood stem cell transplantation; EBRT external beam radiotherapy; NA not applicable
Surgical treatment
Of the patients who underwent enucleation, the ON surgical margin was positive in case 1, which preceded enucleation. In cases 3–5, we checked for the absence of ON invasion on MRI after neoadjuvant chemotherapy and then performed enucleation. The ON surgical margins were negative in all of them. Case 5 underwent enucleation using a double approach [8] that included both the anterior and neurosurgical approaches to ensure that ON surgical margins were negative.
Histopathologic results
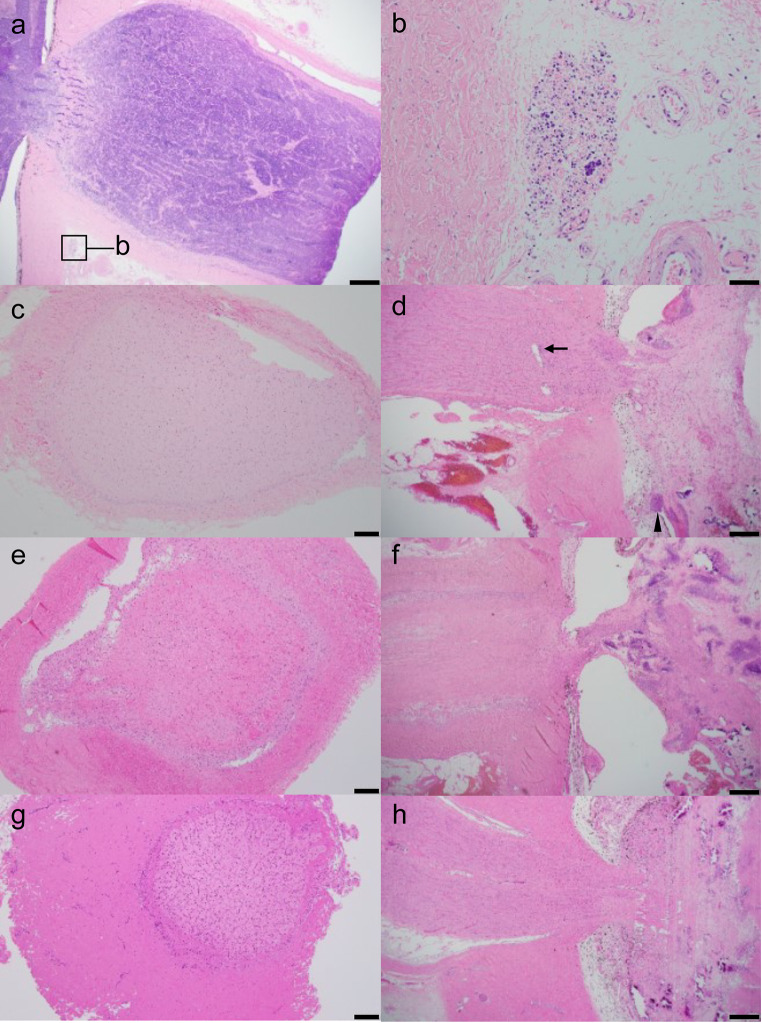
All enucleated eyes were examined pathologically. In case 1, the tumor cells were continuously infiltrating beyond the cribriform plate to the surgical margin (Fig. 4a). Tumor cells were observed in the epidural tissue (Fig. 4b). In case 2, the biopsy established a diagnosis of RB, and small round cells formed a small cluster. In case 3, the ON showed fibrotic changes due to neoadjuvant chemotherapy. No viable tumor cells were seen in the surgical margin (Fig. 4c) or arachnoid space, while there were tumor cells in the ON and rosettes within the choroid (Fig. 4d). In case 4, the ON showed fibrotic changes due to neoadjuvant chemotherapy. No viable tumor cells were seen in the surgical margin (Fig. 4e), ON, or arachnoid space (Fig. 4f). In case 5, no viable tumor cells were seen in the ON, surgical margin (Fig. 4g), or arachnoid space. The ON was retracted into the eye due to neoadjuvant chemotherapy (Fig. 4h).
Fig. 4.
Histopathological images of case 1 (a, b), case3 (c, d), case 4 (e, f) and case 5 (g, h). a ON with tumor invasion. Tumor cells observed in epidural tissue (box). Hematoxylin and Eosin (H&E) staining. Bar = 500 μm. b Tumor cells in epidural tissue. H&E staining. Bar = 50 μm. c No viable tumor cells are seen in the surgical margin. H&E staining. Bar = 200 μm. d ON showed fibrotic changes. Tumor cells found in the ON (arrow) and the choroid (arrowhead). H&E staining. Bar = 500 μm. e No viable tumor cells are seen in the surgical margin. H&E staining. Bar = 200 μm. f ON with fibrotic change. H&E staining. Bar = 500 μm. g ON with negative surgical margin. H&E staining. Bar = 200 μm. h ON retracted into the eye. Arachnoid space and ON without viable cells. H&E staining. Bar = 500 μm
Adjuvant treatment
All enucleated patients underwent chemotherapy postoperatively based on a high-risk preoperative evaluation that there was ON invasion. High-dose chemotherapy was administered to the cases with a positive ON margin or positive CSF. Case 1 had a positive ON surgical margin, and case 4 had a positive CSF after enucleation. When high-dose chemotherapy was administered, autologous bone marrow transplantation or autologous peripheral blood stem cell transplantation was performed.
Radiotherapy
Cases 1-4 were treated with radiotherapy (total dose of external beam radiotherapy, 40–45 Gy). Radiotherapy was administered to the orbit whenever the disease was contained within the orbit and to the whole brain and spinal cord if dissemination to the CSF had occurred. Radiotherapy was administered to distant metastases (the liver in case 2).
Case 5 with bilateral RB did not undergo radiotherapy; MRI was performed and showed 5 mm of ON invasion and the patient achieved a negative surgical margin and no residual viable tumor cells in the pathological findings. The patient was younger than 1 year and had bilateral RB that seemed to have a germline mutation. Therefore, case 5 was at high risk for a secondary cancer.
Treatment-related toxicity
Late complications included central endocrine abnormalities and cataracts due to radiotherapy in cases 1 and 4. Neurologic symptoms due to multiple cerebral infarctions and hemorrhage developed in case 2. No relapse or second cancer occurred in any cases.
Prognoses
Four patients were alive at the last follow-up. Case 2, which had intracranial invasion seen at the initial diagnosis, died 11 months after the initial diagnosis (Table 2).
Discussion
Although numerous reports have been published regarding the poor prognoses of RB with ON invasion, the treatment has not been standardized. We summarized the previously published reports of RB with extraocular invasion (Table 4). The rate of radiotherapy and survival rates vary among the reports. Depending on the report, there are cases with and without extraocular invasion on imaging evaluation. All reports that preceded enucleation in Table 4, except for that of Choucair et al. [8] and Bellaton et al. [14], describe no extraocular invasion on the preoperative imaging evaluation, but postoperative pathological evaluation showed extraocular invasion and adjuvant chemotherapy was administered. However, if preoperative imaging showed extraocular invasion, neoadjuvant chemotherapy was either administered or excluded in these reports. Choucair et al. report a relatively low rate of radiotherapy (45.5%) and a good survival rate (70%) for patients with ON invasion on imaging, similar to the current subjects; their patients were treated with neoadjuvant chemotherapy and the double approach enucleation [8].
Table 4.
Summary of reports
| Reports | Stage | No. cases | No. of extraocular invasions on CT or MRI | Treatment and no. of cases | Radiation therapy | Survival rate (follow-up period) |
|
|---|---|---|---|---|---|---|---|
| Chantada et al. [5] |
IRSS Stages II, III |
26 | 5 (CT or MRI) | Enucleation + adjuvant chemotherapy | 21 |
26 cases (100%) |
70% (5 years) |
| Neoadjuvant chemotherapy + enucleation + adjuvant chemotherapy | 5 | ||||||
| Radhakrishnan et al. [6] |
IRSS Stage III |
28 | 28 (MRI) | Neoadjuvant chemotherapy + enucleation + adjuvant chemotherapy | 21 |
19 cases (67.9%) |
40.4% (14.75 months) |
| Neoadjuvant chemotherapy + enucleation | 1 | ||||||
| Chemotherapy | 6 | ||||||
| Künkele et al. [7] |
IRSS Stage II |
6 | 1 (MRI) | Enucleation + adjuvant chemotherapy | 5 |
6 cases (100%) |
80% (5 years) |
| Neoadjuvant chemotherapy + enucleation + adjuvant chemotherapy | 1 | ||||||
| Choucair et al. [8] | ON invasion on CT or MRI | 11 | 11 (MRI) | Enucleation + adjuvant chemotherapy | 1 |
5 cases (45.5%) |
70% (2 years) |
| Enucleation + adjuvant chemotherapy + high-dose chemotherapy | 1 | ||||||
| Neoadjuvant chemotherapy + double approached enucleation + adjuvant chemotherapy | 7 | ||||||
| Neoadjuvant chemotherapy + enucleation + adjuvant chemotherapy | 1 | ||||||
| Chemotherapy | 1 | ||||||
| Zhao et al. [9] |
IRSS Stage II |
22 | 0 | Enucleation + adjuvant chemotherapy | 22 |
3 cases (13.6%) |
40.9% (5 years) |
| Bellaton et al. [14] | ON invasion on CT or MRI | 4 | 4 (CT or MRI) | Neoadjuvant chemotherapy + double approached enucleation + adjuvant chemotherapy | 3 |
0 case (0%) |
75% (12–40 months) |
| Chemotherapy | 1 | ||||||
IRSS International retinoblastoma staging system
A limitation of the current report is that the five cases were treated at different times (from 2005 to 2022) and with different chemotherapy regimens. Over time, at NCCHD more emphasis has been placed on neoadjuvant chemotherapy for extraocular invasion. Although we used the pathology results after neoadjuvant chemotherapy as a guide, we also performed adjuvant therapy based on a high-risk preoperative evaluation that showed ON invasion. Because we tried to avoid radiation therapy as much as possible and the risk of down-staging, we planned adjuvant therapy for high-risk cases with ON invasion. If the margins are positive, radiotherapy is definitely needed. We prefer to avoid radiotherapy for secondary cancer prevention, especially in young patients with bilateral RB who seemed to have a germline mutation.
Bellaton et al. report neoadjuvant chemotherapy and double approach enucleation in 3 patients with ON invasion seen on imaging, and no patients received radiotherapy [14]. Choucair et al. report that of the 7 patients who underwent neoadjuvant chemotherapy and double approach enucleation, 2 patients underwent radiotherapy. One patient who underwent neoadjuvant chemotherapy and anterior enucleation was positive with a surgical margin of 5 mm and underwent postoperative radiation therapy [8]. No major complications, such as infection, developed in these reports. In addition, the small size of the orbit in young patients occasionally may cause difficulty in removing the ON margin by an anterior approach as in case 5, even though it was 5 mm. Although the double approach was invasive, we avoided radiotherapy, at least in the young patient in case 5 who had bilateral RB and seemed to have a germline mutation. Although the number of current cases is too small to be definitive, it is possible that the double approach may help patients avoid radiation therapy.
We performed secondary enucleation only after confirming the negative results of ON invasion on MRI. Chawla et al. report a high negative predictive rate (100%) in determining ON invasion by MRI after neoadjuvant chemotherapy [15]. Therefore, when MRI after neoadjuvant chemotherapy shows no ON invasion, enucleation is recommended. However, in case 4, the ON invasion progressed in front of the optic canal and the CSF was positive postoperatively. Even though MRI showed no ON invasion after neoadjuvant chemotherapy, the CSF could not be evaluated. In cases with intracranial invasion, the prognosis is poor, and the choice of treatment remains an issue.
B-mode ultrasonography was helpful for detecting the presence of tumor at the primary diagnosis when the fundus cannot be evaluated. However, it could not detect ON invasion in all 5 cases and was inferior to MRI and CT for diagnosing ON invasion.
Considering the differences in results of ON invasion between CT and MRI images from cases 3 and 5, compared to MRI CT may not be able to detect ON invasion adequately. Although the MRI findings were correlated with the pathology results, when compared the positive predictive values of CT is reported to be 66.67% [16]. Gadolinium enhancement on MRI is essential. In the current study, ON invasion also was found by an enhancement effect in all cases. Some studies report that the contrast patterns were correlated with pathology [17] and that MRI after chemotherapy can evaluate ON invasion [15]. In the current study, 133 eyes of 130 patients underwent enucleation after imaging without ON invasion. Of these, no eyes had a positive surgical ON margin, and 13 eyes of 13 cases (9.8%) had laminar cribrosa invasion pathologically. Only 5 of the 13 cases were evaluated by MRI imaging, while the remaining 8 cases were only evaluated by CT imaging.
Considering the current outcomes of the 5 cases, enhanced MRI, rather than CT is recommended to evaluate ON invasion. For RB with ON invasion observed on imaging, appropriate selection of neoadjuvant or adjuvant therapy would be helpful to avoid radiotherapy, especially for young patients or for those with a germline mutation.
Acknowledgements
This research was supported by AMED under Grant Number 24ck0106902h0002.
Declarations
Conflict of interest
T. Onishi, None; S. Nishina, None; T. Yokoi, None; T. Yoshida, None; S. Hayashi, None; H. Anzai, None; N. Azuma, None; C. Kiyotani, None; K. Terashima, None; T. Yoshioka, None; H. Ogiwara, None; H. Fuji, None; M. Kitamura, None; Y. Tsutsumi, None.
Footnotes
Corresponding author: Sachiko Nishina
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dimaras H, Kinami K, Dimba EA, Gronsdhal P, White A, Chan HS, Gallie BL. Retinoblastoma. Lancet. 2012;379:1436–46. [DOI] [PubMed] [Google Scholar]
- 2.The Global Retinoblastoma Study Group. The global retinoblastoma outcome study: a prospective, cluster-based analysis of 4064 patients from 149 countries. Lancet Glob Health. 2022;10:e1128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniels AB, Patel SN, Milam RW, Kohanim S, Friedman DL, Koyama T. Effect of intravenous chemotherapy regimen on globe salvage success rates for retinoblastoma based on disease class-a meta-analysis. Cancers (Basel). 2021;13:2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry JL, Kogachi K, Aziz HA, McGovern K, Zolfaghari E, Linn Murhee A, et al. Risk of metastasis and orbital recurrence in advanced retinoblastoma eyes treated with systemic chemoreduction versus primary enucleation. Pediatr Blood Cancer. 2017;64:101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chantada GL, Guitter MR, Fandiño AC, Raslawski EC, DeDavila MTG, Vaiani E, et al. Treatment results in patients with retinoblastoma and invasion to the cut end of optic nerve. Pediatr Blood Cancer. 2009;52:218–22. [DOI] [PubMed] [Google Scholar]
- 6.Radhakrishanan V, Kashyap S, Pschker N, Sharma S, Pathy S, Mohanti BK, et al. Outcome, pathologic finings, and compliance in orbital retinoblastoma (international retinoblastoma staging system stage III) treated with neoadjuvant chemotherapy: a prospective study. Ophthalmology. 2012;119:1470–7. [DOI] [PubMed] [Google Scholar]
- 7.Künkele A, Wilm J, Holdt M, Lohman D, Bornfeld N, Eggert A, et al. Neoadjuvant/adjuvant treatment of high-risk retinoblastoma: a report from the German retinoblastoma referral centre. Br J Ophthalmol. 2015;99:949–53. [DOI] [PubMed] [Google Scholar]
- 8.Choucair ML, Brisse HJ, Freneaux P, Desjardins L, Dorfmüller G, Puget S, et al. Management of advanced uni- or bilateral retinoblastoma with macroscopic optic nerve invasion. Pediatr Blood Cancer. 2020;67:e27998. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Feng Z, Leung G, Gallie BL. Retinoblastoma survival following primary enucleation by AJCC staging. Cancers (Basel). 2021;13:6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The international classification of retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–80. [DOI] [PubMed] [Google Scholar]
- 11.Okada K, Soejima T, Sakamoto H, Hirato J, Hara J. Phase II study of reduced-dose craniospinal irradiation and combination chemotherapy for children with newly diagnosed medulloblastoma: a report from the Japanese pediatric brain tumor consortium. Pediatr Blood Cancer. 2020;67:e28572. [DOI] [PubMed] [Google Scholar]
- 12.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–8. [DOI] [PubMed] [Google Scholar]
- 13.Okada S, Hongo T, Sakaguchi K, Suzuki K, Nishizawa S, Ohzeki T. Pilot study of ifosfamide/carboplatin/etoposide (ICE) for peripheral blood stem cell mobilization in patients with high-risk or relapsed medulloblastoma. Childs Nerv Syst. 2007;23:407–13. [DOI] [PubMed] [Google Scholar]
- 14.Bellaton E, Bertozzi A, Behar C, Suzuki K, Nishizawa S, Ohzeki T. Neoadjuvant chemotherapy for extensive unilateral retinoblastoma. Br J Ophthalmol. 2003;87:327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chawla B, Chaurasia S, Sharma S, Pattebahadur R, Hasan F, Seth R, et al. Magnetic resonance imaging for tumor restaging after chemotherapy in retinoblastoma with optic nerve invasion. Ophthalmic Genet. 2018;39:584–8. [DOI] [PubMed] [Google Scholar]
- 16.Kim U, Rathi G, Chowdhary G, Srinavasan KG, Shanthi R, Prabhu Krishna RS. Accuracy of preoperative imaging in predicting optic nerve invasion in retinoblastoma: a retrospective study. Indian J Ophthalmol. 2019;67:2019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiranthan M, Meel R, Sharma S, Lomi N, Kashyap S, Bajaj MS. Can enhancement pattern in normal-sized optic nerves on magnetic resonance imaging better predict tumor invasion in retinoblastoma eyes? Ocul Oncol Pathol. 2023;9:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]