Abstract
Background
Acute respiratory distress syndrome (ARDS) is a life-threatening condition associated with the inflammatory activation of alveolar macrophages. Here, we examined the role of circVAPA in regulating inflammasome activation and macrophage inflammatory polarization in an ARDS model.
Methods
circVAPA expression levels were analyzed in macrophages isolated from healthy controls and patients with ARDS. In vitro cell models of mouse alveolar macrophages and an in vivo mouse ARDS model were established through Lipopolysaccharide (LPS) stimulation. The effects of circVAPA knockdown on macrophage inflammatory polarization, inflammasome activation, and pulmonary tissue damage were investigated in both cell and animal models. The interaction between circVAPA and downstream factors was verified through a luciferase reporter assay and by silencing circVAPA.
Results
circVAPA upregulation in alveolar macrophages was associated with the inflammation in ARDS patients. circVAPA was also upregulated in LPS-stimulated mouse alveolar macrophages (MH-S cells). Additionally, circVAPA knockdown attenuated the inflammatory activation of MH-S cells and reduced the expression of pyroptosis-related proteins. circVAPA silencing also mitigated the inflammatory effects of LPS-stimulated MH-S cells on lung epithelial cells (MLE-12), and alleviated the inflammatory damage in the pulmonary tissue of ARDS mouse model. We further showed that miR-212-3p/Sirt1 axis mediated the functional role of circVAPA in the inflammatory polarization of MH-S cells.
Conclusion
Our data suggest that circVAPA promotes inflammasome activity and macrophage inflammation by modulating miR-212-3p/Sirt1 axis in ARDS. Targeting circVAPA may be employed to suppress the inflammatory activation of alveolar macrophages in ARDS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10735-024-10312-3.
Keywords: ARDS, Macrophage, Inflammation, Inflammasome, circVAPA, miR-212-3p, Sirt1
Introduction
Acute respiratory distress syndrome (ARDS) a life-threatening pathogenic damages in pulmonary tissues which that allows fluid leakage into the lungs (Meyer et al. 2021). Unattended progression of ARDS could trigger pulmonary edema, which culminates in respiratory failure and poor oxygenation (Williams et al. 2021). ARDS is commonly caused by infections such as respiratory syncytial virus and SARS-CoV-2 (Luyt et al. 2020). Certain pathophysiological conditions, such as sepsis, traumatic damages and pneumonia, are also risk factors for ARDS (4–5). Currently, there are no effective therapeutic treatment for ARDS due to the lack of molecular target, although the underlying mechanisms of ARDS progression is an area of intensive research (Kaku et al. 2020).
Acute inflammatory damage caused by the infiltration of immune cells in the alveoli and microcirculation of the lung tissues is a major detrimental factor in ARDS (Saguil and Fargo 2020). Activated immune cells can induce inflammatory and oxidative damages in vascular endothelium and alveolar epithelium, which leads to pulmonary edema, impaired lung compliance and gas exchange capacity (Saguil and Fargo 2020; Sivapalan et al. 2020). Inflammatory macrophages and neutrophils are two groups of innate immune cells playing a paramount role in ARDS related pulmonary damages. Neutrophil counts in alveolar tissues have been shown to be positively correlated with disease severity in ARDS (Yang et al. 2021). The inflammatory activation of alveolar macrophages not only stimulate the local pro-inflammatory microenvironment, but also recruit blood macrophages and neutrophils to exacerbate the inflammatory condition (Tao et al. 2023). There is convincing evidence showing that the activation of alveolar macrophages triggers the early inflammation and mediate the pathogenesis of ARDS (Zhang et al. 2021; Chen et al. 2020; Liu et al. 2022). Since functionally plastic macrophages can be polarized into M1 inflammatory phenotype or differentiated into M2 anti-inflammatory condition (Zeng et al. 2023), understanding the mechanism governing macrophage polarization can provide insights into the development of intervention strategy to curb inflammation in ARDS.
Pyroptosis is a pro-inflammatory form of cell death event associated with over-activation of macrophages during inflammatory responses (Chai et al. 2023). The execution of pyroptosis depends on inflammasome activity, which induces the activation of caspase-1 and Gasdermin D (GSDMD). The cleaved GSDMD proteins form pore on the cell membrane to release pro-inflammatory cytokines including interleukin-1β (IL-1β) and interleukin-18 (IL-18) (Man et al. 2017; Zhao et al. 2018). During pyroptosis activation, NOD-like receptor family pyrin domain containing 3 (NLRP3) protein serves as the scaffold of inflammasome to recruits apoptosis-related spot-like proteins for caspase-1 activation and GSDMD cleavage (Zheng and Li 2020; Swanson et al. 2019). Inflammasome activation has been implicated in the inflammatory damages in acute lung injury and ARDS (McVey et al. 2021; Dolinay et al. 2012). However, the mechanisms regulating inflammasome activation in ARDS-associated macrophages remain unclear.
A growing body of evidence suggests that non-coding RNAs such as circular RNAs (circRNAs) and microRNAs (miRNAs) modulates the inflammatory activation of macrophages (Xu et al. 2020; Sprenkle et al. 2023). CircVAPA is a novel circular RNA which is implicated in lung cancer progression (Hua et al. 2022; 2.Liu et al. 2021). However, whether circVAPA regulates the inflammatory activation of macrophages in ARDS remains to be elucidated. Here, we examined the functional role of circVAPA in dictating inflammasome activation and macrophage inflammatory polarization in ARDS model. circVAPA expression pattern was analyzed in macrophages isolated from healthy controls and ARDS patients. In vitro cell model of mouse alveolar macrophages and in vivo animal model of ARDS were established by Lipopolysaccharide (LPS) treatment. The effects of circVAPA knockdown on macrophage inflammatory polarization, inflammasome activation, and pulmonary tissues damages were investigated in both the cell and animal models.
Methods
Clinical samples
The bronchoalveolar lavage fluid (BALF) samples were collected from age and sex-matched healthy controls and ARDS patients (male, age 36–42, n = 10 in each category). ARDS patients were diagnosed according to the Berlin Definition criteria, which include: Onset within 1 week of a known clinical insult or new/worsening respiratory symptoms; Bilateral opacities on chest imaging not fully explained by effusions, lobar/lung collapse, or nodules; Respiratory failure not fully explained by cardiac failure or fluid overload; PaO2/FiO2 ratio ≤ 300 mm Hg with PEEP or CPAP ≥ 5 cm H2O. BALF samples were collected within 24 h of ARDS diagnosis during clinically indicated bronchoscopy procedures. Patients with a history of pre-existing lung diseases or other medical conditions that could compromise pulmonary function were excluded from the study.
Alveolar macrophages were isolated from fresh BALF samples using the antibody-magnetic method with a commercial kit (EasySep™ Direct Human Monocyte Isolation Kit, catalog #19669, STEMCELL Technologies, Vancouver, Canada) according to the manufacturer’s instructions. The isolated alveolar macrophages were immediately processed for RNA extraction and flow cytometry analysis. Remaining BALF samples were aliquoted and stored at -80 °C for subsequent molecular analysis. The utilization of clinical samples gained approval from the Medical Ethics Committee of Kunming Children’s Hospital (2024-03-011-K01). All the enrolled subjected provided singed informed consent.
Enzyme-linked immunoassay (ELISA)
Lung lavage fluid or cell lysates were used for the measurement of inflammatory cytokines, including TNF-α, IL-1β and IL-6 by ELISA. The commercial ELISA kits for IL-1β (PI301), TNF-α (PT513) and IL-6 (PI326) were purchased from Beyotime Biotechnology (Beijing, China) for mouse sample analysis. The following kits were used for human sample analysis: IL-1β (PI305), TNF-α (PT518) and IL-6 (PI330) from Beyotime Biotechnology (Beijing, China). 50 µL sample was utilized to examine the concentrations of each cytokine following the producer’s protocols. The absorbance of each sample was detected at 450 nm using a microplate reader (Infinite 200 PRO, Tecan, Männedorf Switzerland). Quantification was based on the standard curve generated from the serially diluted standards.
Reactive oxygen species (ROS) and myeloperoxidase (MPO) activity
MPO activity in the cell and BALF samples was determined using a MPO activity assay kit (KCW20262, Mlbio, Shanghai, China) according to the supplier’s protocol. MPO activity was recorded at 460 nm absorbance. ROS level was determined using a ROS fluorescence assay kit (R0105, Jiancheng Bioengineering Institute, Nanjing, China), and ROS staining intensity was measured using on the microplate reader (Infinite 200 PRO, Tecan, Männedorf Switzerland) at the excitation/absorption wavelength 485/530 nm.
Cell culture
Mouse alveolar macrophage cell line (MH-S) was purchased from Coweldgen Scientific (KMCC-001-0546, Shanghai, China). Mouse lung epithelial cells (MLE-12) was obtained from Kanglang Biotech (KL036M, Shanghai, China). Both cell lines were examined to be mycoplasma-free and validated by the supplier via STR profiling. Cells were cultivated with DMEM high glucose medium (E600005, Sangon, Shanghai, China) supplemented with 10% fetal bovine serum (26140087, Thermo Fisher Scientific, CA, USA), 1 x penicillin/streptomycin solution (PB180120, Procell, Wuhan, China) at at 37 ℃ and CO2 (5%). at 37 ℃ and CO2 (5%). Lipopolysaccharide (LPS, HY-D1056, MedChemExpress, Shanghai, China) was used to induce the cells at 2 µg/mL for 48 h. For co-culture experiments, different groups of MH-S cells were co-cultured with MLE-12 cells in a Transwell system using Polyester Membrane Transwell® Permeable Supports (24 mm inserts, 0.8 μm pore size, catalog number 3450, Corning Inc., Corning, NY, USA) for 48 h.
Transfection
The delivery of pladmis, siRNA and miRNA mimic/inhibitor into MH-S cells was conducted using Lipofectamine™2000 (11668019, Invitrogen, Shanghai, China). Control siRNA, CircVAPA siRNA, miR-212-3p mimic, miR-212-3p inhibitor, and miRNA controls were customer-designed and synthesized from Daweike Biotechnology (Shanghai, China). 100 nM of each molecule was used to perform transfection in a 6-well plate containing 5 × 105 cells per well, and the cells were subjected to further analysis after 48 h.
Quantitative real-time PCR (qRT-PCR)
Total RNA sample was collected using Trizol extraction kit (R0016, Beyotime, Beijing, China), and 500 ng of the purified RNA was used to conduct reverse transcription by iScript cDNA Synthesis Kit (1708891, Xinjing Biotech, Hangzhou, China) or Taqman™ microRNA reverse transcription kit (4366596, Thermo Fisher Scientific, CA, USA). Quantitative PCR was performed using SYBR Green reagent (B110031-0001, Sangon, Shanghai, China) on the QuantStudio qPCR platform (BioRad, CA, USA). Target gene expression was normalized with GAPDH or U6 snRNA via 2−ΔΔCt method.
Western blot
Cells were lysed using cellular protein collection reagent (P0013C, Beyotime, Beijing, China) on ice for 15 min. The protein contents were examined by a BCA Protein assay kit (P0009, Beyotime, Beijing, China). 10 µg of protein sample was separated by electrophoresis in 10% polyacrylamide gel, and separated protein bands were transferred to the PVDF membrane (P2005, Beyotime, Beijing, China). After blocking, the membranes were probed using the following antibodies (Abcam, Cambridge, UK): Sirt1 (ab12193, 1 µg/mL), GSDMD-N (ab215203, 1 µg/mL), iNOS (ab3523, 1 µg/mL), CD86 (ab220188, 1 µg/mL), CD80 (ab254579, 1 µg/mL), NLRP3 (ab263889, 1 µg/mL), caspase 1 p20/p22 (ab207802, 1 µg/mL), pro-caspase 1 (ab286125, 1 µg/mL), anti-actin (ab209857, 0.5 µg/mL) for 24 h at 4 °C, followed by further labeling with the secondary antibodies (ab131368, 0.33 µg/mL and ab96899, 0.33 µg/mL) for 1 h at ambient temperature. Signals of protein bands were developed using Beyo ECL Star kit (R0024FT, Beyotime, Beijing, China). The quantification of protein band intensity was performed with Image J software (NIH, Bethesda, MD, USA).
CCK-8 cell growth assay
A cell proliferation assay kit (CA1210, Solarbio, Beijing, China) was utilized to assess cell growth ability under different experimental conditions. Cells were cultivated at 2,000 cells/well in a 96-well plate for different durations. Afterward 10 µL of CCK-8 solution was applied in each well at indicated time point for 3 h at 37oC. Light absorbance in each sample was recorded at 450 nm wavelength using the microplate reader (Infinite 200 PRO, Tecan, Männedorf Switzerland).
Flow cytometry analysis
The Annexin-V-fluorescein isothiocyanate (FITC) cell apoptosis detection kit (K201-100, BioVision, Palo Alto, USA) was used to detect cellular apoptosis. Briefly, the Annexin-V-FITC, propidium iodide (PI), hydroxyethyl piperazine ethanesulfonic acid (HEPES) buffer was mixed at the ratio of 1: 1: 48 to prepare the staining solution. 1 × 106 cells were incubated in 200 µL staining solution for 15 min, and then washed in 1 mL HEPES buffer before flow cytomery analysis. For surface staining of macrophage markers, cells were first incubated with Fc-Block (αCD16/32, 1 µg/mL, Biolegend, CA USA) for 10 min at 4 °C. After washing with PBS, the cells were further stained using FITC anti-mouse CD206 MMR antibody (141703, 2.5 µg/mL), FITC anti-human CD206 (MMR) antibody (321103, 2.5 µg/mL), FITC anti-human CD86 antibody (374203, 2.5 µg/mL), FITC anti-mouse CD86 antibody (105005, 2.5 µg/mL), PE anti-human CD14 antibody (301805, 2 µg/mL), and PE anti-mouse F4/80 antibody (123109, 2 µg/mL) for 15 min. After two wahses with PBS, cell samples were analyzed on an LSRII flow cytometer (BD Biosciences, CA, USA).
Dual luciferase reporter assay
Wild-type (WT) fragment of predicted interacting sites or the mutated sequences (MUT) was cloned into pGL4-luciferase plasmid (PGL4-001, Huayueyang Biological, Beijing, China). WT or MUT plasmid was transfected into cells together with miRNA mimic or miR-NC for 48 h. The relative luciferase activities of WT and MUT reporters were analyzed using Dual Glo Luciferase Reporter Gene Assay Kit (11405ES60, Yeasen Biotech, Shanghai, China).
Animal study
SPF grade C57bl/6 mice (male, 8w-10w old, 30–35 g in weight) were reared in a pathogen-free animal facility, with 12 h dark/light cycle and free access to food and water. Animals were randomly assigned into 4 experimental groups (n = 6 in each group): sham group: mice treated with sterile saline; Model group: mice stimulated with LPS as the ARDS model; Model + sh-NC group: model group administrated with lentivirus carrying control shRNA. Model + sh-circVAPA group: model group administrated with lentivirus carrying circVAPA shRNA. To establish the ARDS model, animals were anesthetized by intraperitoneal injection of pentobarbital (25 mg/kg). After tracheotomy exposure, LPS was instilled into the lungs through the trachea (administration dose 6 mg/kg). The animal was maintained in upright position and rotated vertically to make the LPS evenly distributed in the lung tissues. The intervention group received tail vein injection of lentivirus: (sh-NC and sh-CircVAPA) three days before LPS instillation. 72 h after LPS administration, the mice were sacrificed and BALF samples and lung tissues were collected for further analysis. Macrophages in fresh BALF samples were isolated using the EasySep™ Mouse F4/80 Positive Selection Kit (catalog #100–0659, STEMCELL Technologies, Vancouver, Canada). The left lung tissue fixed with formalin and dehydrated with ethanol, followed by the embedding in paraffin. 5 μm tissue sections were deparaffinized and rehydrated, and the histological analysis was conducted using an Hematoxylin and Eosin (H&E) staining kit (C1015S, Beyotime, Beijing, China). The animal procedures in this study were approved by the animal use and welfare committee of the Ethical Review Committee for Animal Experiments of Kunming Medical University (kmmu20240767).
Statistics
The results in this study were analyzed using GraphPad Prism 6.0. (GraphPad software, NY, USA). Two-group comparisons were analyzed by unpaired student’s t tests, and multiple condition comparisons were conducted via one-way analysis of variance (ANOVA). All experimental data were expressed as mean and standard deviation. P < 0.05 indicates the statistical significance.
Results
High level expression of circVAPA in ARDS patients is associated with inflammation and oxidative stress
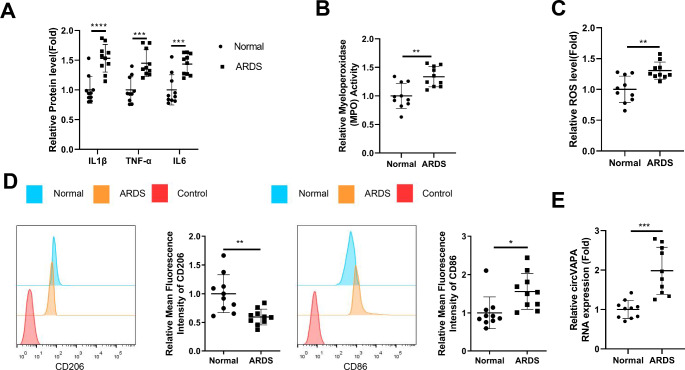
We initially collected the BALF samples from the healthy controls and ARDS patients to analyze the inflammatory and oxidative parameters, as well as the expression of circVAPA in macrophages. As expected, there was a significant increase of inflammatory factors (TNF-α, IL-1β and IL-6) in the BALF samples of ARDS patients (Fig. 1A). Myeloperoxidase (MPO) activity (an indicator of inflammation and oxidative stress induced by neutrophils) was also elevated in the BALF samples of ARDS patients, as well as the ROS level (Fig. 1B C). After purification, more than 90% cells were CD14 + monocytes (Figure S1A). Furthermore, we found an increase of cellular contents and percentage of CD14 + monocytes in BALF samples from the ARDS patients compared to the normal counterparts (Figure S1B and S1C). Flow cytometry analysis of isolated monocytes from BALF samples demonstrated the increased expression of M1 marker (CD86) and the decreased level of M2 marker (CD206) (Fig. 1D). qRT-PCR assay revealed an upregulation of circVAPA in the macrophage samples from ARDS patients (Fig. 1E). Together, these data suggest that circVAPA might be implicated in the inflammatory activation of macrophages in the BALF samples of ARDS patients.
Fig. 1.
High level expression of circVAPA in ARDS patients is associated with inflammation and oxidative stress
ELISA analysis of inflammatory factors (TNF-α, IL-1β and IL-6) in the BALF samples of ARDS patients and healthy controls (n = 10 in each group). (B) Myeloperoxidase (MPO) activity and (C) ROS level measurement in the BALF samples of ARDS patients and healthy controls. (D) Flow cytometry analysis of the expression of M1 marker (CD86) and M2 marker (CD206) in macrophages from BALF samples. (E) qRT-PCR detection of circVAPA expression in the macrophage samples from ARDS patients and healthy controls. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
Silencing circVAPA attenuates LPS-induced inflammatory polarization and pyroptosis in mouse alveolar macrophage cells (MH-S)
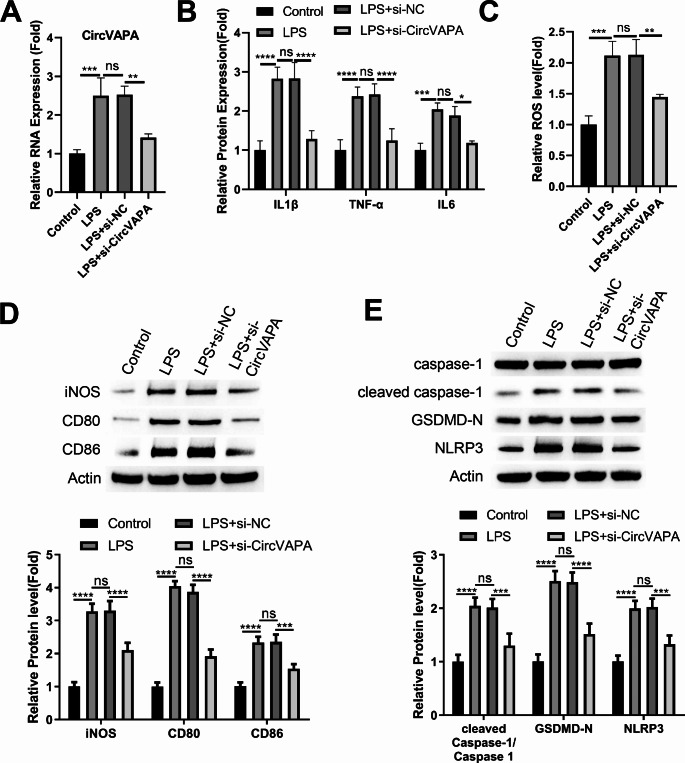
To verify the role of circVAPA in the inflammatory differentiation of alveolar macrophages, mouse alveolar macrophage cells (MH-S) with the transfection of control siRNA (si-NC) or circVAPA targeting siRNA (si-circVAPA) were stimulated with LPS. LPS induction heavily increased circVAPA expression, which was dampened upon the transfection of si-circVAPA (Fig. 2A). ELISA analysis results revealed that silencing circVAPA suppressed the production of inflammatory cytokines (Fig. 2B) and oxidative stress (Fig. 2C) in LPS-stimulated MH-S cells. Besides, Western blot detection of multiple M1 inflammatory markers of macrophages demonstrated that circVAPA knockdown inhibited the upregulations of iNOS, CD86 and CD80 in LPS-stimulated MH-S cells (Fig. 2D). We also examined pyroptosis-related proteins and the results showed that, circVAPA knockdown can reduce the expression levels of NLRP3, cleaved caspase-1, and GSDMD-N caspase-1 (p20 activated form) in LPS-induced MH-S cells (Fig. 2E). These data suggest that circVAPA overexpression contributes to LPS-induced inflammatory polarization and pyroptosis in MH-S cells.
Fig. 2.
Silencing circVAPA attenuates LPS-induced inflammatory polarization and pyroptosis in mouse alveolar macrophage cells (MH-S)
Mouse alveolar macrophage cells (MH-S) with the transfection of control siRNA (si-NC) or circVAPA targeting siRNA (si-circVAPA) were stimulated by LPS. (A) qRT-PCR analysis of circVAPA expression. (B) ELISA analysis inflammatory cytokines (TNF-α, IL-1β and IL-6). (C) ROS level measurement. (D) Western blot detection of M1 inflammatory markers (iNOS, CD86 and CD80). (E) Western blot detection of pyroptosis-related proteins (NLRP3, cleaved caspase-1, and GSDMD-N). n = 3. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
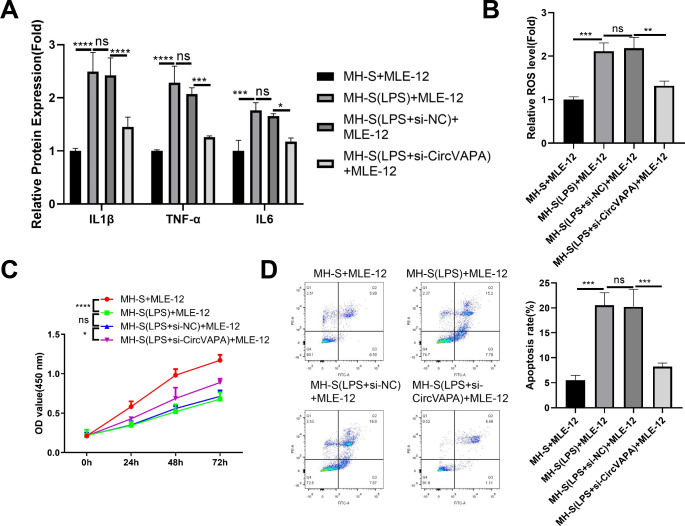
Silencing circVAPA in MH-S cells suppresses the inflammatory damages to lung epithelial cells (MLE-12)
We next investigated whether silencing circVAPA in MH-S cells could protect against the inflammatory damages in lung epithelial cells (MLE-12). MLE-12 cells were co-cultured with control MH-S cells, LPS-stimulated MH-S cells, LPS-stimulated MH-S cells with si-NC transfection or LPS-stimulated MH-S cells with si-circVAPA transfection of 48 h. Silencing circVAPA in LPS-stimulated MH-S cells reduced the production of inflammatory cytokines (Fig. 3A) and the ROS level (Fig. 3B) in the co-culture cell medium. CCK-8 cell growth assay showed that the co-culture with LPS-stimulated MH-S cells significantly impaired cell proliferation of MLE-12 cells, while silencing circVAPA in MH-S cells partially rescued cell growth of MLE-12 cells (Fig. 3C). The co-culture with LPS-stimulated MH-S cells also increased the apoptotic events in MLE-12 cells, which was reduced upon the silencing of circVAPA in MH-S cells (Fig. 3D). These data indicate that silencing circVAPA in MH-S cells suppresses the inflammatory damages to lung epithelial cells (MLE-12).
Fig. 3.
Silencing circVAPA in MH-S cells suppresses the inflammatory damages to lung epithelial cells (MLE-12)
MLE-12 cells were co-cultured in Transwell cassette with control MH-S cells, LPS-stimulated MH-S cells, LPS-stimulated MH-S cells with si-NC transfection or LPS-stimulated MH-S cells with si-circVAPA transfection of 48 h. (A) ELISA analysis of inflammatory cytokines in the cell culture medium. (B) ROS level measurement in the co-culture cell medium. (C) CCK-8 cell growth assay in MLE-12 cells after the co-culture with different groups of MH-S cells. (D) Flow cytometry analysis of apoptotic events in MLE-12 cells after the co-culture with different groups of MH-S cells. n = 3. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
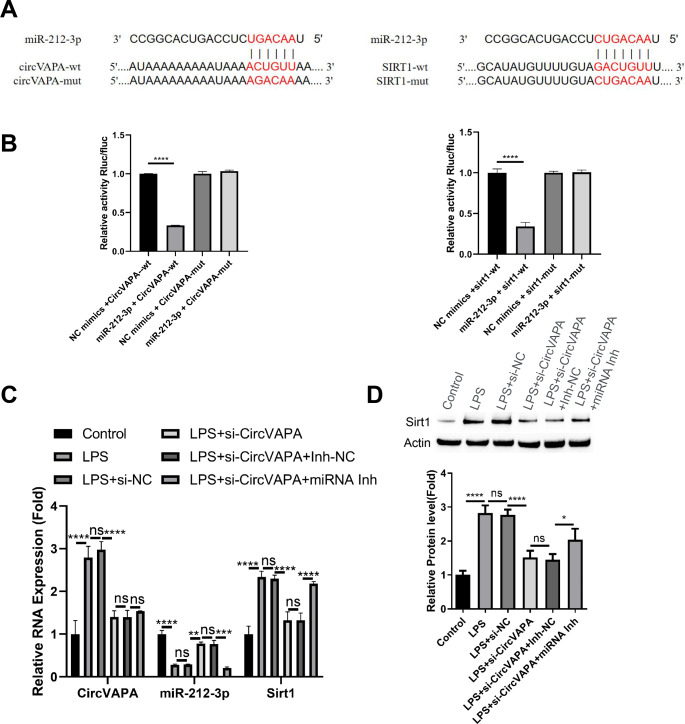
circVAPA targets mir-212-3p and Sirt1 axis in MH-S cells
We next sought to find the mechanism by which circVAPA regulates the inflammatory polarization in MH-S cells. TargetScan online resource analysis revealed that circVAPA might interact with miR-212-3p, and Sirtuin 1 (Sirt1) mRNA 3’ untranslated region (UTR) harbors potential binding sites to miR-212-3p (Fig. 4A). To confirm the molecular interactions, we cloned the wildtype (WT) binding sequences or the mutated sequences (MUT) of each pair into a luciferase reporter vector. The analysis of luciferase activity in MH-S cells showed that, the presence of miR-212-3p mimic could largely abrogated the activity of WT reporter, but miR-212-3p mimic did not significantly affect the activity of MUT reporter (Fig. 4B), indicating the interaction of molecular pairs through predicted WT interaction sites. To further validate the regulatory pattern of circVAPA, miR-212-3p and Sirt1, MH-S cells were transfected with si-NC, si-circVAPA, si-circVAPA + miRNA inhibitor control (inh-NC) or si-circVAPA + miRNA inhibitor. qRT-PCR analysis showed that LPS stimulation promoted the expression of circVAPA and Sirt1, while miR-212-3p was suppressed. si-circVAPA application partially restored miR-212-3p expression level and suppressed Sirt1 level in LPS stimulated cells. The effect of si-circVAPA on Sirt1 and miR-212-3 expression was largely abrogated by miR-212-3p inhibitor (Fig. 4C). Western blot analysis of Sirt1 protein levels showed consistent results with the data in qRT-PCR analysis (Fig. 4D). Together, these findings suggest that circVAPA modulates miR-212-3p and Sirt1 axis in MH-S cells.
Fig. 4.
circVAPA targets miR-212-3p and Sirt1 axis in MH-S cells
miR-212-3p/Sirt1 axis mediates the role of circVAPA in MH-S cells
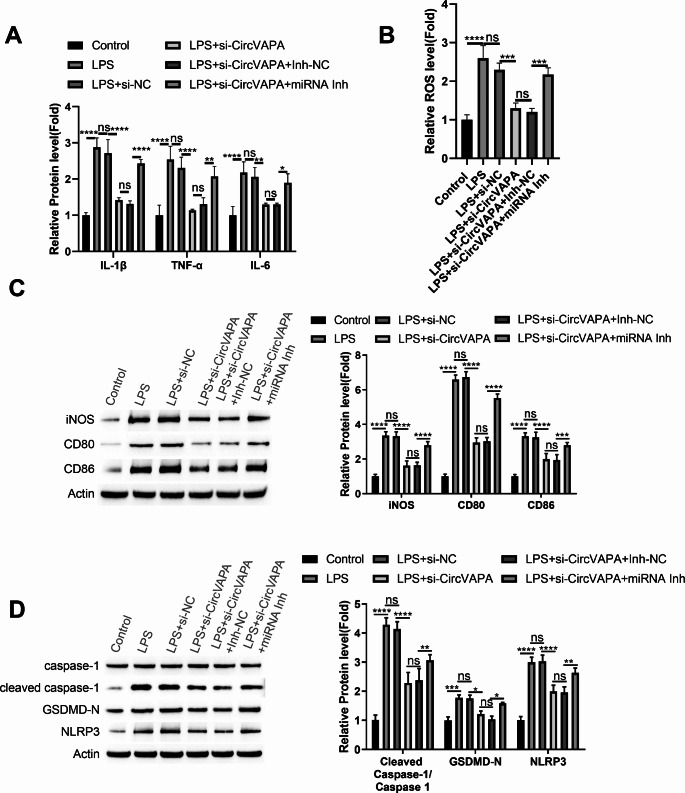
To confirm that miR-212-3p/Sirt1 axis is circVAPA downstream effector involved in the inflammatory regulation of macrophages, MH-S cells transfected with si-NC, si-circVAPA, si-circVAPA + miRNA inhibitor control (inh-NC) or si-circVAPA + miRNA inhibitor were stimulated with LPS. ELISA analysis showed that the suppressive effect of si-circVAPA on LPS-induced inflammatory cytokines was largely abolished by miR-212-3p inhibitor (Fig. 5A). Similar results were observed in the detection of cellular ROS levels (Fig. 5B). si-circVAPA transfection reduced the expression of M1 markers (iNOS, CD86, CD80) and suppressed the protein levels of pyroptosis-related proteins in LPS-stimualted cells, an effect which was attenuated upon the co-transfection of miR-212-3p inhibitor (Fig. 5C and D).
Fig. 5.
miR-212-3p/Sirt1 axis mediates the role of circVAPA in MH-S cells
MH-S cells transfected with si-NC, si-circVAPA, si-circVAPA + miRNA inhibitor control (inh-NC) or si-circVAPA + miRNA inhibitor were stimulated with LPS. (A) ELISA analysis of inflammatory cytokines. (B) Detection of cellular ROS levels. (C) Western blot analysis of M1 markers (iNOS, CD86, CD80). (D) Western blot detection of pyroptosis-related proteins (NLRP3, cleaved caspase-1, and GSDMD-N). n = 3. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
Targeting circVAPA alleviates inflammatory damages in the pulmonary tissues of ARDS mouse model
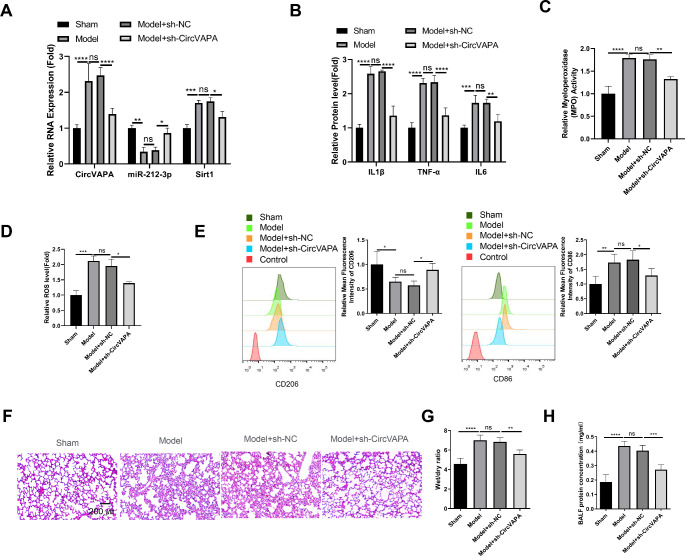
The ARDS model was established by pulmonary instillation of LPS through the trachea. The animals were assigned into 4 groups: sham group: mice treated with sterile saline; Model group: mice stimulated with LPS as the ARDS model; Model + sh-NC group: model group administrated with lentivirus carrying control shRNA. Model + sh-circVAPA group: model group administrated with lentivirus carrying circVAPA shRNA. qRT-PCR analysis in the BALF samples showed that circVAPA and Sirt1 levels were elevated in the Model group, and miR-212-3p level was decreased. Knocking down circVAPA partially reversed these changes (Fig. 6A). The analysis of inflammatory cytokines, MPO activities, and ROS levels in the BALF samples also demonstrated the protective effect of circVAPA silencing in the Model group (Fig. 6B and D). We also isolated F4/80 + macrophages from the BALF samples, which yielded a purity of more than 90% F4/80 + cells (Figure S2A). There was an increase of cellular contents and percentage of F4/80 + macrophages in BALF samples from the model group, which was suppressed by circVAPA knockdown (Figure S2B and S2C). The analysis of M1 (CD86) and M2 (CD206) marker in purified F4/80 + macrophages showed that silencing circVAPA reduced the expression levels of CD86 and increased CD206 levels in the Model group (Fig. 6E). Further, H&E staining in the pulmonary tissues revealed that circVAPA silencing could alleviate LPS-induced tissue damages, including disrupted lumen, alveolar congestion, alveolar wall thickening and edema (Fig. 6F). Consistently, circVAPA silencing significantly reduced the wet/dry ratio of the lung tissues (Fig. 6G) and decreased the protein levels in the BALF samples of the model group (Fig. 6H), indicting that circVAPA knockdown mitigates pulmonary edema and ameliorates alveolar barrier permeability in the model group. Collectively, these results imply that silencing circVAPA attenuates inflammatory damages in the pulmonary tissues of ARDS model by suppressing macrophage activation.
Fig. 6.
Targeting circVAPA alleviates inflammatory damages in the pulmonary tissues of ARDS mouse model
ARDS model was established by pulmonary instillation of LPS through the trachea in mice. Animals were assigned into 4 groups: sham group: mice treated with sterile saline; Model group: mice stimulated with LPS as the ARDS model; Model + sh-NC group: model group administrated with lentivirus carrying control shRNA. Model + sh-circVAPA group: model group administrated with lentivirus carrying circVAPA shRNA. (A) qRT-PCR analysis of circVAPA, Sirt1 and miR-212-3p levels in the BALF samples. (B) ELISA analysis of inflammatory cytokines in the BALF samples. (C) MPO activities detection in the BALF samples. (D) ROS level measurement in the BALF samples. (E) Flow cytometry analysis of M1 (CD86) and M2 (CD206) marker expression in macrophages isolated from the BALF samples. (F) H&E staining in the pulmonary tissues. (G) Summary of wet/dry ratios of lung tissues. (H) Quantification of protein concentrations in the BALF samples. n = 6 animals in each group. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
Discussion
In this study, we showed that the upregulation of circVAPA in alveolar macrophages was associated with the inflammation in ARDS patients. circVAPA was also upregulated in LPS-stimulated mouse alveolar macrophages (MH-S cells). Silencing circVAPA attenuated the inflammatory activation of MH-S cells and dampened the expression of pyroptosis-related proteins. Knocking down circVAPA mitigated the inflammatory effects of LPS-stimulated MH-S cells on lung epithelial cells (MLE-12), and alleviated the inflammatory damages in the mouse model of ARDS. We further showed that miR-212-3p/Sirt1 axis mediated the functional role of circVAPA in the inflammatory polarization of MH-S cells. Our findings indicate that circVAPA promotes inflammasome activity and macrophage inflammatory polarization by modulating miR-212-3p/Sirt1 axis in ARDS. Targeting circVAPA may be employed to inhibit the inflammatory activation of alveolar macrophages in ARDS.
circVAPA is a novel circRNA which has been implicated in lung cancer development and progression (Hua et al. 2022, 2.Liu et al. 2021). Our data suggest that circVAPA is involved in inflammatory regulation by affecting the expression and activity of inflammasome. Although little is know about the role of circVAPA in pathophysiological conditions with acute inflammation, there is evidence that circVAPA overexpression in psoriatic lesional tissues promotes inflammation in keratinocytes (Chen et al. 2023a, b). Nevertheless, the downstream mediators of circVAPA are known to modulate inflammatory responses. For example, a previous study reported that miR-212-3p suppresses LPS-stimulated inflammatory activation in mouse macrophages (Chen et al. 2017). A recent study also demonstrated that miR-212-3p mitigates neuroinflammation in the rat model of Alzheimer’s disease by regulating inflammasome-related pathway (Nong et al. 2022). Further, there is convincing evidence supporting the immunomodulatory role of Sirt1 by regulating inflammasome activity (Li et al. 2017; Park et al. 2020; Chen et al. 2023a, b; Xia et al. 2021). Sirt1 was also found to dampen inflammasome activation and ameliorate inflammatory damages of lung tissues in the murine sepsis model (Gao et al. 2015). Therefore, these findings and our data imply that circVAPA overexpression promotes macrophage activation by regulating inflammasome activity in ARDS through miR-212-3p/Sirt1 axis.
Macrophages are resident innate immune cells in alveoli, which engulf foreign particle or antigens and eliminate excessive surfactant secreted by alveolar cells to maintain the homoeostasis in alveolar microenvironment (Martin et al. 2021). After the onset of ARDS, macrophages are stimulated to secrete pro-inflammatory cytokines for mounting immunity against pathogens (Tao et al. 2023; Fan and Fan 2018). Paradoxically, the activation of macrophages and immune cell infiltration lead to damages in alveolar cell structure and result in cell death. During the progression of ARDS, the anti-inflammatory polarization of macrophages are required to resolve inflammation and facilitate alveolar structure regeneration (Dang et al. 2022). We also demonstrated that LPS-activated macrophages can suppress the proliferation and induce apoptosis in lung epithelial cells. circVAPA knockdown alleviated the inflammatory activation and damaging effect of LPS-stimulated macrophages. Thus, the in vitro finding may explain the protective effect of circVAPA knockdown against LPS induced pulmonary tissue damages in the mouse model.
It is worth mentioning that clinical evidence showed the connection between alveolar macrophage activation status and the outcomes in ARDS patients (Morrell et al. 2019). M1-related pro-inflammatory gene sets such as Janus kinase/signal transducer and activator of transcription 5 were enriched in macrophages isolated in alive subjects on day 1 of admission. However, by Day 8 the same gene sets were enriched in macrophages collected from dead subjects (Morrell et al. 2019). These findings suggest that persistent inflammation mediated by alveolar macrophages is a risk factor of mortality in ARDS patient. Metabolic reprogramming has been proposed as a strategy to re-polarize M1 macrophages into anti-inflammatory M2 phenotype (Guo et al. 2021). Since circVAPA was found to regulate glucose metabolism in cancer cells (Zhang et al. 2020), and Sirt1 also functions as an important regulator of mitochondrial metabolism including the TCA cycle and oxidative phosphorylation (Chuang et al. 2019; Tang 2016; Elesela et al. 2020), future works are warranted to further decipher whether targeting circVAPA and Sirt1 could induce metabolic rewiring in macrophages.
A key limitation of this study is that we did not investigate the specific mechanisms leading to circVAPA upregulation in alveolar macrophages during ARDS. Recent studies have provided some insights into potential mechanisms of circVAPA regulation in inflammatory conditions. Huang et al. demonstrated that circVAPA modulates macrophage pyroptosis in sepsis-induced acute lung injury through the miR-212-3p/Sirt1/Nrf2/NLRP3 axis (Huang et al. 2024). This suggests that inflammatory stimuli associated with sepsis and acute lung injury may trigger circVAPA expression through activation of these pathways. Additionally, Chen et al. showed that circVAPA contributes to inflammation in keratinocytes via the miR-125b-5p/sirt6 axis in psoriasis indicating that circVAPA regulation may involve multiple miRNA-mediated mechanisms in different cell types and inflammatory conditions. In the context of ARDS and LPS stimulation, several possibilities warrant further investigation regrading circVAPA upregulation. First, LPS may directly activate transcription factors that promote circVAPA expression. Moreover, the pro-inflammatory cytokines produced in response to LPS or during ARDS progression could induce circVAPA as part of a positive feedback loop. Given the role of oxidative stress in ARDS pathogenesis, ROS-sensitive transcription factors might contribute to circVAPA upregulation. Future studies should aim to elucidate these potential mechanisms through analysis of the putative transcription factor binding sites in the circVAPA genomic locus and validate the regulators of circVAPA through experimental manipulation.
It should be noted that the intra-tracheal LPS administration used to induce acute lung injury in the mouse model represents a sterile inflammatory condition, which differs from the infectious etiology that commonly triggers ARDS in clinical settings. Therefore, this model may not fully recapitulate the complex pathophysiology of human ARDS, which is predominantly caused by infections or sepsis. Additionally, in our study, circVAPA was knocked down in the mouse model three days before LPS administration. While this approach allowed us to examine the preventive effects of circVAPA inhibition, it does not directly mimic a therapeutic intervention after the onset of lung injury. Future studies using infection-induced ARDS models and evaluating the therapeutic effects of circVAPA knockdown after the establishment of lung injury would be valuable to further validate the clinical relevance of our findings.
Conclusion
To sum up, our findings suggest that circVAPA upregulation contributes to the inflammatory activation of alveolar macrophages in ARDS. circVAPA promotes the inflammasome activity in macrophages through modulating miR-212-3p/Sirt1 axis. circVAPA may serve as a therapeutic target to suppress the inflammatory damages mediated by alveolar macrophages in ARDS.
-
(A)
TargetScan online resource analysis the interaction sequences between circVAPA/miR-212-3p and Sirt1 mRNA 3’ untranslated region (UTR)/miR-212-3p. (B) Luciferase reporter assay using the WT reporters and MUT reporters. MH-S cells were transfected with si-NC, si-circVAPA, si-circVAPA + miRNA inhibitor control (inh-NC) or si-circVAPA + miRNA inhibitor. (C) qRT-PCR analysis of circVAPA and Sirt1, while miR-212-3p in each experimental group. (D) Western blot analysis of Sirt1 protein levels in each experimental group. n = 3. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
All authors were involved in the conceptualization and design of the study. ML provided experimental ideas. LB completed the first draft of the manuscript. JL performed the material preparation, data collection and analysis. JG was mainly responsible for reviewing the final manuscript. All authors participated in the editorial revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Scientific research projects of Kunming Health Care Commission (2022-06-03-007).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The use of human samples gained the approval of the Medical Ethics Committee of Kunming Children’s Hospital (2024-03-011-K01). The parents of all the recruited subjects signed the informed consent. All the sample handling and data processing steps were following the Declaration of Helsinki. Animal protocols were in compliance with the guidelines of animal care and welfare and were approved by the Ethical Review Committee for Animal Experiments of Kunming Medical University (kmmu20240767).
Consent for publication
All authors are aware of and have consented to publication.
Competing interests
The authors declare no competing interests.
Footnotes
Lingyun Bao is regarded as a first author.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Liu X, Cheng Y, Wang Y, Zhang Y (2021) Circular RNA circVAPA contributes to non-small-cell lung cancer progression via mir-342-3p-dependent regulation of ZEB2. World J Surg Oncol 19(1):335 [DOI] [PMC free article] [PubMed]
- Chai R, Li Y, Shui L, Ni L, Zhang A (2023) The role of pyroptosis in inflammatory diseases. Front Cell Dev Biol 11:1173235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Ma X, Zhang P, Li Q, Liang X, Liu J (2017) MiR-212-3p inhibits LPS-induced inflammatory response through targeting HMGB1 in murine macrophages. Exp Cell Res 350(2):318–326 [DOI] [PubMed] [Google Scholar]
- Chen X, Tang J, Shuai W, Meng J, Feng J, Han Z (2020) Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm Res 69(9):883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xu Z, Wang Y, Xu J, He K, Wang H, Bai X, Xiang G (2023a) CircVAPA contributes to hyper-proliferation and inflammation of keratinocytes through miR-125b-5p/sirt6 axis in psoriasis. Int Immunopharmacol 115:109632 [DOI] [PubMed] [Google Scholar]
- Chen H, Deng J, Gao H, Song Y, Zhang Y, Sun J, Zhai J (2023b) Involvement of the SIRT1-NLRP3 pathway in the inflammatory response. Cell Commun Signal 21(1):185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YC, Chen SD, Jou SB, Lin TK, Chen SF, Chen NC, Hsu CY (2019) Sirtuin 1 regulates mitochondrial Biogenesis and provides an endogenous neuroprotective mechanism against Seizure-Induced neuronal cell death in the Hippocampus following Status Epilepticus. Int J Mol Sci 20(14):3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang W, Tao Y, Xu X, Zhao H, Zou L, Li Y (2022) The role of lung macrophages in acute respiratory distress syndrome. Inflamm Res 71(12):1417–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, Choi AM (2012) Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 185(11):1225–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elesela S, Morris SB, Narayanan S, Kumar S, Lombard DB, Lukacs NW (2020) Sirtuin 1 regulates mitochondrial function and immune homeostasis in respiratory syncytial virus infected dendritic cells. PLoS Pathog 16(2):e1008319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan EKY, Fan J (2018) Regulation of alveolar macrophage death in acute lung inflammation. Respir Res 19(1):50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Ma Z, Hu Y, Chen J, Shetty S, Fu J (2015) Sirt1 restrains lung inflammasome activation in a murine model of sepsis. Am J Physiol Lung Cell Mol Physiol 308(8):L847–L853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Islam R, Zhang S, Fang J (2021) Metabolic reprogramming of macrophages and its involvement in inflammatory diseases. EXCLI J 20:628–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Wang X, Ma L, Li J, Cao G, Zhang S, Lin W (2022) CircVAPA promotes small cell lung cancer progression by modulating the mir-377-3p and miR-494-3p/IGF1R/AKT axis. Mol Cancer 21(1):123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Lin J, Wu Z, Li Y (2024) Circular RNA circVAPA modulates macrophage pyroptosis in sepsis-induced acute lung injury through targeting miR-212-3p/Sirt1/Nrf2/NLRP3 axis. Int J Exp Pathol 105(1):21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku S, Nguyen CD, Htet NN, Tutera D, Barr J, Paintal HS, Kuschner WG (2020) Acute respiratory distress syndrome: etiology, Pathogenesis, and Summary on Management. J Intensive Care Med 35(8):723–737 [DOI] [PubMed] [Google Scholar]
- Li Y, Yang X, He Y, Wang W, Zhang J, Zhang W, Jing T, Wang B, Lin R (2017) Negative regulation of NLRP3 inflammasome by SIRT1 in vascular endothelial cells. Immunobiology 222(3):552–561 [DOI] [PubMed] [Google Scholar]
- Liu C, Xiao K, Xie L (2022) Advances in the regulation of macrophage polarization by mesenchymal stem cells and implications for ALI/ARDS treatment. Front Immunol 13:928134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyt CE, Bouadma L, Morris AC, Dhanani JA, Kollef M, Lipman J, Martin-Loeches I, Nseir S, Ranzani OT, Roquilly A, Schmidt M, Torres A, Timsit JF (2020) Pulmonary infections complicating ARDS. Intensive Care Med 46(12):2168–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Karki R, Kanneganti TD (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277(1):61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FP, Jacqueline C, Poschmann J, Roquilly A (2021) Alveolar macrophages: adaptation to their anatomic niche during and after inflammation. Cells 10(10):2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Ware LB, Zimmerman GA (2012) The acute respiratory distress syndrome. J Clin Invest 122(8):2731–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey MJ, Steinberg BE, Goldenberg NM (2021) Inflammasome activation in acute lung injury. Am J Physiol Lung Cell Mol Physiol 320(2):L165–L178 [DOI] [PubMed] [Google Scholar]
- Meyer NJ, Gattinoni L, Calfee CS (2021) Acute respiratory distress syndrome. Lancet 398(10300):622–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell ED, Bhatraju PK, Mikacenic CR, Radella F 2nd, Manicone AM, Stapleton RD, Wurfel MM, Gharib SA (2019) Alveolar Macrophage Transcriptional Programs Are Associated with outcomes in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 200(6):732–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong W, Bao C, Chen Y, Wei Z (2022) Mir-212-3p attenuates neuroinflammation of rats with Alzheimer’s disease via regulating the SP1/BACE1/NLRP3/Caspase-1 signaling pathway. Bosn J Basic Med Sci 22(4):540–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeyemi Y, Moraes AGD, Gajic O (2020) What factors predispose patients to acute respiratory distress syndrome? Evidence-Based Pract Crit Care.:103–108e1
- Park S, Shin J, Bae J, Han D, Park SR, Shin J, Lee SK, Park HW (2020) SIRT1 alleviates LPS-Induced IL-1β production by suppressing NLRP3 inflammasome activation and ROS production in Trophoblasts. Cells 9(3):728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saguil A, Fargo MV (2020) Acute respiratory distress syndrome: diagnosis and management. Am Fam Physician 101(12):730–738 [PubMed] [Google Scholar]
- Sivapalan P, Bonnesen B, Jensen JU (2020) Novel perspectives regarding the Pathology, inflammation, and biomarkers of Acute Respiratory Distress Syndrome. Int J Mol Sci 22(1):205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenkle NT, Serezani CH, Pua HH (2023) MicroRNAs in macrophages: regulators of activation and function. J Immunol 210(4):359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KV, Deng M, Ting JP (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19(8):477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL (2016) Sirt1 and the Mitochondria. Mol Cells 39(2):87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Xu Y, Zhang S (2023) The role of macrophages and alveolar epithelial cells in the development of ARDS. Inflammation 46(1):47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GW, Berg NK, Reskallah A, Yuan X, Eltzschig HK (2021) Acute Respiratory Distress Syndrome Anesthesiology 134(2):270–282 [DOI] [PMC free article] [PubMed]
- Xia DY, Yuan JL, Jiang XC, Qi M, Lai NS, Wu LY, Zhang XS (2021) SIRT1 promotes M2 Microglia polarization via reducing ROS-Mediated NLRP3 Inflammasome Signaling after Subarachnoid Hemorrhage. Front Immunol 12:770744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Xie F, Tang X, Wang T, Wang S (2020) Insights into the role of circular RNA in macrophage activation and fibrosis disease. Pharmacol Res 156:104777 [DOI] [PubMed] [Google Scholar]
- Yang SC, Tsai YF, Pan YL, Hwang TL (2021) Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed J 44(4):439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Li F, Jin S, Ho PC, Liu PS, Xie X (2023) Functional polarization of tumor-associated macrophages dictated by metabolic reprogramming. J Exp Clin Cancer Res 42(1):245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xu Y, Yamaguchi K, Hu J, Zhang L, Wang J, Tian J, Chen W (2020) Circular RNA circVAPA knockdown suppresses colorectal cancer cell growth process by regulating miR-125a/CREB5 axis. Cancer Cell Int 20:103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Chu C, Wu Z, Liu F, Xie J, Yang Y, Qiu H (2021) IFIH1 contributes to M1 Macrophage polarization in ARDS. Front Immunol 11:580838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Shi J, Shao F (2018) Inflammatory caspases: activation and cleavage of Gasdermin-D in Vitro and during pyroptosis. Methods Mol Biol 1714:131–148 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Li G (2020) Mechanisms and therapeutic regulation of pyroptosis in inflammatory diseases and Cancer. Int J Mol Sci 21(4):1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.