Abstract
Background:
Cognitive impairment (CI) is one of the main features of multiple sclerosis (MS). Cognitive rehabilitation (CR) programs are crucial for improving cognition and computer-assisted cognitive rehabilitation is considered as an effective method for cognition rehabilitation. To assess the effects of computer-based cognitive rehabilitation program on cognition in patients with multiple sclerosis (MS).
Methods:
We performed a comprehensive search in PubMed, Scopus, Web of Science, EMBASE, and Google Scholar databases along with gray literature up to September 2021. Randomized clinical trials, articles had been published in the English language. We evaluated the risk of potential bias via the Cochrane Collaboration's tool for assessing the risk of bias. Standardized mean difference (SMD) was calculated.
Results:
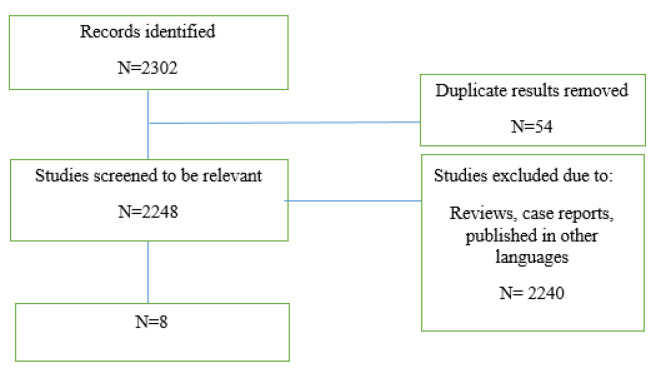
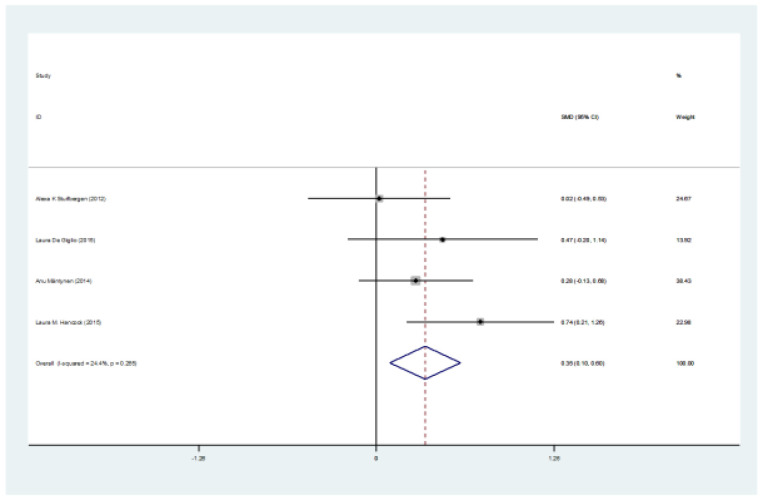
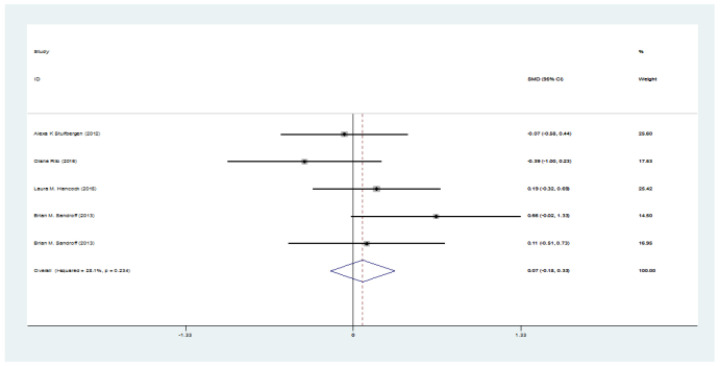
The preliminary search retrieved 2302 articles by literature search, after deleting duplicates 2248 remained. Eight articles remained for meta-analysis. Totally, 235 patients in intervention group and 192 in control group were evaluated. Mean age ranged from 43.5-52 years. The SMD of PASAT (Paced Auditory Serial Addition Test) (Case-control) test was 0.35 (95%CI:0.1-0.66) (I2:24.4%, P=0.2). The SMD of SDMT (Case-control) test was 0.07 (95%CI:-0.18-0.33). The SMD of PASAT before and after intervention in case group was 0.68 (95%CI:0.45-0.91) (I2:40%, P=0.15). The SMD of SDMT before and after intervention in case group was 0.44 (95%CI:0.21-0.66) (I2:40%, P=0.15).
Conclusions:
The results of this systematic and meta-analysis showed that computerized cognitive rehabilitation program is effective in improving PASAT score.
Key Words: Multiple sclerosis, Cognition, Rehabilitation
Multiple sclerosis, a demyelinating disease of the central nervous system (CNS), affects youths all over the world with a wide range of physical and mental complications (1). MS progressing over time and more than two thirds of the patients’ experience cognitive impairment (CI) (2, 3) affecting negatively the quality of life (4). CI is associated with unemployment, job loss, poor adherence to medications, difficulty with everyday tasks, and social/marital dysfunction (5). Different aspects of cognition are impaired in patients with MS such as recent memory, attention, information processing (processing speed), executive functions (verbal learning), and visuospatial abilities (6-8). Medications such as memantine, rivastigmine and donepezil are used for cognition rehabilitation in MS cases while their efficacy is not satisfactory (9-11). Cognitive rehabilitation (CR) programs are crucial for improving cognition in MS cases which include restitution strategies to rehabilitate cognitive skills and improving coping strategies (12). CR also improves functional and structural neuroplasticity, decreases CI, and finally enhances fatigue, mood, and quality of life (13, 14). Computer-assisted cognitive rehabilitation is considered as an effective method for rehabilitation (15) and is supported by functional MRI, showing neuroplasticity (16).
It focuses on repeated practice on controlled learning actions over arranged sessions, and could target specific cognitive processes (17). Today, there are studies evaluating the effects of computerized cognitive rehabilitation (CCR) on cognitive status of MS patients. We designed this systematic review and meta-analysis to assess the effects of computer-based cognitive rehabilitation program on cognition in patients with MS.
Methods
Data search: Two independent researchers comprehensively searched PubMed, Scopus, EMBASE, Web of Science, and Google Scholar along with gray literature up to September 2021. The study is written based on Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA). The search strategy was: (“Multiple Sclerosis” OR “MS” OR “Relapsing-Remitting Multiple Sclerosis” OR “Chronic Progressive Multiple Sclerosis” OR “demyelinating diseases” OR “demyelinating disorders” OR “autoimmune demyelinating disease" AND “Cognitive Behavior Therapy” OR “Cognitive Therapy” OR “Cognitive Behavior Therapy” OR “Cognitive Psychotherapy” OR “Cognitive Therapy” OR “Cognition Therapy” OR (cognitive* AND behavior* AND therapy*)).
Inclusion criteria were: Randomized clinical trials which has computer-based cognitive rehabilitation program in one arm, articles which had been published in the English language.
Exclusion criteria: Cohort, cross-sectional, case-report, letters.
Selection and data collection: After obtaining the first results, the duplicates were deleted. Then titles and abstracts were evaluated by two researchers and full texts of eligible studies were evaluated. Full texts of eligible studies were evaluated by two researchers and data were extracted by each one and entered in Excel files. Data regarding first author, country of origin, number of enrolled patients, mean age, F/M ratio, mean EDSS, mean duration of the disease, and mean scores of cognition tests. In case of discrepancies, the third one solved the problem.
We collected risk of bias assessment: Two independent researchers evaluated the risk of potential bias using the Cochrane Collaboration's tool for assessing the risk of bias (includes comprising the six domains related to selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases). Each domain is scored as low risk, high risk, or unclear risk (18). In the case of disagreement, the third one solved the problem.
Statistical analysis: All statistical analyses were performed using STATA (Version 14.0; Stata Corp LP, College Station, TX, USA). We used random effects model.
To determine heterogeneity, inconsistency (I2) was calculated. Standardized mean difference (SMD) was calculated. Sandroff et al. compared the control group one with PDDS between 0-2 and another time with PDDS 3-6.
Results
The preliminary search retrieved 2302 articles by literature search, after deleting duplicates 2248 remained. Finally, eight articles remained for meta-analysis (figure 1).
Figure 1.
Flow diagram summarizing the selection of eligible studies
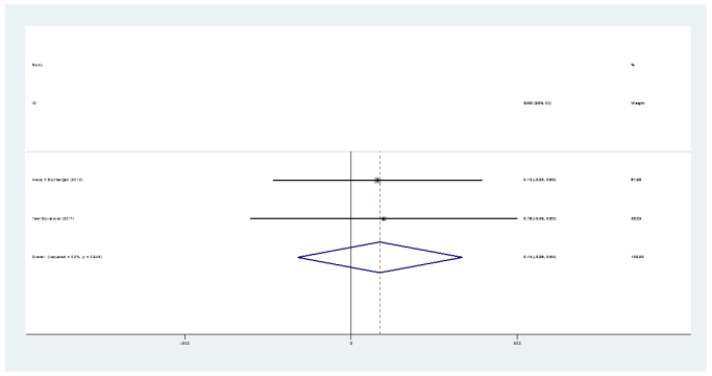
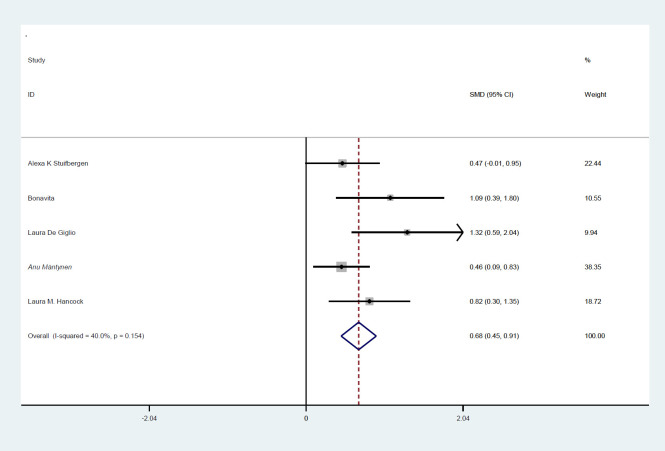
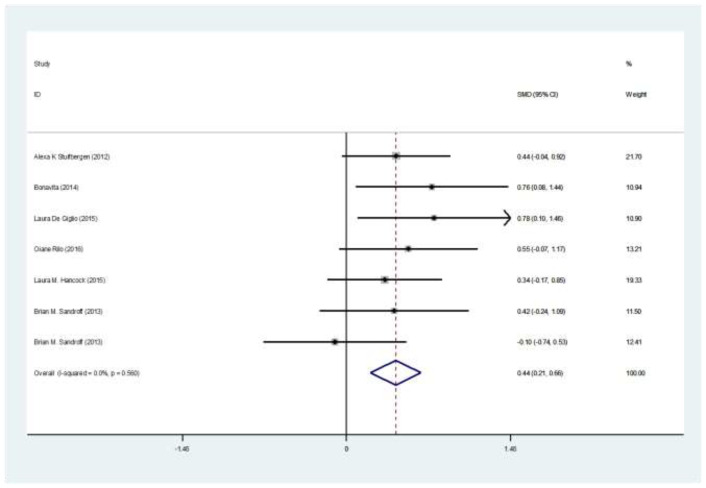
Totally, 235 patients in intervention group and 192 in control group were evaluated. Mean age ranged from 43.5 to 52 years. Five studies were from the USA, one from Finland, and two from Italy. The mean age ranged between 44, and 51 years, and EDSS ranged between 3.2 and 5 (table 1). The SMD of PASAT (case-control) test was 0.35 (95%CI:0.1-0.66) (I2:24.4%, P=0.2) (figure 2). The SMD of SDMT (case-control) test was 0.07 (95%CI: -0.18-0.33) (I2:28.1%, P=0.2) (figure 3). The SMD of CVLT (case-control) test was 0.14 (95%CI: -0.26-0.54) (I2:0%) (figure 4). The SMD of PASAT before and after intervention in case group was 0.68 (95%CI:0.45-0.91) (I2:40%, P=0.15) (figure 5). The SMD of SDMT before and after intervention in case group was 0.44 (95%CI:0.21-0.66) (I2:40%, P=0.15) (figure 6). The quality assessment of included studies are summarized in table 2.
Table 1.
Data extracted from included studies
| Author | Year | Country |
# MS
CASE - CONTROL |
EDSS | Age | Education Level | Disease Duration | PASAT |
SDMT
Case/control |
CVLT
long delay |
Key Findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alexa K Stuifbergen (19) | 2012 | USA | 34 F: 29 M: 5 |
27 F: 25 M: 2 |
42.6 (10.7) 47.4 (9.6) |
41.9 (12.4) 47.2 (10.70 |
44.6 (10.4) 4 9.7 (12.7) |
46.3 (13.7) 50.6 (13.1) |
11.3 (3.8) 12.5 (4.1) |
10.9 (4.0) 11.4 (4.1) |
Given the large relative increase in use of compensatory strategies by the intervention group, it holds promise for enhancing cognitive function in persons with multiple sclerosis. | ||||||||
| S. Bonavita (20) | 2014 | Italy | 18 F: 18 M: 0 |
- | 5 (1) | - | 49 (8) | - | 11 (4) | - | 22 (8) | - | 30.62 (9.41) 40.00 (7.76) |
23.4 (4.2) 28.2 (7.9) |
This exploratory study suggests that in cognitively impaired RRMS patients, computer-aided cognitive rehabilitation improves cognitive performances (i.e., processing speed and visual and verbal sustained memory), and increases FC in the PCC and IPC of the DMN. | ||||
| Laura De Giglio (21) | 2015 | Italy | 18 F: 14 M: 4 |
17 F: 12 M:5 |
3.25 (2-6) median – range |
2 (2-4) median - range |
44.64 (7.63) |
42.99 (9.42) | 13.94 (2.90) | 14.06 (3.57) | 13.28 (8.28) | 11.4 (7.45) | 24.83 (6.35) 36.28 (10.5) |
32.12 (9.82) 31.69 (9.06) |
39.22 (9.68) 47.44 (11.465) |
34.56 (8.03) 38.59 (8.6) |
Results of this study provides that a home-based cognitive rehabilitation program may improve cognitive functions, some aspects of QoL, and cognitive fatigue in MS patients. | ||
| Yael Goverover (22) | 2017 | USA | 19 F: 13 M: 6 |
18 F: 12 M: 4 |
50.15 (9.121) | 48.5 (8.8) |
16 (2.1) | 15.2 (2.3) | 11.1 (6.5) | 11.4 (7.1) |
10.1 (3.9) 11.6 (3.4) |
8.8 (3.7) 10.5 (4) |
The results of this study proved that the self-GEN behavioral intervention improves memory, self-regulation, functional status, affective symptomatology, and QOL in MS patients. | ||||||
| Oiane Rilo (23) | 2016 | USA | 21 F: 13 M: 8 |
21 F: 14 M: 7 |
3.52 (1.59) | 2.50 (1.85) | 43.90 (9.51) |
43.67 (6.89) |
13.00 (3.03) | 13.95 (3.12) | 9.95 (7.84) | 10.67 (5.79) | 36.67 (9.23) 42.62 (12.46) |
46.43 (13.46) 47.52 (13) |
Patients receiving cognitive rehabilitation showed improvements in several cognitive domains. This preliminary study thus provides evidence supporting the efficacy of this integrative group-based cognitive rehabilitation intervention in MS. | ||||
| Anu Mäntynen (24) | 2014 | Finland | 58 F: 45 M: 13 |
40 F: 31 M: 9 |
43.5 (8.7) | 44.1 (8.8) | 13.6 (2.3) | 13.8 (2.6) | 9.2 (6.6) | 10.1 (7.1) | 41.3 (11.7) 46.7 (11.8) |
37.4 (11.9) 43.5 (11) |
Strategy-oriented neuropsychological rehabilitation did not improve cognitive performance but reduced perceived cognitive deficits in MS. | ||||||
| Laura M. Hancock (25) | 2015 | USA | 30 | 30 | 50.65 (6.32) | 49.13 (10.09) | 14.65 (2.06) | 16.33 (3.11) | 80.79 (15.99) 93.64 (15.49) |
76.00 (27.06) 78.57 (24.65) |
49.40 (11.02) 53.13 (10.79) |
49.40 (19.16) 50.67 (15.86) |
The current study supports that cognitive training with MS patients may produce moderate improvement in select areas of cognitive functioning. | ||||||
| Brian M. Sandroff (26) | 2013 | USA | 18 | 18 | 45.4 (10.1 | 49.0 (10.0) | 9.0 (7.2) | 12.7 (9.7) | 60.83 (13.6) 66.44 (12.8) |
57.25 (11.7) 58.38 (11.9) |
PDDS 0–2 The results showed that physical activity is a promising tool for managing cognitive impairment and impaired walking performance in patients with MS. Furthermore, physical activity might affect cognition specifically and walking performance non-specifically. |
||||||||
| Brian M. Sandroff (26) | 2013 | USA | 19 | 21 | 52.1 (6.4) | 51.6 (6.7) | 12.3 (6.3) | 14.0 (9.0) | 54.56 (10.8) 53.31 (12.3) |
51.10 (11.1) 52.15 (10.1) |
PDDS 3–6 The results showed that physical activity is a promising tool for managingcognitive impairment and impaired walking performancein patients with MS. Further, physical activity might affect cognition specifically and walking performance non-specifically. |
||||||||
MS: Multiple Sclerosis, PASAT: Paced Auditory Serial Addition Test, SDMT: The Symbol Digit Modalities Test, CVLT: California Verbal Learning Test, RRMS: Relapsing-Remitting Multiple Sclerosis, FC: Functional Connectivity, QoL: Quality of Life, PCC: Posterior Cingulate Cortex, IPC: Inferior Parietal Cortex, DMN: Default Mode Network, Patient Determined Disease Steps (PDDS).
Figure 2.
The SMD of PASAT (intervention-control) test
Figure 3.
The SMD of SDMT (intervention-control) test
Figure 4.
The SMD of CVLT (intervention-control) test
Figure 5.
The SMD of PASAT (Case-control) test before and after intervention in case group
Figure 6.
The SMD of SDMT before and after intervention in case group
Table 2.
The quality assessment of included studies
| Author | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and researchers (Performance bias) |
Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) |
|---|---|---|---|---|---|---|
| Alexa K Stuifbergen | HRB | URB | HRB | URB | URB | LRB |
| S. Bonavita | HRB | HRB | HRB | LRB | LRB | LRB |
| Laura De Giglio | LRB | LRB | LRB | LRB | LRB | LRB |
| Yael Goverover | LRB | URB | HRB | LRB | URB | LRB |
| Oiane Rilo | LRB | LRB | HRB | HRB | LRB | LRB |
| Anu Mäntynen | LRB | LRB | URB | HRB | LRB | LRB |
| Laura M. Hancock | HRB | HRB | LRB | LRB | LRB | LRB |
| Brian M. Sandroff | LRB | LRB | URB | HRB | URB | LRB |
Discussion
The results of the current study show that the pooled SMD of PASAT after intervention was 0.36(95%CI:0.1-0.6) indicating that computerized cognitive rehabilitation improved cognition significantly. We also found that the rehabilitation grogram improved SDMT and CVLT scores in intervention group more than controls although the differences were not significant.
Stuifbergen enrolled 61 MS patients (34 in intervention group and 27 in control group). The intervention group underwent the eight-week MAPSS-MS program (Memory, Attention, and Problem Solving Skills for Persons with Multiple Sclerosis) which is a computer-assisted cognitive rehabilitation program. They found that all cognition scores (PASAT, DSMT, CVLT) improved in both groups, but the differences between the two groups were not significant (19). Bonavita et al. enrolled 32 RRMS patients (18 in computer-based cognitive rehabilitation program and 14 in aspecific cognitive training (aCT) program). They reported significant improvement in PASAT, and SDMT tests after intervention (20). Sandroff et al. enrolled 37 cases in intervention (digital a physical activity behavioral intervention) and 41 in control groups. They investigated that cognitive improvement was better in intervention group with PDDS between 0-2. Comparing with control group, the SMD of patients with PDDS between 0-2 was 0.66 and the SMD of the patients with PDDS between 3-6 was 0.11 (26). Cognitive impairment is common in MS, and is present since the early stages of the disease (20). It was first suggested that cognitive impairment is the white matter dysfunction in MS while gray matter atrophy is detected in cases with cognitive impairment (27). Disease-modifying therapies (DMT) are suitable for reducing neurodegeneration and cognitive impairment in MS (28) while medications such as rivastigmine and donepezil are considered to have less effects for cognitive impairment in MS (9, 10).
There is not enough evidence confirming the effects of memantine, a non-competitive N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, for improving MS-related cognitive impairment (27). Cognitive rehabilitation is a beneficial, neurobehavioral therapeutic option for MS patients even in the late stages of the disease (29). Computerized cognitive rehabilitation programs are safe, cost effective programs that help patients to recover soon, but the problem is that after ceasing the sessions, the effects may disappear. Different tests have been developed for cognition assessment in MS. Both PASAT and SDMT are used for cognition evaluation in MS while they provide data regarding information processing speed and working memory (30, 31). Williams et al. found that PASAT and SDMT evaluate different domains of the cognition (32) while Drake et al. found that SDMT could discriminate MS cases from controls in a better way and it takes less time, needs no electrical equipment, and level of stress is low (33). In another study, Brochet et al. found that SDMT is correlated with EDSS consistently (34), while others support the strong correlation between SDMT and brain MRI pathologies (35-38). This study highlights the gap of knowledge of small amount of studies in this field. So, larger clinical trials are recommended. This systematic review had some limitations. First, the number of enrolled patients in each study was limited. Second, all studies did not use the same cognitive assessment methods. Larger, multicentric original studies with a unique test is recommended. The results of this systematic review highlights the positive impact of computerized cognitive rehabilitation program on cognitive status of patients with MS, which could be used in clinical practice to help patients, but more multicentric larger studies are needed to evaluate the effects of computerized cognitive rehabilitation program on cognitive status of different MS population.
Acknowledgments
None.
Funding:
The authors declare no funding.
Conflict of Interests:
The authors declare no conflict of interest.
Authors’ contribution:
ANM: study conception, article writing. OM: data gathering, article writing. SB: data gathering, article writing. MAS: data gathering, article writing. MG: study design, data analysis, article writing.
References
- 1.Ghajarzadeh M, Jalilian R, Eskandari G, et al. Fatigue in multiple sclerosis: relationship with disease duration, physical disability, disease pattern, age and sex. Acta Neurologica Belgica. 2013;113:411–4. doi: 10.1007/s13760-013-0198-2. [DOI] [PubMed] [Google Scholar]
- 2.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–51. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 3.Benedict RH, Zivadinov R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol. 2011;7:332–42. doi: 10.1038/nrneurol.2011.61. [DOI] [PubMed] [Google Scholar]
- 4.Langdon DW. Cognition in multiple sclerosis. Curr Opin Neurol. 2011;24:244–9. doi: 10.1097/WCO.0b013e328346a43b. [DOI] [PubMed] [Google Scholar]
- 5.Munger KC, Martinez AP, Hyland MH. The impact of cognitive rehabilitation on quality of life in multiple sclerosis: A pilot study. Mult Scler J Exp Transl Clin. 2021;7:20552173211040239. doi: 10.1177/20552173211040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierson SH, Griffith N. Treatment of cognitive impairment in multiple sclerosis. Behav Neurol. 2006;17:53–67. doi: 10.1155/2006/545860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien AR, Chiaravalloti N, Goverover Y, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: a review of the literature. Arch Phys Med Rehabil. 2008;89:761–9. doi: 10.1016/j.apmr.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Goretti B, Portaccio E, Zipoli V, Hakiki B, Siracusa G, Sorbi S, et al. Impact of cognitive impairment on coping strategies in multiple sclerosis. Clin Neurol Neurosurg. 2010;112:127–30. doi: 10.1016/j.clineuro.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Wiebenga OT, Heilman KM, Krupp LB, Hulst H, Kooi EJ, Killestein J, et al. Multicenter randomized clinical trial of donepezil for memory impairment in multiple sclerosis. Neurology. 2011;77:1998–2000. [PubMed] [Google Scholar]
- 10.Huolman S, Hämäläinen P, Vorobyev V, et al. The effects of rivastigmine on processing speed and brain activation in patients with multiple sclerosis and subjective cognitive fatigue. Mult Scler. 2011;17:1351–61. doi: 10.1177/1352458511412061. [DOI] [PubMed] [Google Scholar]
- 11.Turalde CWR, Espiritu AI, Anlacan VMM. Memantine for multiple sclerosis: A systematic review and meta-analysis of randomized trials. Front Neurol. 2020;11:574748. doi: 10.3389/fneur.2020.574748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cicerone KD, Goldin Y, Ganci K, et al. Evidence-based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Arch Phys Med Rehabil. 2019;100:1515–33. doi: 10.1016/j.apmr.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Flachenecker P, Meissner H, Frey R, Guldin W. Neuropsychological training of attention improves MS-related fatigue: Results of a randomized, placebo-controlled, double-blind pilot study. Eur Neurol. 2017;78:312–7. doi: 10.1159/000481941. [DOI] [PubMed] [Google Scholar]
- 14.Tacchino A, Podda J, Bergamaschi V, Pedullà L, Brichetto G. Cognitive rehabilitation in multiple sclerosis: Three digital ingredients to address current and future priorities. Front Hum Neurosci. 2023;17:1130231. doi: 10.3389/fnhum.2023.1130231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell J, Langdon D, Cercignani M, Rashid W. A randomised controlled trial of efficacy of cognitive rehabilitation in multiple sclerosis: a cognitive, behavioural, and MRI study. Neural Plast. 2016;2016:4292585. doi: 10.1155/2016/4292585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filippi M, Riccitelli G, Mattioli F, et al. Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures an explorative study. Radiology. 2012;262:932–40. doi: 10.1148/radiol.11111299. [DOI] [PubMed] [Google Scholar]
- 17.Keshavan MS, Vinogradov S, Rumsey J, Sherrill J, Wagner A. Cognitive training in mental disorders: update and future directions. Am J Psychiatry. 2014;171:510–22. doi: 10.1176/appi.ajp.2013.13081075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ . 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuifbergen AK, Becker H, Perez F, et al. A randomized controlled trial of a cognitive rehabilitation intervention for persons with multiple sclerosis. Clin Rehabil. 2012;26:882–93. doi: 10.1177/0269215511434997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonavita S, Sacco R, Della Corte M, et al. Computer-aided cognitive rehabilitation improves cognitive performances and induces brain functional connectivity changes in relapsing remitting multiple sclerosis patients: an exploratory study. J Neurol. 2015;262:91–100. doi: 10.1007/s00415-014-7528-z. [DOI] [PubMed] [Google Scholar]
- 21.De Giglio L, De Luca F, Prosperini L, et al. A low-cost cognitive rehabilitation with a commercial video game improves sustained attention and executive functions in multiple sclerosis: a pilot study. Neurorehabil Neural Repair. 2015;29:453–61. doi: 10.1177/1545968314554623. [DOI] [PubMed] [Google Scholar]
- 22.Goverover Y, Chiaravalloti N, Genova H, DeLuca J. A randomized controlled trial to treat impaired learning and memory in multiple sclerosis: The self-GEN trial. Mult Scler. 2018;24:1096–104. doi: 10.1177/1352458517709955. [DOI] [PubMed] [Google Scholar]
- 23.Rilo O, Peña J, Ojeda N, et al. Integrative group-based cognitive rehabilitation efficacy in multiple sclerosis: a randomized clinical trial. Disabil Rehabil. 2018;40:208–16. doi: 10.1080/09638288.2016.1250168. [DOI] [PubMed] [Google Scholar]
- 24.Mäntynen A, Rosti-Otajärvi E, Koivisto K, et al. Neuropsychological rehabilitation does not improve cognitive performance but reduces perceived cognitive deficits in patients with multiple sclerosis: a randomised, controlled, multi-centre trial. Mult Scler. 2014;20:99–107. doi: 10.1177/1352458513494487. [DOI] [PubMed] [Google Scholar]
- 25.Hancock LM, Bruce JM, Bruce AS, Lynch SG. Processing speed and working memory training in multiple sclerosis: A double-blind randomized controlled pilot study. J Clin Exp Neuropsychol. 2015;37:113–27. doi: 10.1080/13803395.2014.989818. [DOI] [PubMed] [Google Scholar]
- 26.Sandroff BM, Klaren RE, Pilutti LA, Dlugonski D, Benedict RH, Motl RW. Randomized controlled trial of physical activity, cognition, and walking in multiple sclerosis. J Neurol. 2014;261:363–72. doi: 10.1007/s00415-013-7204-8. [DOI] [PubMed] [Google Scholar]
- 27.Turalde CWR, Espiritu AI, Anlacan VMM. Memantine for Multiple Sclerosis: A Systematic Review and Meta-Analysis of Randomized Trials. Front Neurol. 2021;11:574748. doi: 10.3389/fneur.2020.574748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kappos L, Freedman MS, Polman CH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 2009;8:987–97. doi: 10.1016/S1474-4422(09)70237-6. [DOI] [PubMed] [Google Scholar]
- 29.Vilou I, Bakirtzis C, Artemiadis A, et al. Computerized cognitive rehabilitation for treatment of cognitive impairment in multiple sclerosis: an explorative study. J Integr Neurosci. 2020;19:341–7. doi: 10.31083/j.jin.2020.02.35. [DOI] [PubMed] [Google Scholar]
- 30.Parmenter B, Weinstock-Guttman B, Garg N, Munschauer F, Benedict RH. Screening for cognitive impairment in multiple sclerosis using the Symbol Digit Modalities Test. Mult Scler. 2007;13:52–7. doi: 10.1177/1352458506070750. [DOI] [PubMed] [Google Scholar]
- 31.Possa MF. Neuropsychological measures in clinical practice. Neurol Sci. 2010;31:219–22. doi: 10.1007/s10072-010-0374-6. [DOI] [PubMed] [Google Scholar]
- 32.Williams J, O’Rourke K, Hutchinson M, Tubridy N. The face-symbol test and the symbol-digit test are not reliable surrogates for the Paced Auditory Serial Addition Test in multiple sclerosis. Mult Scler. 2006;12:599–604. doi: 10.1177/1352458506070752. [DOI] [PubMed] [Google Scholar]
- 33.Drake A, Weinstock-Guttman B, Morrow S, et al. Psychometrics and normative data for the multiple sclerosis functional composite: replacing the PASAT with the symbol digit modalities test. Mult Scler. 2010;16:228–37. doi: 10.1177/1352458509354552. [DOI] [PubMed] [Google Scholar]
- 34.Brochet B, Deloire M, Bonnet M, et al. Should SDMT substitute for PASAT in MSFC? A 5-year longitudinal study. Mult Scler. 2008;14:1242–9. doi: 10.1177/1352458508094398. [DOI] [PubMed] [Google Scholar]
- 35.Christodoulou C, Krupp L, Liang Z, et al. Cognitive performance and MR markers of cerebral injury in cognitively impaired MS patients. Neurology. 2003;60:1793–8. doi: 10.1212/01.wnl.0000072264.75989.b8. [DOI] [PubMed] [Google Scholar]
- 36.Benedict RH, Weinstock-Guttman B, Fishman I, et al. Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol. 2004;61:226–30. doi: 10.1001/archneur.61.2.226. [DOI] [PubMed] [Google Scholar]
- 37.Bermel RA, Bakshi R, Tjoa C, Puli SR, Jacobs L. Bicaudate ratio as a magnetic resonance imaging marker of brain atrophy in multiple sclerosis. Arch Neurol. 2002;59:275–80. doi: 10.1001/archneur.59.2.275. [DOI] [PubMed] [Google Scholar]
- 38.Houtchens M, Benedict R, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69:1213–23. doi: 10.1212/01.wnl.0000276992.17011.b5. [DOI] [PubMed] [Google Scholar]