Abstract
Background:
Preeclampsia (PE) caused 30%–40% of maternal and newborn deaths worldwide. Despite unclear exact cause, strategies exist to mitigate less severe PE effects. This review explores PE etiology, pathophysiology, risk factors, impact, and prevention.
Methods:
Searching Scopus, PubMed, ProQuest, Google Scholar, and Science Direct for “preeclampsia and pregnancy” and “prevention” yielded 2012–2022 articles.
Results:
Preeclampsia features abnormal placental changes, altered immunity response, trophoblast apoptosis, and reduced uterine perfusion. Risk factors include hypertension history, nulliparity, age over 40, BMI over 35 kg/m2, family history, amniotic pregnancy, and long pregnancy interval. This condition risks cardiovascular and neonatal morbidity, straining health resources. Prevention involves aspirin, vitamin D, exercise, folic acid, diet, early screening, and antenatal care.
Conclusion:
Findings emphasize enhancing health literacy and preeclampsia education in prenatal care to mitigate PE risk among women. Further research, novel therapies, and assessing prevention strategies with accessible educational materials and multidisciplinary approaches are warranted to enhance pregnant women’s health literacy and decrease PE risk.
Keywords: Preeclampsia, Etiology, Risk factor, Impact, Prevention
Introduction
Preeclampsia (PE) is a key public health problem. It happens after 20 wk of gestation. It causes high blood pressure and proteinuria (1, 2). PE is a multisystem disorder of pregnancy characterized by dysfunction of the placenta and maternal vasculature, and is significantly associated with maternal and fetal morbidity and mortality, affecting more than 10 million women and annually contributing to more than 76,000 maternal deaths and 500,000 infant deaths (3,4). PE is significant in increasing infant and maternal mobility and mortality rates in the range of 30–40% worldwide (5–7). PE is often associated with complications involving other organs. These can arise in pregnancy and the postpartum period. PE occurs in about 4.6% of pregnancies worldwide (8). Preeclampsia affects 2.8% of live births on average in developing countries compared to 0.4% in developed countries, a seven-fold increase from previous incidence rates (9).
Research conducted by Rachael Fox et al. (10), along with the N. Stitterich et al. Another study (11), highlight knowledge gaps in the diagnosis, management and prevention of preeclampsia. Wainstock et al. and Stitterich et al. examined risk factors, including previous pregnancy complications and provided insights into the prevention of preeclampsia.
These studies contribute to comprehending the risk, impact, and prevention strategies of preeclampsia, advocating interventions like antenatal care, lifestyle adjustments, and nutritional supplementation to lessen preeclampsia incidence and enhance pregnancy outcomes.
Search Method
This study systematically reviewed preeclampsia’s causes, occurrence, risk factors, impact, and prevention. It searched multiple databases including Scopus, PubMed, ProQuest, Google Scholar, and Science Direct using keywords “preeclampsia and pregnancy” and “preeclampsia prevention” from 2012 to 2022.
Inclusion criteria included articles on preeclampsia etiology, pathophysiology, risk factors, impact, and prevention, published in English and available in full text. Exclusion criteria involved articles single case studies, and editorial reviews or commentaries. Study selection included screening titles and abstracts.
Etiology of Preeclampsia
The etiology of PE typically entails placental abnormalities and suboptimal maternal-placental interactions (12). The placenta contributes to maternal pregnancy adaptation and fetal development (13). The precise cause of PE remains uncertain (14, 15) . Epidemiological and experimental evidence shows placental angiogenic factor imbalance contributes to maternal preeclampsia symptoms (16, 17). PE and intrauterine growth restriction (IUGR) are frequent pregnancy complications linked to abnormal placental development (18–20). In preeclampsia, chronic stresses such as renal, metabolic, or autoimmune diseases appear prevalent (21). Maternal vascular and endothelial responses may be particularly sensitive to placental factors (22). Sequence variants associated with preeclampsia in the maternal genome at ZNF831/20q13 and FTO/16q12 have been identified in European and Central Asian mothers (23). Key immune checkpoint receptors like T-cell immunoglobulin mucin-3 (Tim-3) and Programmed cell death-1 (PD-1) regulate maternal antibodies response to infant antigens in pregnancy, where disrupted immune regulatory mechanisms contribute to adverse outcomes like preeclampsia (24). Pro- and anti-inflammatory cytokines influence placental development and function, potentially contributing to various pregnancy-related disorders like preeclampsia (25). Several studies have reported using biochemical markers, like pregnancy-associated protein and placental growth factor, in PE screening (26). In the third trimester, PE correlates with altered gut microbiota, hypertension, liver dysfunction, and lower birth weight compared to uncomplicated pregnancies (27). PE and gestational diabetes correlate with elevated neutrophil to lymphocyte ratio (NLR), but the direct causal link remains unclear (28). These findings the importance of understanding biological interactions to advance preeclampsia prevention and treatment strategies.
Pathophysiology of Preeclampsia
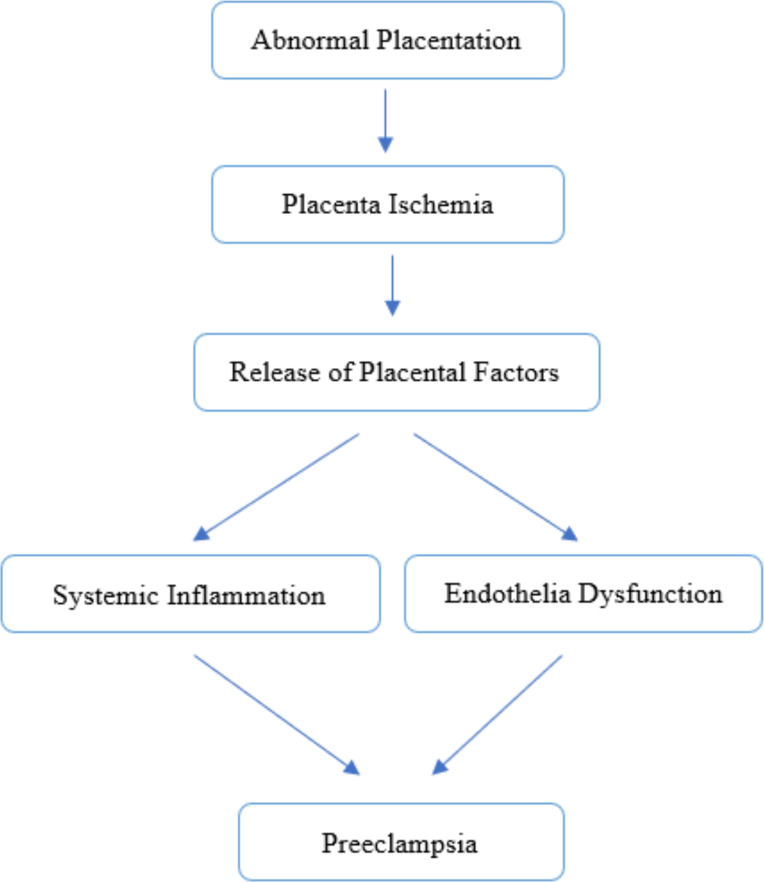
PE develops in two stages: placental abnormalities in the first trimester, followed by “mother syndrome” in the second and third trimesters characterized by an excess of anti-angiogenic factors (29). PE involves heightened immune response, trophoblast apoptosis, reduced trophoblast infiltration, altered placental morphology like spiral artery remodeling, decreased uterine perfusion pressure (RUPP) (30) and placental ischemia or hypoxia. The RUPP condition can result from an imbalance in anti-angiogenic factors (such as fms-like tyrosekinase-1/SFlit-1 and soluble endoglin/SEng) and proangiogenic factors (such as vascular endothelial growth factor/VEGF and placental growth factor/PlGF). The stimulates the release of bioactive factors circulating in the blood, such as cytokines, hypoxia-inducible factor-1/HIF-1, reactive oxygen species/ROS, and angiotensin receptor agonist/AT1 AA autoantibodies (31, 32). Abnormal placenta in early pregnancy is considered an important early event in the development of PE, it causes the release of anti-angiogenic factors [mainly tyrosine kinase-1 (sFlt-1)] and soluble cytokines, leading to widespread vascular dysfunction (33). Systematic morphologic studies indicate that preeclampsia is associates with incomplete transformation of spiral arteries and the presence of endothelial and myogenic cells in endometrial segments (34). Angiotensin peptide imbalance might contribute to PE pathophysiology and fetal growth issues (35). Decidual macrophage dysfunction is pivotal in PE pathogenesis (36). These findings underscore the need for deeper understanding to develop more effective therapeutic approaches in managing PE. Figure 1 provides a simplified overview of the complex processes involved in the pathogenesis of preeclampsia.
Fig. 1:
Pathophysiology of Preeclampsia
Risk Factors for Preeclampsia
According to the 2019 NICE Guidelines, women at high risk of preeclampsia include those with a history of hypertensive disease in prior pregnancies or maternal conditions like chronic kidney disease, autoimmune disease, diabetes, or chronic hypertension. Moderate-risk women include nulliparous, aged ≥ 40, BMI ≥ 35 kg/m2, with family history of preeclampsia, amniotic pregnancy, or pregnancy interval > 10 years (37, 38) . Additionally, primigravida status and alcohol consumption correlate with risk of PE. Alternatively, consuming vegetables protects against PE development (39) and there is an association of ABO blood group in pregnant women with pregnancy complications including preeclampsia (40).
Other risk factors, encompassing socio-demographic and clinical aspects, contribute to PE occurrence (41). Preeclamptic mothers face elevated lead exposure risks both at work and home, correlating with higher blood lead levels and increased preeclampsia risk (42). Preeclampsia prevalence is higher in couples using condoms, shorter cohabitation, first pregnancy in multiparous women with partner change, and pregnancy post-assisted reproduction with gamete donation. In most developed countries with a “Western lifestyle,” 80–90% of preeclampsia cases are strongly linked to maternal metabolic syndrome variables (43). Identifying risks and implementing preventive strategies, such as lifestyle modifications and close medical monitoring, is crucial for reducing preeclampsia.
Impact of Preeclampsia
Preeclampsia and Cardiovascular Disease
Cardiovascular disease (CVD) is the leading cause of pregnancy-related mortality (44, 45). Women with prior hypertensive pregnancy disorders face a 5%–10% increased risk of cardiovascular disease (46–50). First-pregnancy hypertension correlates with a 63% higher incidence of cardiovascular disease later in life. Over 80% of this risk is attributed to developing chronic hypertension post-delivery, suggesting that preeclampsia may heighten cardiovascular risk (50, 51). Epidemiologic studies support these findings, indicating that gestational hypertension and preeclampsia elevate the risk of future cardiovascular disease compared to women without pregnancy-related hypertension (48). Besides short-term impacts, preeclampsia has long-term repercussions for both mothers and offspring, as indicated by abundant epidemiological evidence. Studies demonstrate heightened susceptibility to cardiovascular, metabolic, and neurologic disorders, and other maladies among offspring affected by preeclampsia (49). The occurrence of cardiometabolic conditions in pregnancy, like gestational diabetes and hypertension, elevates the likelihood of subsequent cardiovascular events (50). Preeclampsia and small-for-gestational-age infants correlate with CVD (51).
Preeclampsia and neonatal Outcomes
PE is linked to higher long-term maternal and child morbidity risks related to prematurity (52). PE is linked to immune-based pregnancy issues and atopic abnormalities in newborns (53). PE significantly affects fetal growth restriction by impairing spiral artery remodeling and trophoblast invasion, thereby reducing nutrient supply to the fetus (54). Preeclampsia triggers placental vascular insufficiency, leading to epigenetic and pathological alterations in both maternal and fetal systems. Infants exposed to preeclampsia exhibited diminished weight, height, and BMI compared to controls with normotensive pregnancies at 2 years. Accelerated weight gain in controls potentially supports growth to age 2, influencing long-term cardiometabolic health. Yet, disparities exist regarding the impact of preeclampsia on infant motor and cognitive development or vice versa (55). The intrauterine environment in PE adversely affects fetal lung development, altering gene profiles and postnatal alveolar and bronchial structures (56). Evidence indicates hypertension, gestational diabetes, and preeclampsia are associated with intrauterine growth restriction (IUGR) and potentially small for gestational age (SGA) (57).
Preeclampsia and economic impact
Managing PE incurs substantial costs, imposing a significant burden on healthcare systems, especially regarding hospitalization and addressing maternal and obstetric complications (62, 63). Preeclampsia costs $1.03 billion for moms and $1.15 billion for babies over the first 12 months following delivery. The cost burden per baby varies with gestational age, starting at 26 wk and going up to 36 wk, or $1311, respectively (60).
Preeclampsia impacts maternal and infant health, necessitating research and interventions to mitigate risks and economic burdens.
Prevention of Preeclampsia
Aspirin
Aspirin prevented early PE by 62% in pregnant women at high risk of preeclampsia (65, 66, 67). Aspirin™ (acetylsalicylic acid) stands out as the sole effective medication in lowering PE rates, boasting analgesic properties and anti-cardiovascular effects. It has remained indispensable for over 120 years due and is listed in the WHO Essential Medicines Catalog. Administering low-dose Aspirin (80–150 mg) once nightly is recommended. Evidence indicates increased efficacy with higher doses. While deemed safe for mother and fetus at low doses, usage exceeding 100 mg is uncommon. Furthermore, the safety of a preventive regimen with 150 mg daily remains incompletely validated. (64). Evidence-based studies indicate that administering 150 mg/day Aspirin before 16 wk of gestation, particularly at night, to high-risk individuals identified through combined first-trimester screening, can lower preterm preeclampsia by 62% (65). Low-dose aspirin prevents preeclampsia, with efficacy linked to dosage, reducing postpartum hemorrhage, fetal growth restriction, preterm birth, and cesarean section (66). Aspirin should be recommended pre-16 wk gestation to prevent preeclampsia and preterm birth (67). At all pregnancy ages, aspirin doses led to a 30% reduction in preeclampsia risk (68). The optimal dose of aspirin daily is 75 mg (69).
Vitamin D
Pregnant women with deficiency vitamin D have a higher preeclampsia risk (70). Vitamin D promise for its positive effects on placental attachment, the immune system, and angiogenesis (75). Vitamin D supplementation significantly protects against Preeclampsia incidence (76) (77) (78).
Structured Sports
Women with a preeclampsia history require accurate lifestyle guidance (79). Yoga, with its combination of aerobic, strength, and flexibility elements, offers superior risk reduction for hypertensive disorders of pregnancy (HDP) compared to standalone aerobic exercise. It serves as a low-impact physical activity, potentially providing a safer and more acceptable means for pregnant women to mitigate HDP risk (80). In addition, progressive muscle relaxation is an alternative for pregnancy complications, reducing muscle tension and promoting relaxation in pregnant women (81).
Folic Acid
High-dose folic acid supplements taken three months before conception until delivery can reduce the risk of preeclampsia in pregnant women (82).
Mushroom Diet
The Mushroom Diet aims to prevent gestational hypertension, preeclampsia, and associated comorbidities such as gestational diabetes and excessive pregnancy weight gain (83).
Consumption of Ajwa Dates
Pregnant women prone to preeclampsia may benefit from consuming seven Ajwa dates daily, reducing mean arterial pressure (MAP) and Roll-Over Test (ROT), potentially preventing preeclampsia (84).
Consume Extra Virgin Olive Oil
Preeclampsia induces trophoblast release into maternal circulation, impacting fetal health. Extra virgin olive oil (EVOO) harbors tocopherols (vitamin E) with antioxidant properties, while Haramonting, an Indonesian traditional remedy, prevents DNA damage and exhibits antioxidant activity. Co-administration of EVOO and nano herbal Haramonting augments trophoblast count and improves placental histology (85).
Preeclampsia Screening
The subsequent aspects necessitate meticulous consideration during preeclampsia screening:
1) Early detection of blood pressure changes is crucial for preventing preeclampsia (81). During early pregnancy, women ought to undergo screening for clinical risk indicators of preeclampsia (strong, moderate). When feasible, screening should occur between wk 11 and 14 of gestation utilizing a blend of clinical risk markers, uterine artery pulsatility index, and placental growth factor (PlGF) risk factors to assess preeclampsia risk (strong, moderate) (86).
2) Personal History Screening and History Family for preeclampsia in men and women should be a priority. Mothers with a history of preeclampsia should be alerted to the heightened risk in subsequent pregnancies and commence prenatal monitoring promptly. Factors like blood pressure, multiple gestations, and familial hypertension history enhance the prenatal care link, aiding in early preeclampsia detection and complication prevention (87).
3) Screening for urinary tract infections (UTIs) links to maternal and infant complications like low birth weight, preterm birth, stillbirth, preeclampsia, maternal anemia, sepsis, and amnionitis, even when asymptomatic. UTI occurrence in pregnancy, notably in the third trimester, strongly associates with preeclampsia, hinting at heightened maternal inflammation elevating preeclampsia risk. Preventing UTIs could potentially and affordably curb preeclampsia development (47).
4) Monitoring urinary protein levels, while not diagnostic, correlates with severe preeclampsia symptoms, potentially leading to premature labor (88). Proteinuria, common in preeclampsia patients, predicts adverse outcomes. Monitoring urinary protein levels is advised for follow-up care (89).
Antenatal Care Visit
Enhancing pregnant women’s health-seeking behavior in urban and rural areas offers an opportunity for early preeclampsia diagnosis and complication prevention (90).
Timely intervention and risk mitigation are vital in preventing preeclampsia in both mothers and infants. Table 1 was prepared based on provided information, covering preeclampsia’s etiology, pathophysiology, risk factors, effects, and preventionants.
Table 1:
Summary of preeclampsia: etiology, pathophysiology, risk factors, impact, and prevention and preventive measures
| Summary | Aspects |
|---|---|
| Etiology |
|
| Pathophysiology |
|
| Risk Factors |
|
| Impact |
|
| Preventive measures |
|
Consider various interventions, including hypertension treatment, antenatal care, delivery services, postpartum care, and enhanced education and standardization of hypertension testing during pregnancy, to mitigate and prevent hypertensive disorders in pregnancy (91, 92).
Recommendations include conducting multicentric research to gather diverse data on preeclampsia dynamics, testing new therapies (both pharmacological and non-pharmacological), evaluating preventive strategies like low-dose aspirin and vitamin D supplementation. Intervention studies to enhance pregnant women’s health literacy on preeclampsia .along with assessing its long-term impact on incidence and pregnancy outcomes.
This study was limited by the availability and access to recent literature, potentially overlooking new findings or recommendations in obstetrics and gynecology that emerged after the writing period.
Conclusion
This article elucidates preeclampsia’s etiology, pathophysiology, risk factors, impact, and preventive measures, arising from compromised placental development, vascularization issues, and immune dysregulation.
The implications of this study emphasizes the necessity of enhancing health literacy and integrating preeclampsia education into prenatal care to empower pregnant women in mitigating preeclampsia risk.
Journalism Ethical considerations
Ethical issues (including plagiarism, informed consent, errors, falsification and/or fabrication of data, multiple publications and/or submissions, redundancy, etc.) have been fully addressed by the authors.
Footnotes
Funding
The authors revealed financial and personal conflicts of interest and received financial support but stressed no conflicts affecting manuscript objectivity.
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Mukosha M, Vwalika B, Lubeya MK, et al. (2022). Determinants and neonatal outcomes of preeclampsia among women living with and without HIV at a tertiary hospital in Zambia: a review of medical records. Pan Afr Med J, 43: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazloomi S, Khodadadi I, Alimohammadi S, et al. (2021). Correlation of thioredoxin reductase (TrxR) and nitric oxide synthase (NOS) activities with serum trace elements in preeclampsia. Clin Exp Hypertens, 43(2): 120–4. [DOI] [PubMed] [Google Scholar]
- 3.Rezai H, Ahmad S, Alzahrani FA, et al. (2021). MZe786, a hydrogen sulfide-releasing aspirin prevents preeclampsia in heme oxygenase-1 haplodeficient pregnancy under high soluble flt-1 environment. Redox Biol, 38: 101768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chappell LC, Brocklehurst P, Green ME, et al. (2019). Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet, 394 (10204): 1181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortved D, Hawkins TLA, Johnson JA, et al. (2019). Cost-effectiveness of first-trimester screening with early preventative use of aspirin in women at high risk of early-onset preeclampsia. Ultrasound Obstet Gynecol, 53(2) : 239–44. [DOI] [PubMed] [Google Scholar]
- 6.Jhee JH, Lee S, Park Y, et al. (2019). Prediction model development of late-onset preeclampsia using machine learning-based methods. PLoS One, 14 (8): e0221202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald EJ, Lepine S, Pledger M, et al. (2019). Pre-eclampsia causing severe maternal morbidity – A national retrospective review of preventability and opportunities for improved care. Aust N Z J Obstet Gynaecol, 59(6): 825–30. [DOI] [PubMed] [Google Scholar]
- 8.Tsigas EZ. (2022). The Preeclampsia Foundation: the voice and views of the patient and her family. Am J Obstet Gynecol, 226 (2S): S1254–S1264.e1. [DOI] [PubMed] [Google Scholar]
- 9.Osungbade KO, Ige OK. (2011). Public Health Perspectives of Preeclampsia in Developing Countries: Implication for Health System Strengthening. J Pregnancy, 2011:481095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox R, Kitt J, Leeson P, et al. (2019). Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. J Clin Med, 8(10): 1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stitterich N, Shepherd J, Koroma MM, et al. (2021). Risk factors for preeclampsia and eclampsia at a main referral maternity hospital in Freetown, Sierra Leone: a case-control study. BMC Pregnancy Childbirth, 21(1): 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duhig KE, Myers J, Seed PT, et al. (2019). Placental growth factor testing to assess women with suspected pre-eclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet, 393 (10183): 1807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inno R, Kikas T, Lillepea K, et al. (2021). Coordinated Expressional Landscape of the Human Placental miRNome and Transcriptome. Front Cell Dev Biol, 9: 697947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainstock T, Sergienko R, Sheiner E. (2020). Who Is at Risk for Preeclampsia? Risk Factors for Developing Initial Preeclampsia in a Subsequent Pregnancy. J Clin Med, 9 (4): 1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton GJ, Redman CW, Roberts JM, et al. (2019). Pre-eclampsia: pathophysiology and clinical implications. BMJ, 366:l2381. [DOI] [PubMed] [Google Scholar]
- 16.Ghorbannejad S, MehdizadehTourzani Z, Kabir K, et al. (2022). The effectiveness of Jacobson’s progressive muscle relaxation technique on maternal, fetal and neonatal outcomes in women with non-severe preeclampsia: a randomized clinical trial. Heliyon, 8 (6): e09709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rana S, Burke SD, Karumanchi SA. (2022). Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol, 226 (2S): S1019–S1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awamleh Z, Gloor GB, Han VKM. (2019). Placental microRNAs in pregnancies with early onset intrauterine growth restriction and preeclampsia: Potential impact on gene expression and pathophysiology. BMC Med Genomics, 12 (1): 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dugalic S, Petronijevic M, Stefanovic A, et al. (2019). Comparison of 2 approaches in management of pregnant women with inherited trombophilias: Prospective analytical cohort study. Medicine (Baltimore), 98 (34): e16883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane SL, Doyle AS, Bales ES, et al. (2020). Increased uterine artery blood flow in hypoxic murine pregnancy is not sufficient to prevent fetal growth restriction. Biol Reprod, 102 (3): 660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristensen JH, Basit S, Wohlfahrt J, et al. (2019). Pre-eclampsia and risk of later kidney disease: Nationwide cohort study. BMJ, 365: l1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Than NG, Posta M, Györffy D, et al. (2022). Early pathways, biomarkers, and four distinct molecular subclasses of preeclampsia: The intersection of clinical, pathological, and high-dimensional biology studies. Placenta, 125: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinthorsdottir V, McGinnis R, Williams NO, et al. (2020). Genetic predisposition to hypertension is associated with preeclampsia in European and Central Asian women. Nat Commun, 11 (1): 5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu XH, Li ZH, Muyayal KP. (2022). A newly intervention strategy in preeclampsia: Targeting PD-1/Tim-3 signaling pathways to modulate the polarization of decidual macrophages. FASEB J, 36(1):e22073.. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal R, Jain AK, Mittal P, et al. (2019). Association of pro- and anti-inflammatory cytokines in preeclampsia. J Clin Lab Anal, 33 (4): e22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shraga Y, Pariente G, Rotem R, et al. (2020). Changes in trends over time for the specific contribution of different risk factors for pre-eclampsia. Arch Gynecol Obstet, 302 (4): 977–82. [DOI] [PubMed] [Google Scholar]
- 27.Lv LJ, Li SH, Li SC, et al. (2019). Early-Onset Preeclampsia Is Associated With Gut Microbial Alterations in Antepartum and Postpartum Women. Front Cell Infect Microbiol, 9: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christoforaki V, Zafeiriou Z, Daskalakis G, et al. (2020). First trimester neutrophil to lymphocyte ratio (NLR) and pregnancy outcome. J Obstet Gynaecol, 40 (1): 59–64. [DOI] [PubMed] [Google Scholar]
- 29.Rana S, Lemoine E, Granger J, et al. (2019). Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res, 124 (7): 1094–112. [DOI] [PubMed] [Google Scholar]
- 30.Saif J, Ahmad S, Rezai H, Litvinova K, et al. (2021). Hydrogen sulfide releasing molecule MZe786 inhibits soluble Flt-1 and prevents preeclampsia in a refined RUPP mouse model. Redox Biol, 38:101814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerdeira AS, O’Sullivan J, Ohuma EO, et al. (2019). Randomized Interventional Study on Prediction of Preeclampsia/Eclampsia in Women with Suspected Preeclampsia: INSPIRE. Hypertension, 74 (4): 983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brownfoot FC, Hannan NJ, Cannon P, et al. (2019). Sulfasalazine reduces placental secretion of antiangiogenic factors, up-regulates the secretion of placental growth factor and rescues endothelial dysfunction. EBioMedicine, 41: 636–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saleh L, Verdonk K, Visser W, et al. (2016). The emerging role of endothelin-1 in the pathogenesis of pre-eclampsia., Ther Adv Cardiovasc Dis, 10 (5): 282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris LK, Benagiano M, D’Elios MM, Brosens I, Benagiano G. (2019). Placental bed research: II. Functional and immunological investigations of the placental bed. Am J Obstet Gynecol, 221(5):457–69. [DOI] [PubMed] [Google Scholar]
- 35.Tamanna S, Clifton VL, Rae K, et al. (2020). Angiotensin Converting Enzyme 2 (ACE2) in Pregnancy: Preeclampsia and Small for Gestational Age. Front Physiol, 11:590787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li JH, Wang LL, Liu H, et al. (2019). Galectin-9 alleviates LPS-induced preeclampsia-like impairment in rats via switching decidual macrophage polarization to M2 subtype. Front Immunol, 9: 3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Negi M, Mulla MJ, Han CS, et al. (2020). Allopurinol inhibits excess glucose-induced trophoblast IL-1β and ROS production. Reproduction, 159 (1): 73–80. [DOI] [PubMed] [Google Scholar]
- 38.Chantanahom N, Phupong V. (2021). Clinical risk factors for preeclampsia in twin pregnancies. Laganà AS, editor. PLoS One, 16 (4): e0249555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ngwenya S, Jones B, Mwembe D, et al. (2021). Determinants of eclampsia in women with severe preeclampsia at Mpilo Central Hospital, Bulawayo, Zimbabwe. Pregnancy Hypertens, 25: 235–9. [DOI] [PubMed] [Google Scholar]
- 40.Sharma S, Goyal M, Kumar PK, et al. (2019). Association of raised blood lead levels in pregnant women with preeclampsia: A study at tertiary centre. Taiwan J Obstet Gynecol, 58 (1): 60–3. [DOI] [PubMed] [Google Scholar]
- 41.Robillard PY, Dekker G, Scioscia M, et al. (2022). Progress in the understanding of the pathophysiology of immunologic maladaptation related to early-onset preeclampsia and metabolic syndrome related to late-onset preeclampsia. Am J Obstet Gynecol, 226 (2S): S867–75. [DOI] [PubMed] [Google Scholar]
- 42.Mehta LS, Warnes CA, Bradley E, et al. (2020). Cardiovascular Considerations in Caring for Pregnant Patients: A Scientific Statement from the American Heart Association. Circulation, 141 (23): e884–e903. [DOI] [PubMed] [Google Scholar]
- 43.Brener A, Lewnard I, Mackinnon J, et al. (2020). Missed opportunities to prevent cardiovascular disease in women with prior preeclampsia. BMC Womens Health, 20 (1): 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honigberg MC, Zekavat SM, Aragam K, et al. (2019). Long-Term Cardiovascular Risk in Women With Hypertension During Pregnancy. J Am Coll Cardiol, 74 (22): 2743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haug EB, Horn J, Markovitz AR, et al. (2019). Association of Conventional Cardiovascular Risk Factors with Cardiovascular Disease after Hypertensive Disorders of Pregnancy: Analysis of the Nord-Trøndelag Health Study. JAMA Cardiol, 4 (7): 628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garovic VD, White WM, Vaughan L, et al. (2020). Incidence and Long-Term Outcomes of Hypertensive Disorders of Pregnancy. J Am Coll Cardiol, 75 (18): 2323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riise HKR, Sulo G, Tell GS, Igland J, Egeland G, et al. (2019). Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. Int J Cardiol, 282: 81–87. [DOI] [PubMed] [Google Scholar]
- 48.Almli I, Haugdahl HS, Sandsæter HL, et al. (2020). Implementing a healthy postpartum lifestyle after gestational diabetes or preeclampsia: A qualitative study of the partner’s role. BMC Pregnancy Childbirth, 20 (1): 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnott C, Nelson M, Alfaro Ramirez M, et al. (2020). Maternal cardiovascular risk after hypertensive disorder of pregnancy. Heart, 106 (24): 1927–33. [DOI] [PubMed] [Google Scholar]
- 50.Suvakov S, Bonner E, Nikolic V, et al. (2020). Overlapping pathogenic signalling pathways and biomarkers in preeclampsia and cardiovascular disease. Pregnancy Hypertens, 20: 131–6. [DOI] [PubMed] [Google Scholar]
- 51.Stuart JJ, Tanz LJ, Rimm EB, et al. (2022). Cardiovascular Risk Factors Mediate the Long-Term Maternal Risk Associated With Hypertensive Disorders of Pregnancy. J Am Coll Cardiol, 79 (19): 1901–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Easter SR, Cantonwine DE, Zera CA, et al. (2016). Urinary tract infection during pregnancy, angiogenic factor profiles, and risk of preeclampsia. Am J Obstet Gynecol, 214 (3): 387.e1–7. [DOI] [PubMed] [Google Scholar]
- 53.Seely EW, Celi AC, Chausmer J, et al. (2021). Cardiovascular health after preeclampsia: Patient and provider perspective. J Womens Health (Larchmt), 30 (3): 305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu HQ, Hu R. (2019). Lasting effects of intrauterine exposure to preeclampsia on offspring and the underlying mechanism. AJP Rep, 9 (3): e275–e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thong EP, Ghelani DP, Manoleehakul P, et al. (2022). Optimising Cardiometabolic Risk Factors in Pregnancy: A Review of Risk Prediction Models Targeting Gestational Diabetes and Hypertensive Disorders. J Cardiovasc Dev Dis, 9 (2): 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Markovitz AR, Stuart JJ, Horn J, Williams PL, et al. (2019). Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J, 40 (14): 1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waker CA, Kaufman MR, Brown TL. (2021). Current State of Preeclampsia Mouse Models: Approaches, Relevance, and Standardization. Front Physiol, 12: 681632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu M, Eviston D, Hsu P, et al. (2019). Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nat Commun, 10(1):3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohammad NS, Nazli R, Zafar H, et al. (2022). Effects of lipid based multiple micronutrients supplement on the birth outcome of underweight pre-eclamptic women: A randomized clinical trial. Pak J Med Sci, 38 (1): 219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vakil P, Henry A, Craig ME, et al. (2022). A review of infant growth and psychomotor developmental outcomes after intrauterine exposure to preeclampsia. BMC Pediatr, 22 (1): 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taglauer ES, Fernandez-Gonzalez A, Willis GR, et al. (2022). Antenatal Mesenchymal Stromal Cell Extracellular Vesicle Therapy Prevents Preeclamptic Lung Injury in Mice. Am J Respir Cell Mol Biol, 66 (1): 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luyckx VA, Brenner BM. (2020). Clinical consequences of developmental programming of low nephron number. Anat Rec (Hoboken), 303 (10): 2613–31. [DOI] [PubMed] [Google Scholar]
- 63.Hao J, Hassen D, Hao Q, et al. (2019). Maternal and Infant Health Care Costs Related to Preeclampsia. Obstet Gynecol, 134 (6): 1227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubon Garcia A, Devlieger R, Redekop K, et al. (2021). Cost-utility of a first-trimester screening strategy versus the standard of care for nulliparous women to prevent pre-term pre-eclampsia in Belgium. Pregnancy Hypertens, 25: 219–24. [DOI] [PubMed] [Google Scholar]
- 65.Stevens W, Shih T, Incerti D, et al. (2017). Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol, 217 (3): 237–248.e16. [DOI] [PubMed] [Google Scholar]
- 66.Mendoza M, Bonacina E, Garcia-Manau P, et al. (2023). Aspirin Discontinuation at 24 to 28 Weeks’ Gestation in Pregnancies at High Risk of Preterm Preeclampsia: A Randomized Clinical Trial. JAMA, 329 (7): 542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hastie R, Tong S, Wikström AK, et al. (2021). Aspirin use during pregnancy and the risk of bleeding complications: a Swedish population-based cohort study. Am J Obstet Gynecol, 224 (1): 95.e1–95.e12. [DOI] [PubMed] [Google Scholar]
- 68.Clymer D, Kostadinov S, Catov J, et al. (2020). Decidual Vasculopathy Identification in Whole Slide Images Using Multiresolution Hierarchical Convolutional Neural Networks. Am J Pathol, 190 (10): 2111–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atallah A, Lecarpentier E, Goffinet F, et al. (2017). Aspirin for Prevention of Preeclampsia. Drugs, 77 (17): 1819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rolnik DL, Nicolaides KH, Poon LC. (2022). Prevention of preeclampsia with aspirin. Am J Obstet Gynecol, 226 (2S): S1108–S1119. [DOI] [PubMed] [Google Scholar]
- 71.Gu W, Lin J, Hou YY, et al. (2020). Effects of low-dose aspirin on the prevention of preeclampsia and pregnancy outcomes: A randomized controlled trial from Shanghai, China. Eur J Obstet Gynecol Reprod Biol, 248: 156–63. [DOI] [PubMed] [Google Scholar]
- 72.Hoffman MK, Goudar SS, Kodkany BS, et al. (2020). Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet, 395 (10220): 285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Doorn R, Mukhtarova N, Flyke IP, et al. (2021). Dose of aspirin to prevent preterm preeclampsia in women with moderate or high-risk factors: A systematic review and meta-analysis. PLoS One, 16 (3): e0247782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Guo X, Obore N, et al. (2022). Aspirin for the prevention of preeclampsia: A systematic review and meta-analysis of randomized controlled studies. Front Cardiovasc Med, 9: 936560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cyprian F, Lefkou E, Varoudi K, et al. (2019). Immunomodulatory Effects of Vitamin D in Pregnancy and Beyond. Front Immunol, 10: 2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poniedziałek-Czajkowska E, Mierzyński R. (2021). Could Vitamin D Be Effective in Prevention of Preeclampsia? Nutrients, 13 (11): 3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fogacci S, Fogacci F, Banach M, et al. (2020). Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clin Nutr, 39 6): 1742–52. [DOI] [PubMed] [Google Scholar]
- 78.Fu Z mei, Ma Z zhi, Liu G jie, et al. (2018). Vitamins supplementation affects the onset of preeclampsia. J Formos Med Assoc, 117 (1): 6–13. [DOI] [PubMed] [Google Scholar]
- 79.Palacios C, Kostiuk LK, Peña-Rosas JP. (2019). Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. Cochrane Database Syst Rev, 7(7):CD008873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Wu N, Shen H. (2021). A Review of Research Progress of Pregnancy with Twins with Preeclampsia. Risk Manag Healthc Policy, 14:1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Danielli M, Gillies C, Thomas RC, et al. (2022). Effects of Supervised Exercise on the Development of Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis. J Clin Med, 11 (3): 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng L, Huang J, Kong H, et al. (2020). The effect of folic acid throughout pregnancy among pregnant women at high risk of preeclampsia: A randomized clinical trial. Pregnancy Hypertens, 19: 253–8. [DOI] [PubMed] [Google Scholar]
- 83.Sun L, Niu Z. (2020). A mushroom diet reduced the risk of pregnancy-induced hypertension and macrosomia: A randomized clinical trial. Food Nutr Res, 64: 10.29219/fnr.v64.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Royani I, As’ad S, Mappaware NA, et al. (2019). Effect of Ajwa Dates Consumption to Inhibit the Progression of Preeclampsia Threats on Mean Arterial Pressure and Roll-Over Test. Biomed Res Int, 2019: 2917895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Irianti E, Ilyas S, Hutahaean S, et al. (2020). Placental histological on preeclamptic rats (Rattus norvegicus) after administration of nanoherbal haramonting (Rhodomyrtus tomentosa). Research J Pharm Tech, 13 (8): 3879–82. [Google Scholar]
- 86.Magee LA, Smith GN, Bloch C, et al. (2022). Guideline No. 426: Hypertensive Disorders of Pregnancy: Diagnosis, Prediction, Prevention, and Management. J Obstet Gynaecol Can, 44(5):547–571.e1. [DOI] [PubMed] [Google Scholar]
- 87.Demissie Beketie E, Tesfaye Tafese W, Zeleke Shiferaw B, et al. (2022). Determinants of preeclampsia among mothers attending perinatal care in Gurage zone public hospitals, Ethiopia, matched case control study. Int J Afr Nurs Sci, 17: 100453. [Google Scholar]
- 88.Mateus J, Newman R, Sibai B, Li Q, et al. (2017). Massive Urinary Protein Excretion Associated with Greater Neonatal Risk in Preeclampsia. AJP Rep, 7 (1): e49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lei T, Qiu T, Liao W, et al. (2021). Proteinuria may be an indicator of adverse pregnancy outcomes in patients with preeclampsia: a retrospective study. Reprod Biol Endocrinol, 19 (1): 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Belay AS, Wudad T. (2019). Prevalence and associated factors of pre-eclampsia among pregnant women attending anti-natal care at Mettu Karl referal hospital, Ethiopia: Cross-sectional study. Clin Hypertens, 25: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mou AD, Barman Z, Hasan M, et al. (2021). Prevalence of preeclampsia and the associated risk factors among pregnant women in Bangladesh. Sci Rep, 11 (1): 21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagraj S, Hinton L, Praveen D, et al. (2019). Women’s and healthcare providers’ perceptions of long-term complications associated with hypertension and diabetes in pregnancy: a qualitative study. BJOG, 126 Suppl 4(Suppl Suppl 4):34– 42. [DOI] [PMC free article] [PubMed] [Google Scholar]