Abstract
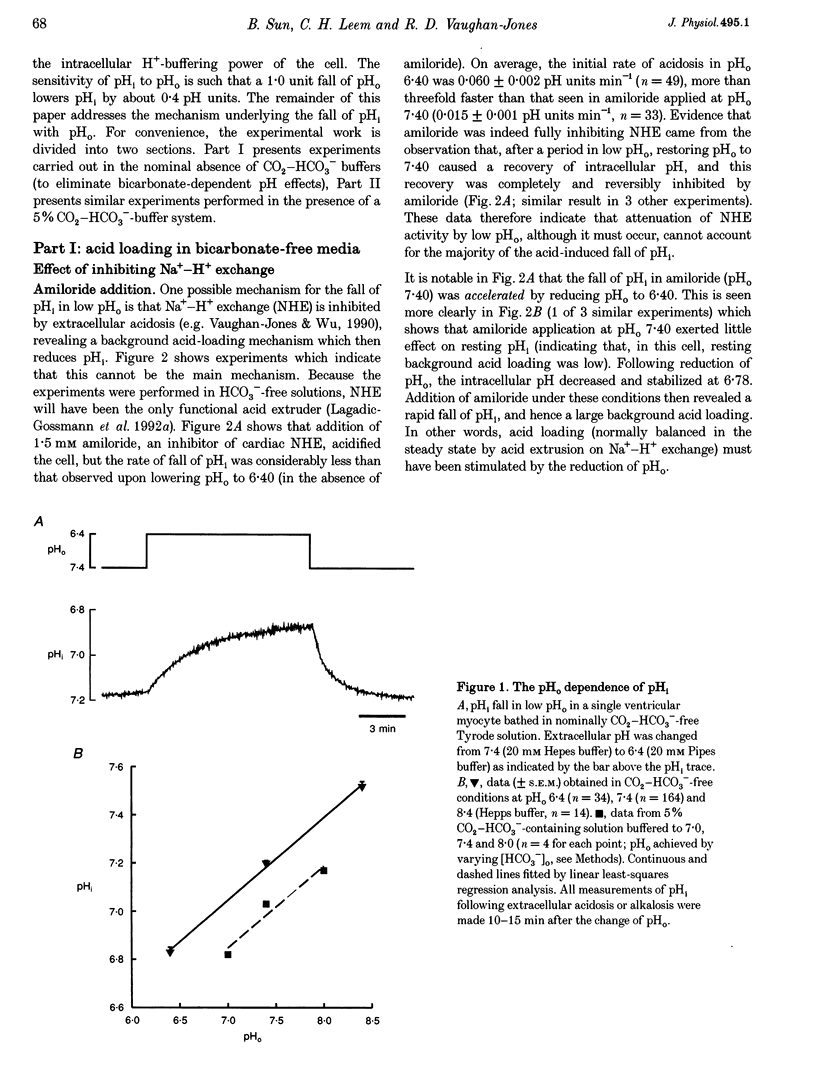
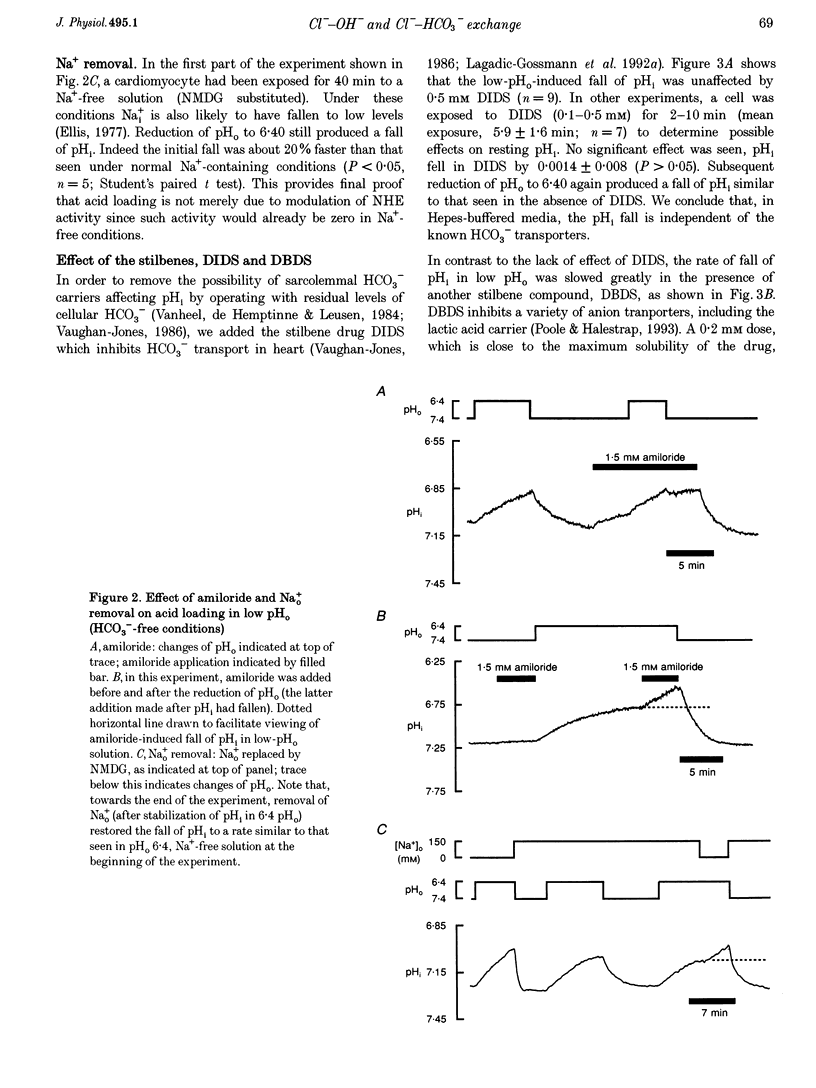
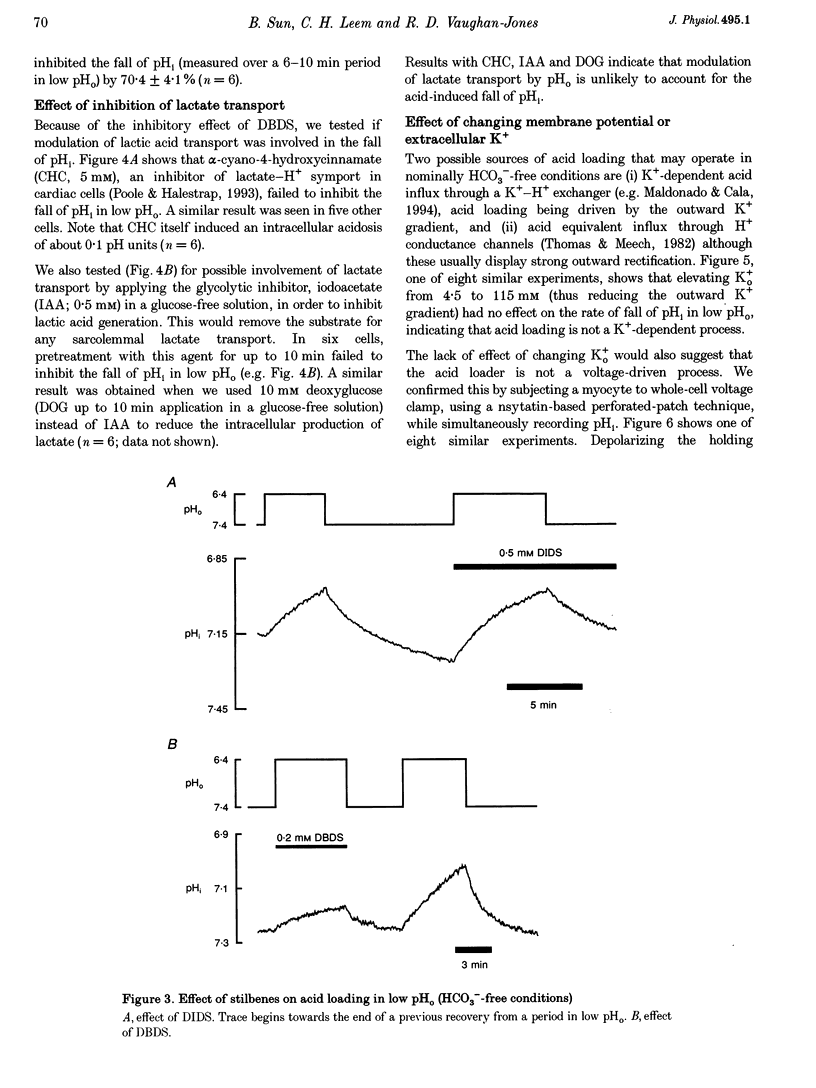
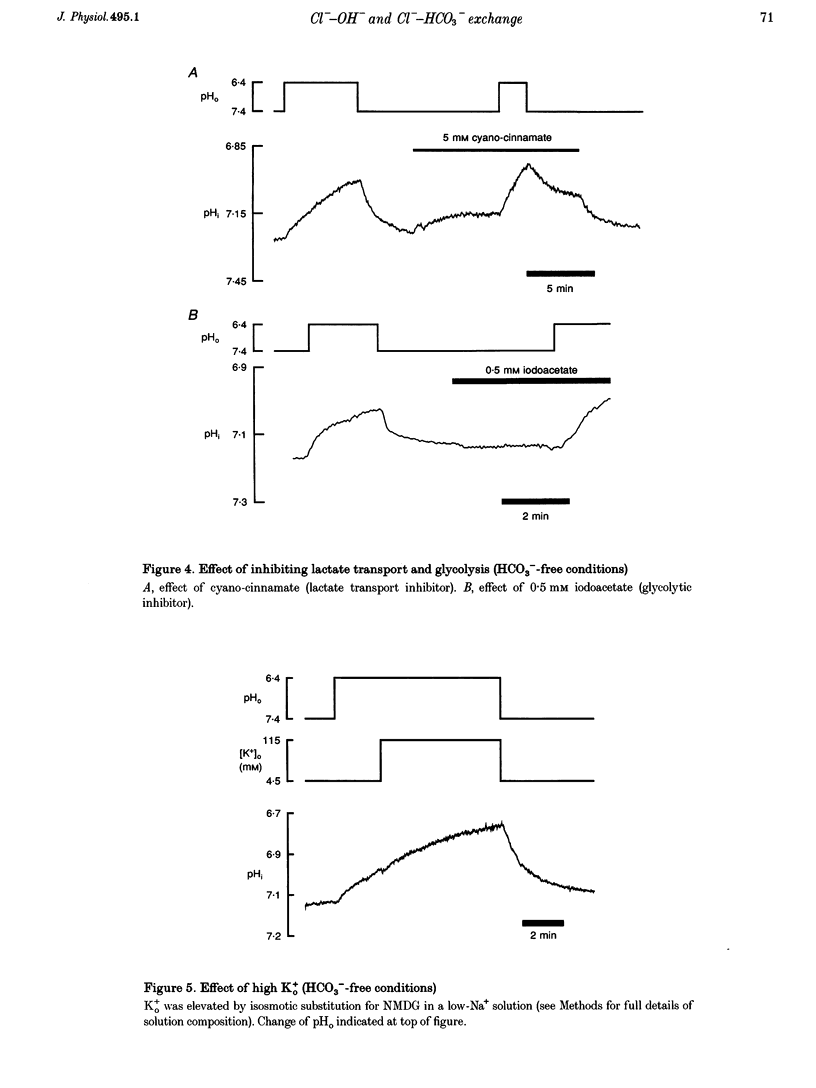
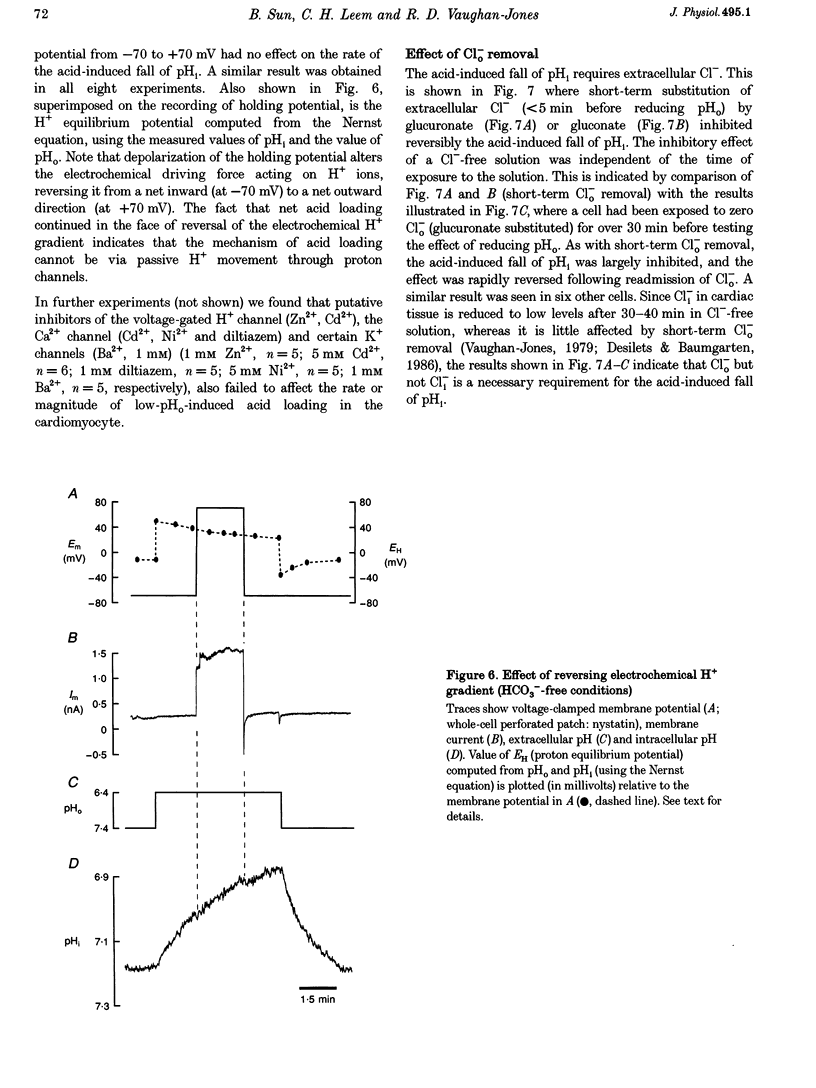
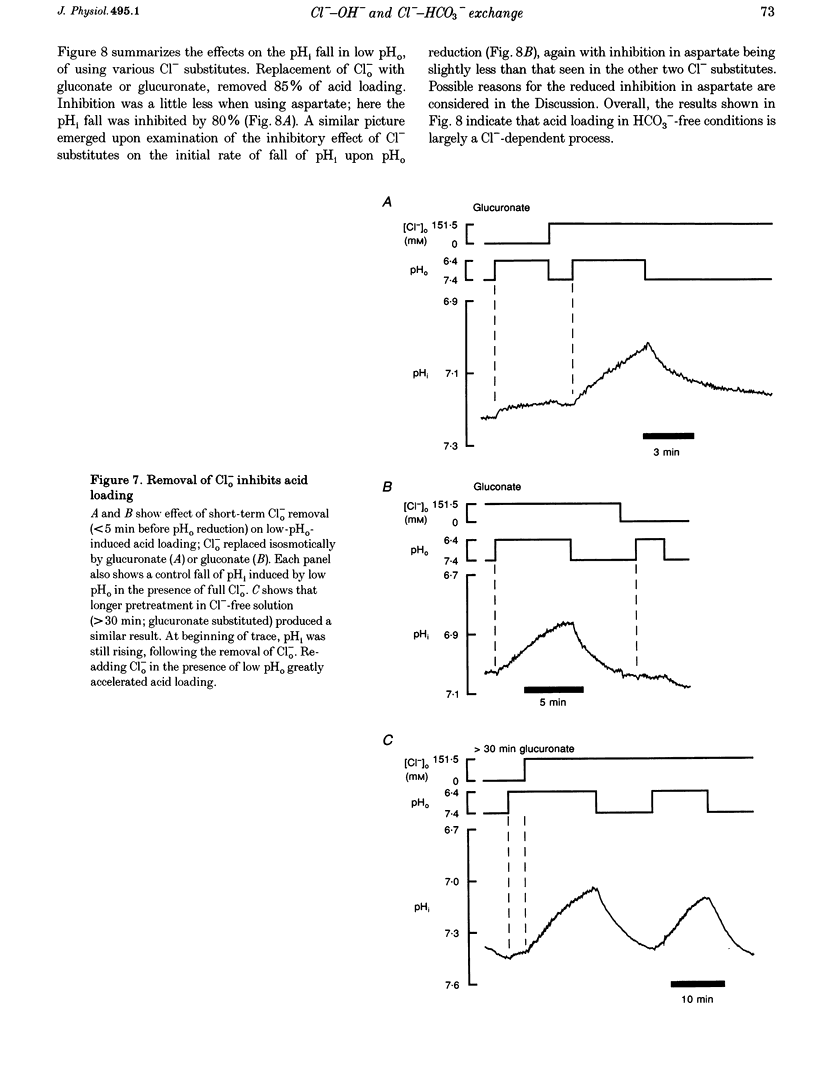
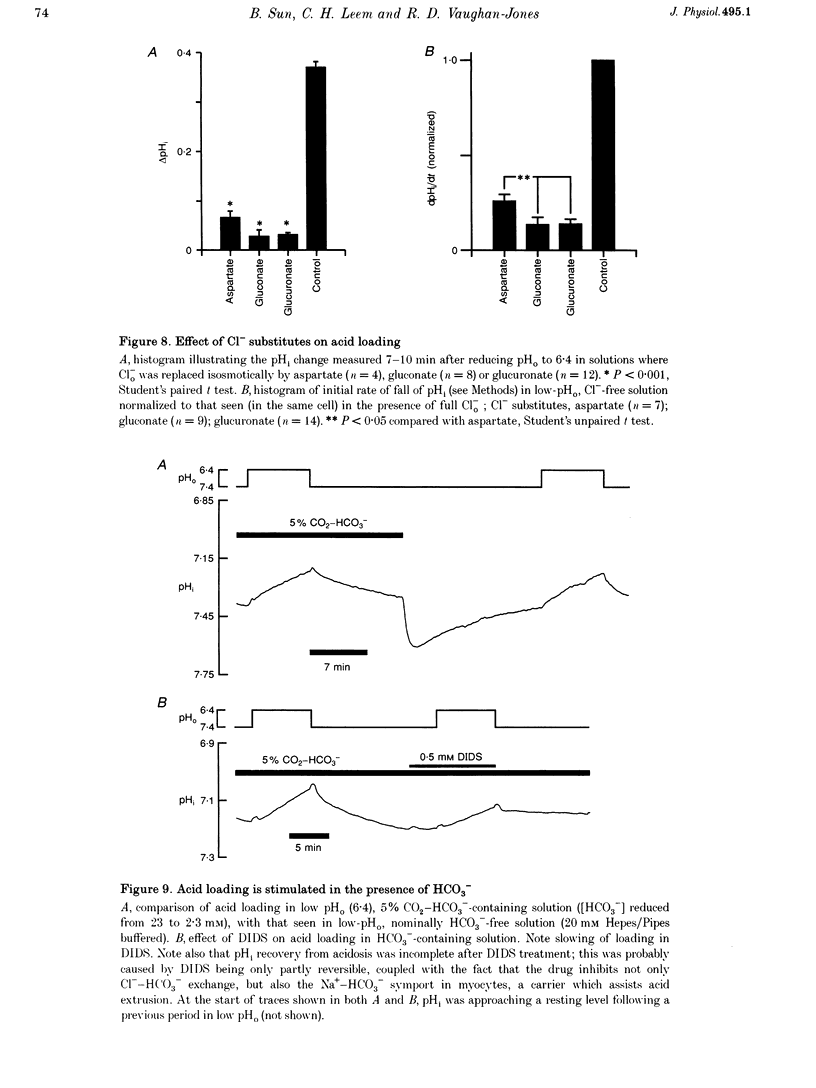
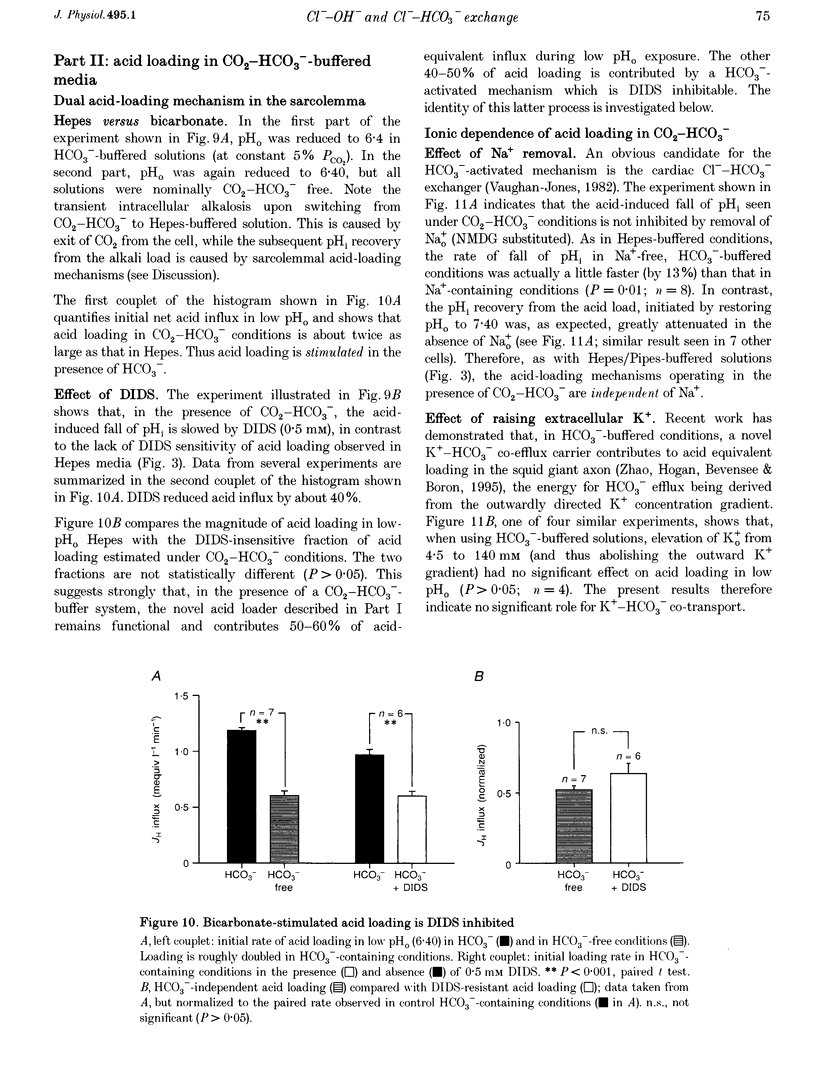
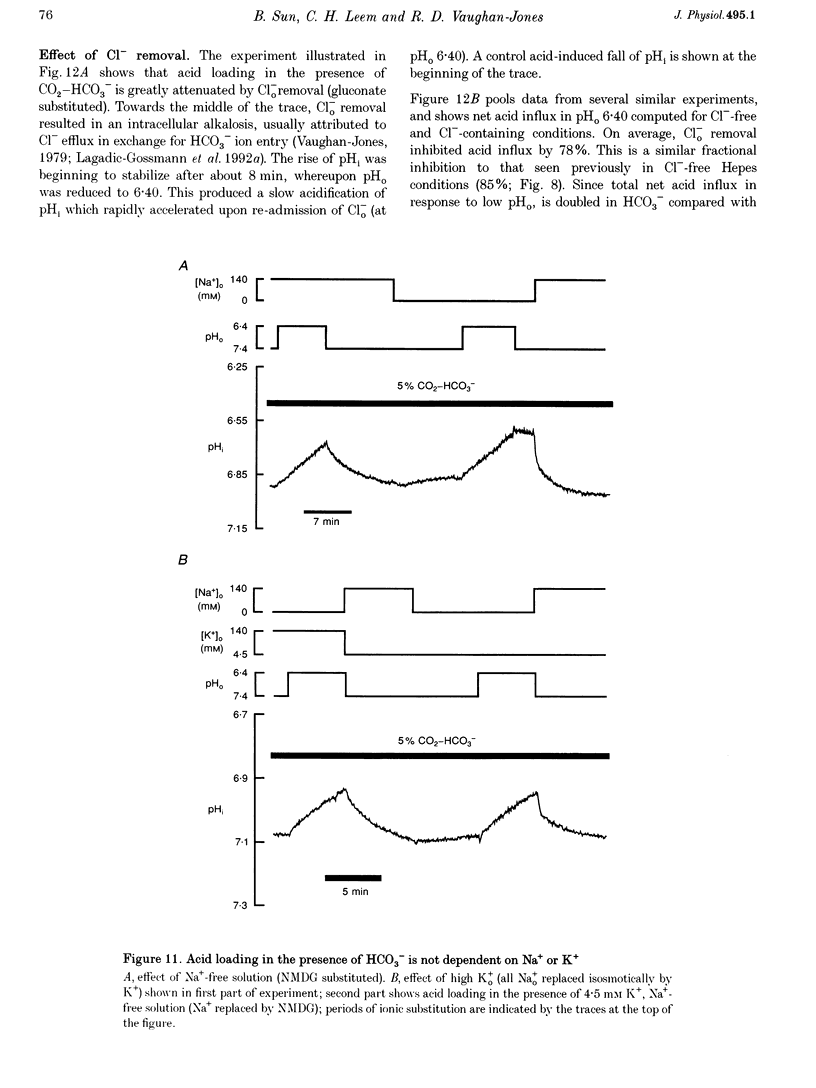
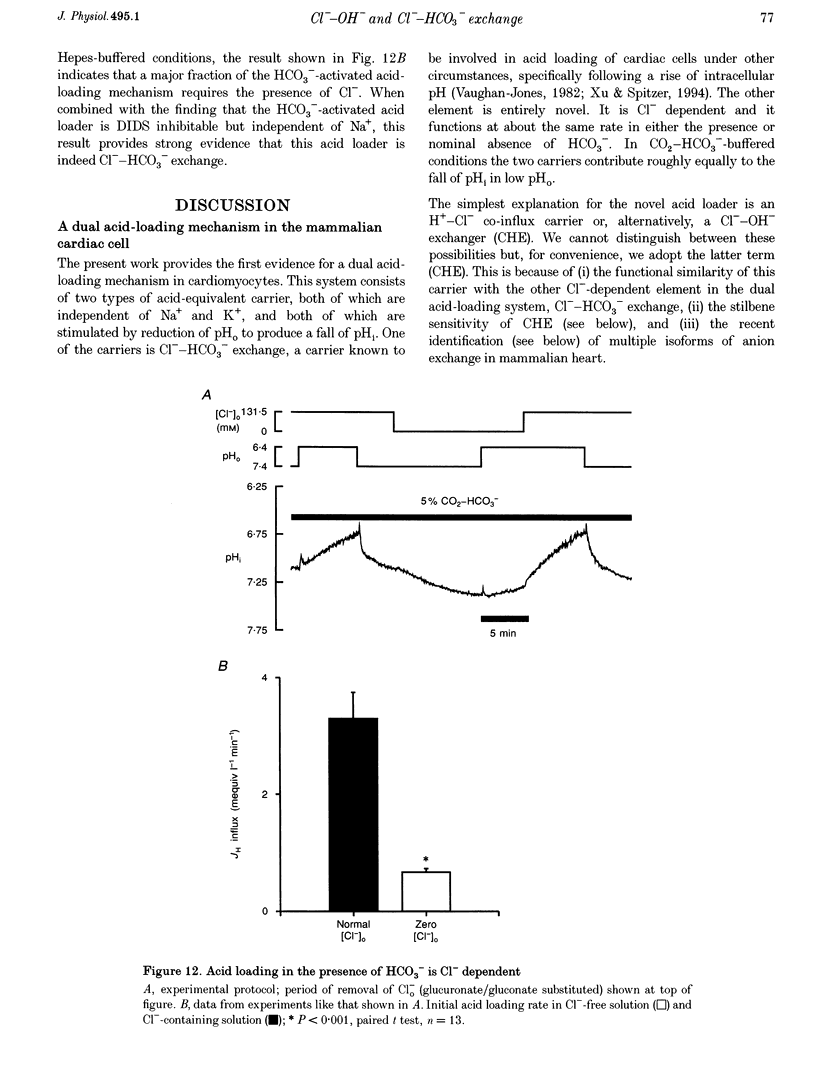
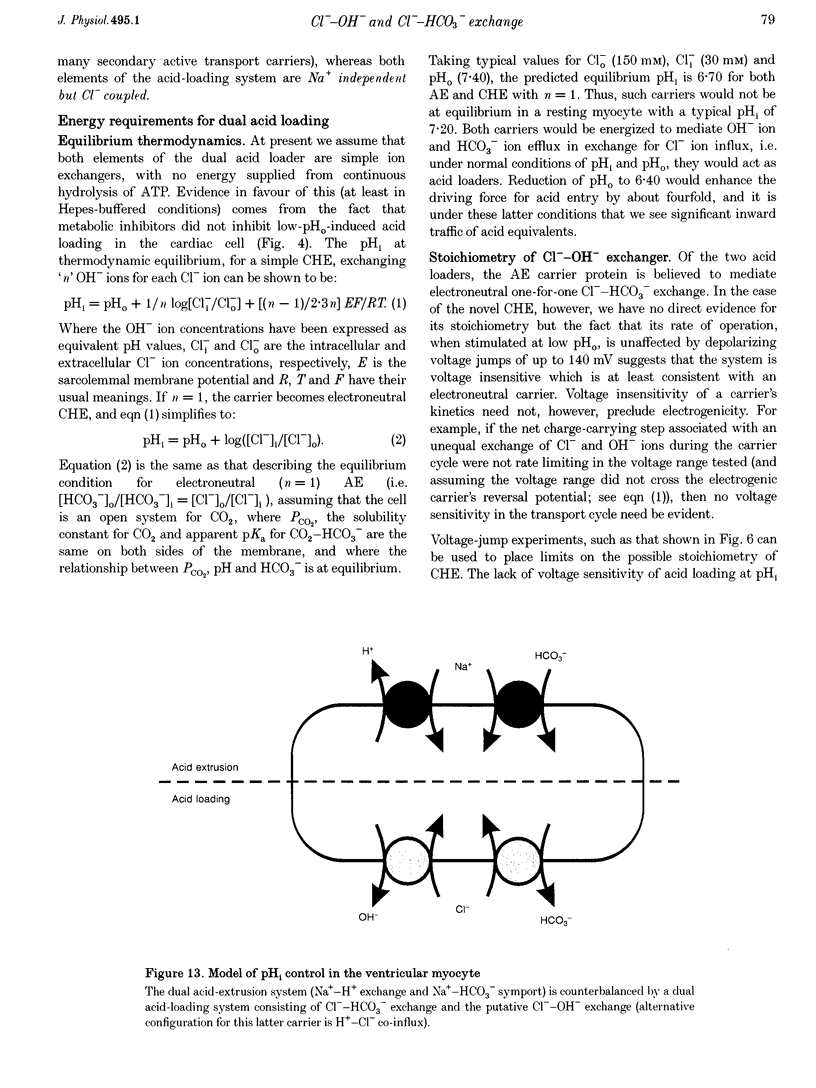
1. The fall of intracellular pH (pH1) following the reduction of extracellular pH (pH0) was investigated in guinea-pig isolated ventricular myocytes using intracellular fluorescence measurements of carboxy-SNARF-1 (to monitor pH1). Cell superfusates were buffered either with a 5% CO2-HCO3- system or were nominally CO2-HCO3-free. 2. Reduction of pH0 from 7.4 to 6.4 reversibly reduced pH1 by about 0.4 pH units, independent of the buffer system used. 3. In HCO3(-)-free conditions, acid loading in low pH0 was not dependent on Na(+)-H+ exchange or on the presence of Na+. It was unaffected by high-K+ solution, by voltage-clamp depolarization, by various divalent cations (Zn2+, Cd2+, Ni2+ and Ba2+) and by the organic Ca2+ channel blocker diltiazem, thus ruling out proton influx through H(+)-or Ca(2+)-conductance channels or influx via a K(+)-H+ exchanger. The fall also persisted in the presence of glycolytic inhibitors, or the lactate transport inhibitor, alpha-cyano-4-hydroxy cinnamate. 4. In HCO3(-)-free conditions, acid loading in low pH0 was reversibly inhibited (by up to 85%) by Cl(-)0 removal and was slowed by the stilbene drug DBDS (dibenzamidostilbene disulphonic acid). In contrast, the Cl(-)-HCO3-exchange inhibitor DIDS (4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid) had no inhibitory effect. Acid loading is therefore mediated by a novel Cl(-)-dependent, acid influx pathway. 5. After switching to CO2-HCO3(-)-buffered conditions, acid loading was doubled. It was still not inhibited by Na(+)-free or high-K+ solutions but was once again inhibited (by 78%) in Cl(-)-free solution. The HCO3(-)-stimulated fraction of acid loading was inhibited by DIDS. 6. We propose a model of acid loading in the cardiomyocyte which consists of two parallel carriers. One is Cl(-)-HCO3-exchange, while we suggest the other to be a novel Cl(-)-OH-exchanger (although we do not rule out the alternative configuration of H(+)-Cl-co-influx). The proposed dual acid-loading mechanism accounts for most of the sensitivity of pH1 to a fall of pH0.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L. The band 3-related anion exchanger (AE) gene family. Annu Rev Physiol. 1991;53:549–564. doi: 10.1146/annurev.ph.53.030191.003001. [DOI] [PubMed] [Google Scholar]
- Blank P. S., Silverman H. S., Chung O. Y., Hogue B. A., Stern M. D., Hansford R. G., Lakatta E. G., Capogrossi M. C. Cytosolic pH measurements in single cardiac myocytes using carboxy-seminaphthorhodafluor-1. Am J Physiol. 1992 Jul;263(1 Pt 2):H276–H284. doi: 10.1152/ajpheart.1992.263.1.H276. [DOI] [PubMed] [Google Scholar]
- Bountra C., Powell T., Vaughan-Jones R. D. Comparison of intracellular pH transients in single ventricular myocytes and isolated ventricular muscle of guinea-pig. J Physiol. 1990 May;424:343–365. doi: 10.1113/jphysiol.1990.sp018071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C., Vaughan-Jones R. D. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989 Nov;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désilets M., Baumgarten C. M. K+, Na+, and Cl- activities in ventricular myocytes isolated from rabbit heart. Am J Physiol. 1986 Aug;251(2 Pt 1):C197–C208. doi: 10.1152/ajpcell.1986.251.2.C197. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Kenning N. A., O'Neill S. C., Pocock G., Richards C. D., Valdeolmillos M. A novel method for absolute calibration of intracellular pH indicators. Pflugers Arch. 1989 Mar;413(5):553–558. doi: 10.1007/BF00594188. [DOI] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., Thomas R. C. Microelectrode measurement of the intracellular pH of mammalian heart cells. Nature. 1976 Jul 15;262(5565):224–225. doi: 10.1038/262224a0. [DOI] [PubMed] [Google Scholar]
- Fan C. C., Faust R. G., Powell D. W. Coupled sodium-chloride transport by rabbit ileal brush-border membrane vesicles. Am J Physiol. 1983 Apr;244(4):G375–G385. doi: 10.1152/ajpgi.1983.244.4.G375. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R., Berkoff H. A., Moss R. L. Metabolic cost of the stimulated beating of isolated adult rat heart cells in suspension. Circ Res. 1983 Mar;52(3):342–351. doi: 10.1161/01.res.52.3.342. [DOI] [PubMed] [Google Scholar]
- Lagadic-Gossmann D., Buckler K. J., Vaughan-Jones R. D. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol. 1992 Dec;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagadic-Gossmann D., Vaughan-Jones R. D., Buckler K. J. Adrenaline and extracellular ATP switch between two modes of acid extrusion in the guinea-pig ventricular myocyte. J Physiol. 1992 Dec;458:385–407. doi: 10.1113/jphysiol.1992.sp019423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado H. M., Cala P. M. Labeling of the Amphiuma erythrocyte K+/H+ exchanger with H2DIDS. Am J Physiol. 1994 Oct;267(4 Pt 1):C1002–C1012. doi: 10.1152/ajpcell.1994.267.4.C1002. [DOI] [PubMed] [Google Scholar]
- Orchard C. H., Kentish J. C. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990 Jun;258(6 Pt 1):C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Probst I., Schwartz P., Hütter F. J., Spieckermann P. G. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982 Jul;14(7):397–412. doi: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- Poole R. C., Halestrap A. P. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol. 1993 Apr;264(4 Pt 1):C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Pucéat M., Clément O., Vassort G. Extracellular MgATP activates the Cl-/HCO3- exchanger in single rat cardiac cells. J Physiol. 1991 Dec;444:241–256. doi: 10.1113/jphysiol.1991.sp018875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucéat M., Korichneva I., Cassoly R., Vassort G. Identification of band 3-like proteins and Cl-/HCO3- exchange in isolated cardiomyocytes. J Biol Chem. 1995 Jan 20;270(3):1315–1322. doi: 10.1074/jbc.270.3.1315. [DOI] [PubMed] [Google Scholar]
- Rajendran V. M., Binder H. J. Cl-HCO3 and Cl-OH exchanges mediate Cl uptake in apical membrane vesicles of rat distal colon. Am J Physiol. 1993 May;264(5 Pt 1):G874–G879. doi: 10.1152/ajpgi.1993.264.5.G874. [DOI] [PubMed] [Google Scholar]
- Shiuan D., Weinstein S. W. Evidence for electroneutral chloride transport in rabbit renal cortical brush border membrane vesicles. Am J Physiol. 1984 Nov;247(5 Pt 2):F837–F847. doi: 10.1152/ajprenal.1984.247.5.F837. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Meech R. W. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982 Oct 28;299(5886):826–828. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A. Intracellular pH in depolarized cardiac Purkinje strands. Pflugers Arch. 1985 Sep;405(2):118–126. doi: 10.1007/BF00584532. [DOI] [PubMed] [Google Scholar]
- Vanheel B., de Hemptinne A., Leusen I. Analysis of Cl- -HCO3(-) exchange during recovery from intracellular acidosis in cardiac Purkinje strands. Am J Physiol. 1984 May;246(5 Pt 1):C391–C400. doi: 10.1152/ajpcell.1984.246.5.C391. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. An investigation of chloride-bicarbonate exchange in the sheep cardiac Purkinje fibre. J Physiol. 1986 Oct;379:377–406. doi: 10.1113/jphysiol.1986.sp016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979 Oct;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. Extracellular H+ inactivation of Na(+)-H+ exchange in the sheep cardiac Purkinje fibre. J Physiol. 1990 Sep;428:441–466. doi: 10.1113/jphysiol.1990.sp018221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. L., Tsai M. L., Tseng Y. Z. DIDS-sensitive pHi regulation in single rat cardiac myocytes in nominally HCO3-free conditions. Circ Res. 1994 Jul;75(1):123–132. doi: 10.1161/01.res.75.1.123. [DOI] [PubMed] [Google Scholar]
- Yannoukakos D., Stuart-Tilley A., Fernandez H. A., Fey P., Duyk G., Alper S. L. Molecular cloning, expression, and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ Res. 1994 Oct;75(4):603–614. doi: 10.1161/01.res.75.4.603. [DOI] [PubMed] [Google Scholar]
- Zhao J., Hogan E. M., Bevensee M. O., Boron W. F. Out-of-equilibrium CO2/HCO3- solutions and their use in characterizing a new K/HCO3 cotransporter. Nature. 1995 Apr 13;374(6523):636–639. doi: 10.1038/374636a0. [DOI] [PubMed] [Google Scholar]