Abstract
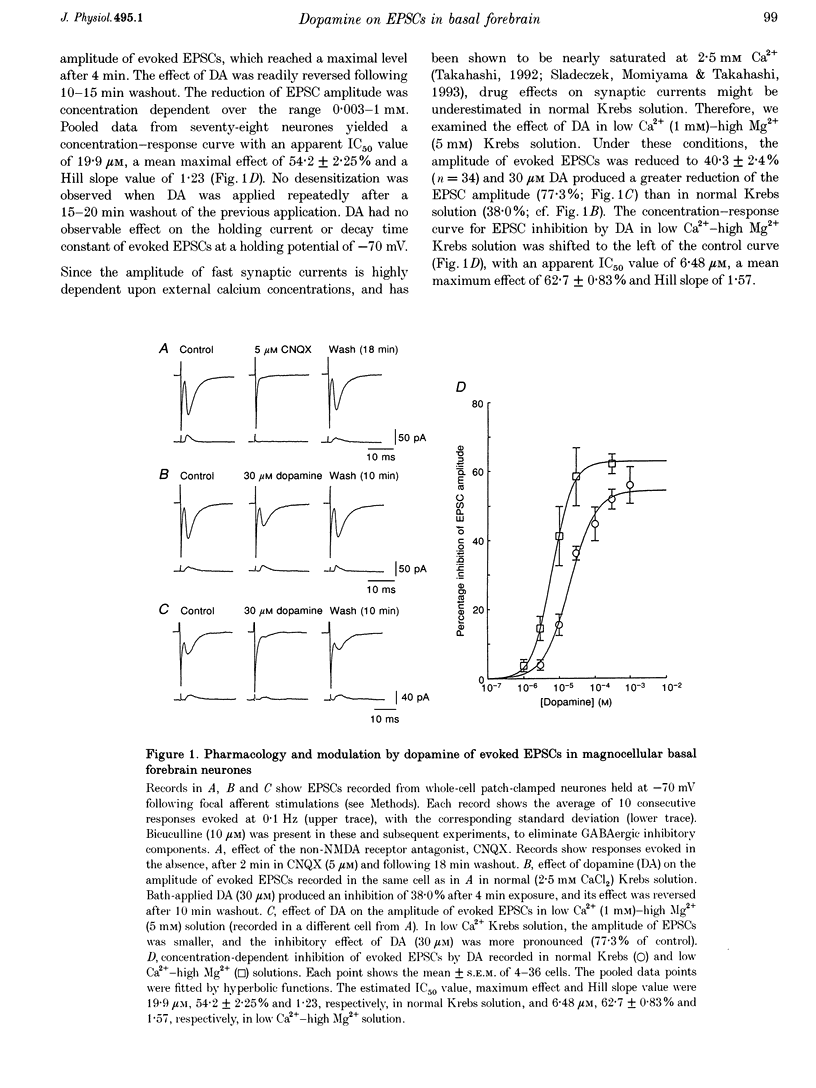
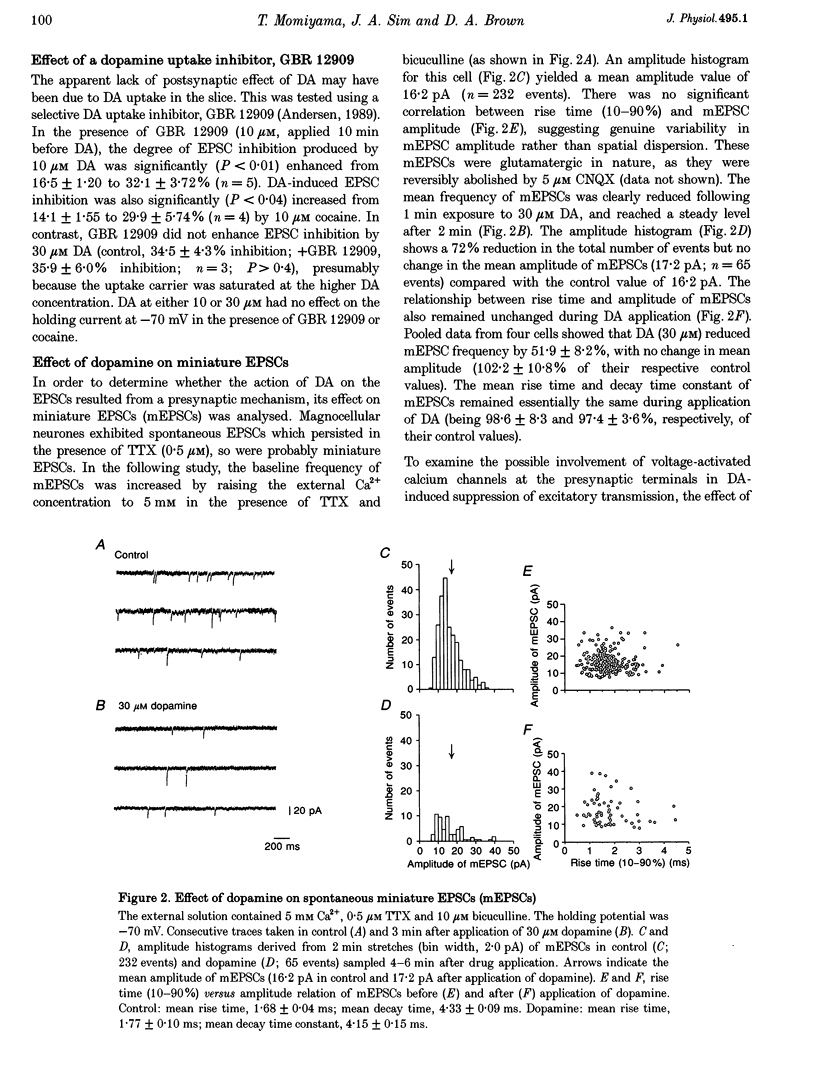
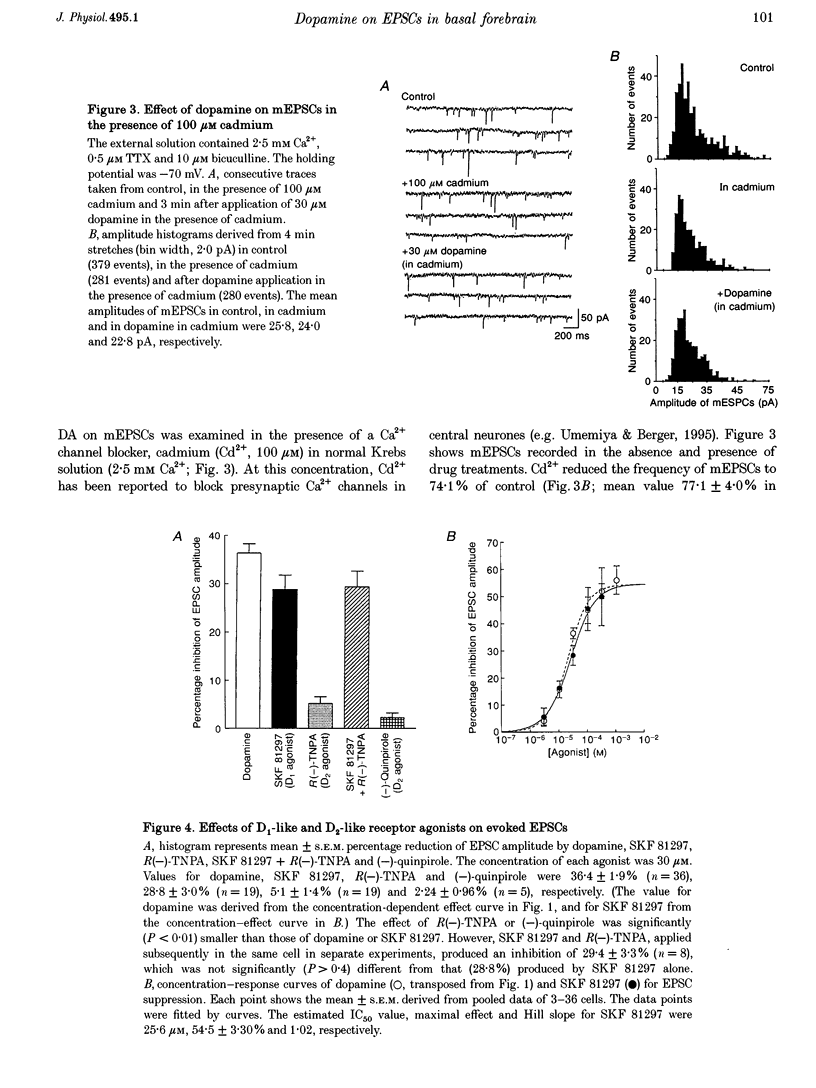
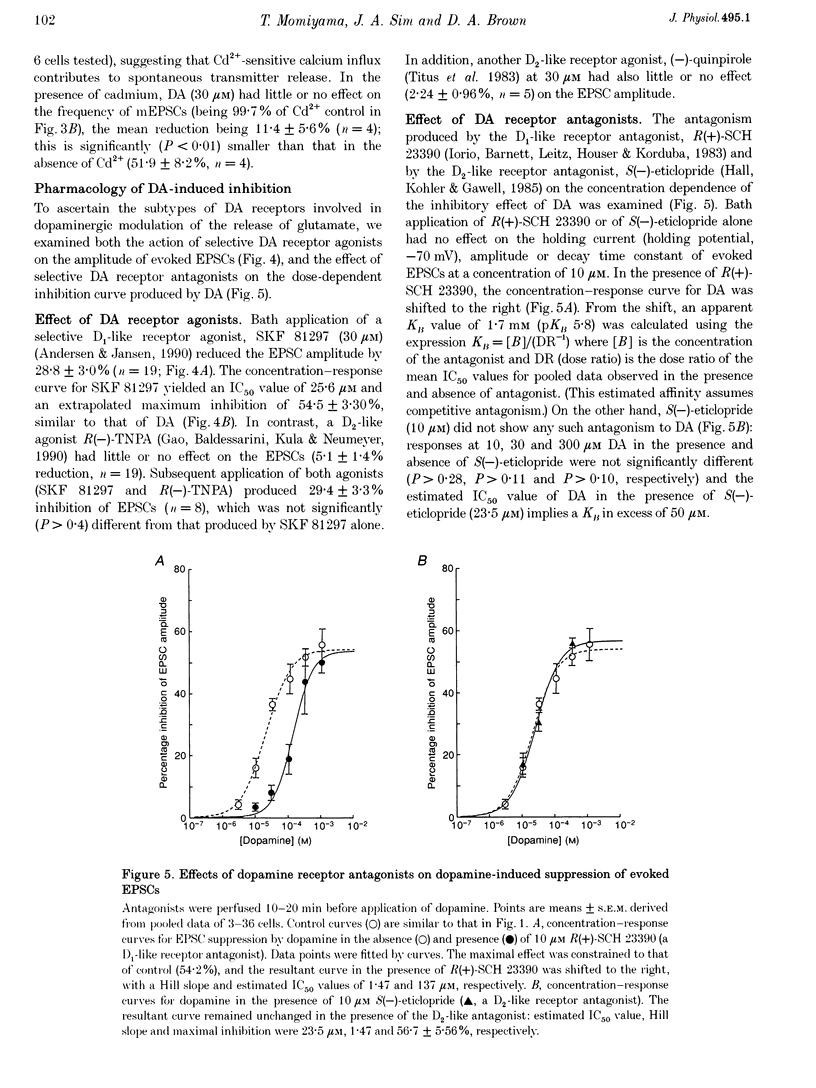
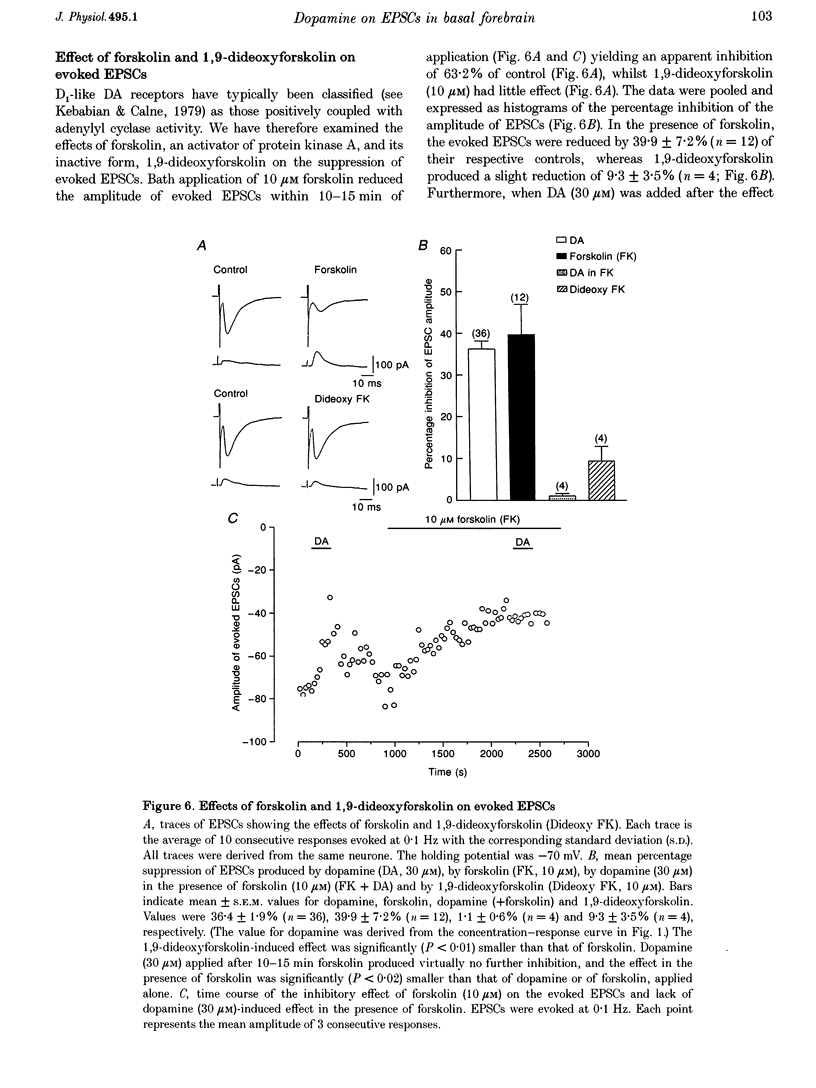
1. Excitatory postsynaptic currents (EPSCs) following focal afferent stimulation were recorded from patch-clamped magnocellular neurones in a thin-slice preparation of the rat basal forebrain. Evoked EPSCs had a mean decay time constant of 3.81 +/- 0.09 ms and were reversibly blocked by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 5 microM). 2. Bath-applied dopamine (DA) reduced evoked EPSC amplitude by up to 54.2 +/- 2.3% with an IC50 of 19.9 microM in normal Krebs solution (2.5 mM Ca2+, 1.2 mM Mg2+) without effect on postsynaptic holding current. 3. DA (30 microM) reduced the mean frequency of spontaneous miniature EPSCs recorded in 0.5 microM tetrodotoxin without affecting their mean amplitude, rise time or decay time constant. This effect was diminished by 100 microM Cd2+. 4. The effect of DA on evoked EPSCs was mimicked by the D1-like receptor agonist, SKF 81297 (IC50 25.6 microM), but not by the D2-like receptor agonist R(-)-TNPA (30 microM) or (-)-quinpirole (30 microM), and was antagonized by the D1-like receptor antagonist R(+)-SCH 23390 (estimated dissociation constant KB = 1.7 microM) but not by the D2-like receptor antagonist S(-)-eticlopride (10 microM). 5. Forskolin (10 microM) reduced evoked EPSCs to approximately 60% of the control amplitude, and occluded the effect of subsequent application of DA. 6. These results suggest that glutamatergic afferents to magnocellular basal forebrain neurones possess presynaptic D1-like DA receptors, and that activation of these receptors reduces excitatory glutamatergic transmission, probably via an adenylyl cyclase-dependent pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. H., Grønvald F. C., Jansen J. A. A comparison between dopamine-stimulated adenylate cyclase and 3H-SCH 23390 binding in rat striatum. Life Sci. 1985 Nov 25;37(21):1971–1983. doi: 10.1016/0024-3205(85)90028-1. [DOI] [PubMed] [Google Scholar]
- Andersen P. H., Jansen J. A. Dopamine receptor agonists: selectivity and dopamine D1 receptor efficacy. Eur J Pharmacol. 1990 Jun 12;188(6):335–347. doi: 10.1016/0922-4106(90)90194-3. [DOI] [PubMed] [Google Scholar]
- Andersen P. H. The dopamine inhibitor GBR 12909: selectivity and molecular mechanism of action. Eur J Pharmacol. 1989 Aug 3;166(3):493–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- Beninger R. J. The role of dopamine in locomotor activity and learning. Brain Res. 1983 Oct;287(2):173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- Blake J. F., Brown M. W., Collingridge G. L. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rat hippocampal slices. Neurosci Lett. 1988 Jun 29;89(2):182–186. doi: 10.1016/0304-3940(88)90378-3. [DOI] [PubMed] [Google Scholar]
- Calabresi P., De Murtas M., Mercuri N. B., Bernardi G. Chronic neuroleptic treatment: D2 dopamine receptor supersensitivity and striatal glutamatergic transmission. Ann Neurol. 1992 Apr;31(4):366–373. doi: 10.1002/ana.410310404. [DOI] [PubMed] [Google Scholar]
- Capogna M., Gähwiler B. H., Thompson S. M. Presynaptic enhancement of inhibitory synaptic transmission by protein kinases A and C in the rat hippocampus in vitro. J Neurosci. 1995 Feb;15(2):1249–1260. doi: 10.1523/JNEUROSCI.15-02-01249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Noriega L. E., Stevens C. F. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J Neurosci. 1994 Jan;14(1):310–317. doi: 10.1523/JNEUROSCI.14-01-00310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O., Bunzow J. R., Grandy D. K. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- Eaton M. J., Wagner C. K., Moore K. E., Lookingland K. J. Neurochemical identification of A13 dopaminergic neuronal projections from the medial zona incerta to the horizontal limb of the diagonal band of Broca and the central nucleus of the amygdala. Brain Res. 1994 Oct 3;659(1-2):201–207. doi: 10.1016/0006-8993(94)90879-6. [DOI] [PubMed] [Google Scholar]
- Gao Y. G., Baldessarini R. J., Kula N. S., Neumeyer J. L. Synthesis and dopamine receptor affinities of enantiomers of 2-substituted apomorphines and their N-n-propyl analogues. J Med Chem. 1990 Jun;33(6):1800–1805. doi: 10.1021/jm00168a040. [DOI] [PubMed] [Google Scholar]
- Hall H., Köhler C., Gawell L. Some in vitro receptor binding properties of [3H]eticlopride, a novel substituted benzamide, selective for dopamine-D2 receptors in the rat brain. Eur J Pharmacol. 1985 May 8;111(2):191–199. doi: 10.1016/0014-2999(85)90756-3. [DOI] [PubMed] [Google Scholar]
- Hori Y., Endo K., Takahashi T. Presynaptic inhibitory action of enkephalin on excitatory transmission in superficial dorsal horn of rat spinal cord. J Physiol. 1992 May;450:673–685. doi: 10.1113/jphysiol.1992.sp019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio L. C., Barnett A., Leitz F. H., Houser V. P., Korduba C. A. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983 Aug;226(2):462–468. [PubMed] [Google Scholar]
- Jonas P., Major G., Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993 Dec;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987 Nov;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law-Tho D., Hirsch J. C., Crepel F. Dopamine modulation of synaptic transmission in rat prefrontal cortex: an in vitro electrophysiological study. Neurosci Res. 1994 Dec;21(2):151–160. doi: 10.1016/0168-0102(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Le Moal M., Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991 Jan;71(1):155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Llano I., Gerschenfeld H. M. Beta-adrenergic enhancement of inhibitory synaptic activity in rat cerebellar stellate and Purkinje cells. J Physiol. 1993 Aug;468:201–224. doi: 10.1113/jphysiol.1993.sp019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Murillo R., Semenenko F., Cuello A. C. The origin of tyrosine hydroxylase-immunoreactive fibers in the regions of the nucleus basalis magnocellularis of the rat. Brain Res. 1988 Jun 7;451(1-2):227–236. doi: 10.1016/0006-8993(88)90767-6. [DOI] [PubMed] [Google Scholar]
- Monti J. M. Minireview. Catecholamines and the sleep-wake cycle. II. REM sleep. Life Sci. 1983 Mar 28;32(13):1401–1415. doi: 10.1016/0024-3205(83)90905-0. [DOI] [PubMed] [Google Scholar]
- Napier T. C., Maslowski-Cobuzzi R. J. Electrophysiological verification of the presence of D1 and D2 dopamine receptors within the ventral pallidum. Synapse. 1994 Jul;17(3):160–166. doi: 10.1002/syn.890170304. [DOI] [PubMed] [Google Scholar]
- Pennartz C. M., Dolleman-Van der Weel M. J., Kitai S. T., Lopes da Silva F. H. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol. 1992 May;67(5):1325–1334. doi: 10.1152/jn.1992.67.5.1325. [DOI] [PubMed] [Google Scholar]
- Pralong E., Jones R. S. Interactions of dopamine with glutamate- and GABA-mediated synaptic transmission in the rat entorhinal cortex in vitro. Eur J Neurosci. 1993 Jun 1;5(6):760–767. doi: 10.1111/j.1460-9568.1993.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Scanziani M., Gahwiler B. H., Thompson S. M. Presynaptic inhibition of excitatory synaptic transmission by muscarinic and metabotropic glutamate receptor activation in the hippocampus: are Ca2+ channels involved? Neuropharmacology. 1995 Nov;34(11):1549–1557. doi: 10.1016/0028-3908(95)00119-q. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R., López-Barneo J., Konnerth A. Excitatory and inhibitory synaptic currents and receptors in rat medial septal neurones. J Physiol. 1992 Jan;445:261–276. doi: 10.1113/jphysiol.1992.sp018923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K., Reiner P. B., McGeer E. G., Fibiger H. C. Brainstem afferents to the magnocellular basal forebrain studied by axonal transport, immunohistochemistry, and electrophysiology in the rat. J Comp Neurol. 1988 Jan 15;267(3):433–453. doi: 10.1002/cne.902670311. [DOI] [PubMed] [Google Scholar]
- Sim J. A., Griffith W. H. Muscarinic inhibition of glutamatergic transmissions onto rat magnocellular basal forebrain neurons in a thin-slice preparation. Eur J Neurosci. 1996 May;8(5):880–891. doi: 10.1111/j.1460-9568.1996.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Momiyama A., Takahashi T. Presynaptic inhibitory action of a metabotropic glutamate receptor agonist on excitatory transmission in visual cortical neurons. Proc Biol Sci. 1993 Sep 22;253(1338):297–303. doi: 10.1098/rspb.1993.0117. [DOI] [PubMed] [Google Scholar]
- Stern P., Edwards F. A., Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J Physiol. 1992 Apr;449:247–278. doi: 10.1113/jphysiol.1992.sp019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara R. K., Guan H. C., O'Dowd B. F., Seeman P., Laurier L. G., Ng G., George S. R., Torchia J., Van Tol H. H., Niznik H. B. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991 Apr 18;350(6319):614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T. The minimal inhibitory synaptic currents evoked in neonatal rat motoneurones. J Physiol. 1992 May;450:593–611. doi: 10.1113/jphysiol.1992.sp019145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. D., Kornfeld E. C., Jones N. D., Clemens J. A., Smalstig E. B., Fuller R. W., Hahn R. A., Hynes M. D., Mason N. R., Wong D. T. Resolution and absolute configuration of an ergoline-related dopamine agonist, trans-4,4a,5,6,7,8,8a,9-Octahydro-5-propyl-1H(or 2H)-pyrazolo[3,4-g]quinoline. J Med Chem. 1983 Aug;26(8):1112–1116. doi: 10.1021/jm00362a005. [DOI] [PubMed] [Google Scholar]
- Umemiya M., Berger A. J. Presynaptic inhibition by serotonin of glycinergic inhibitory synaptic currents in the rat brain stem. J Neurophysiol. 1995 Mar;73(3):1192–1201. doi: 10.1152/jn.1995.73.3.1192. [DOI] [PubMed] [Google Scholar]
- Yamamoto B. K., Davy S. Dopaminergic modulation of glutamate release in striatum as measured by microdialysis. J Neurochem. 1992 May;58(5):1736–1742. doi: 10.1111/j.1471-4159.1992.tb10048.x. [DOI] [PubMed] [Google Scholar]
- Zilles K., Werner L., Qü M., Schleicher A., Gross G. Quantitative autoradiography of 11 different transmitter binding sites in the basal forebrain region of the rat--evidence of heterogeneity in distribution patterns. Neuroscience. 1991;42(2):473–481. doi: 10.1016/0306-4522(91)90390-a. [DOI] [PubMed] [Google Scholar]


