Abstract
1. Studies show that reversible inactivation of the anterior interpositus nucleus (AIP) of the cerebellum with muscimol (a GABAA agonist) prevents acquisition of the classically conditioned nictitating membrane response (NMR) in the rabbit. Here, we have used reversible inactivations of the AIP with muscimol to investigate the role of the cerebellum in the extinction of this response. 2. Experimental subjects were implanted with cannulae targeted to the AIP, through which muscimol could be infused via an injector cannula. This experiment was divided into three phases lasting 4 days, separated by 3 day intervals. Experimental and unoperated control subjects received acquisition training in phase 1; in phases 2 and 3 they received extinction training. 3. Presentation of the conditioned stimulus (CS) alone in phase 2 produced normal extinction in control subjects. Muscimol inactivation of the AIP in experimental subjects during phase 2 prevented extinction of conditioned responses (CRs), shown by initial high CR frequency in the first post-drug session of phase 3, which then extinguished in a manner indistinguishable from controls in phase 2. 4. Our findings support the suggestion that similar cerebellar circuitry is engaged in acquisition and extinction of NMR conditioning.
Full text
PDF



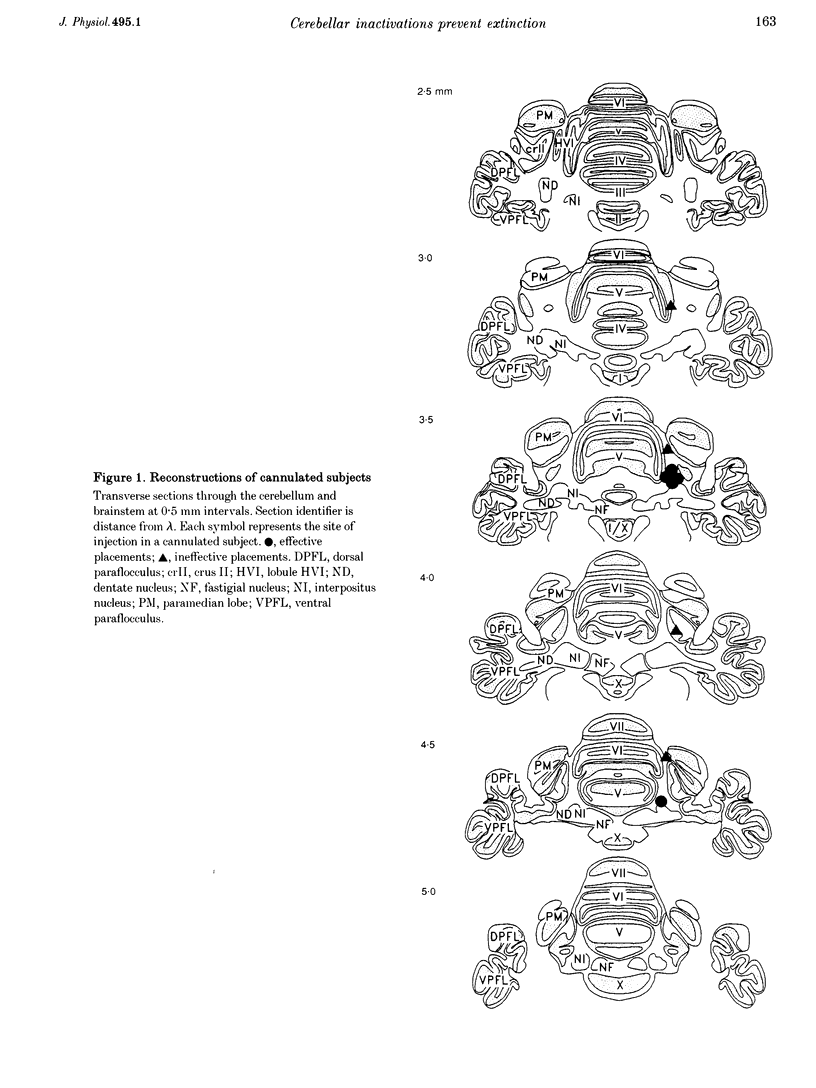

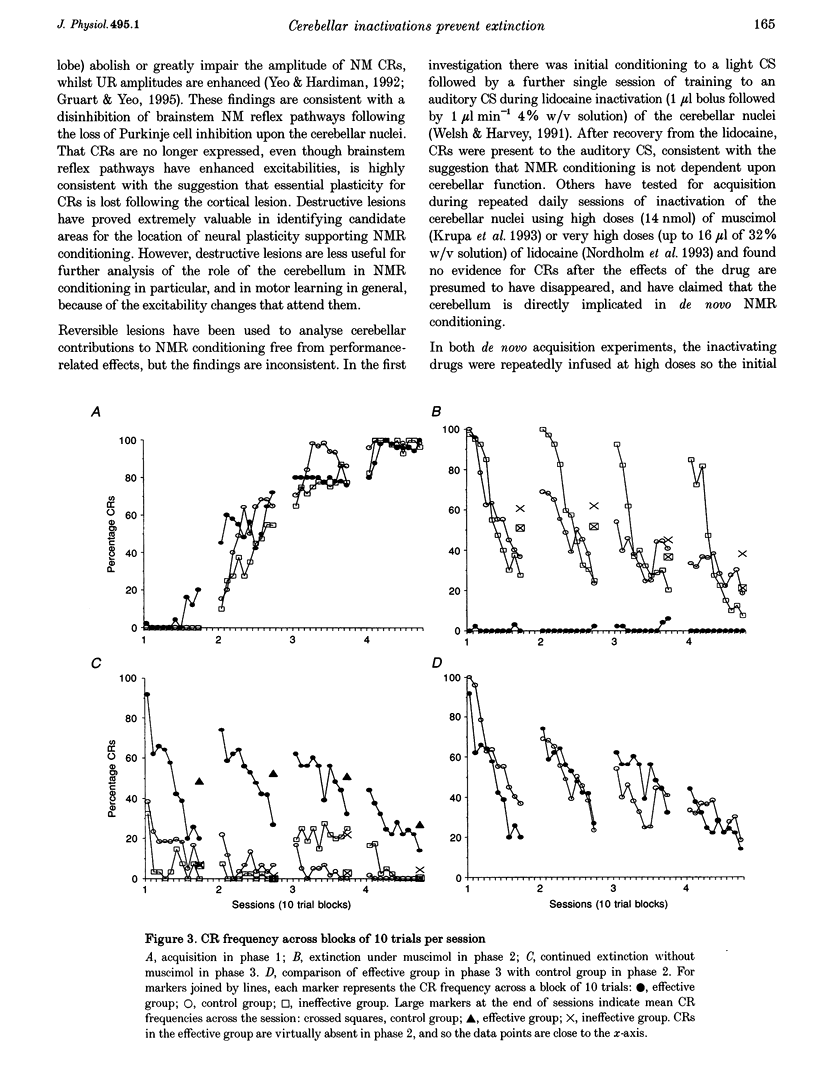



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GORMEZANO I., SCHNEIDERMAN N., DEAUX E., FUENTES I. Nictitating membrane: classical conditioning and extinction in the albino rabbit. Science. 1962 Oct 5;138(3536):33–34. doi: 10.1126/science.138.3536.33. [DOI] [PubMed] [Google Scholar]
- Gilbert P. F. A theory of memory that explains the function and structure of the cerebellum. Brain Res. 1974 Apr 12;70(1):1–18. doi: 10.1016/0006-8993(74)90208-x. [DOI] [PubMed] [Google Scholar]
- Gruart A., Yeo C. H. Cerebellar cortex and eyeblink conditioning: bilateral regulation of conditioned responses. Exp Brain Res. 1995;104(3):431–448. doi: 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- Hardiman M. J., Yeo C. H. The Effect of Kainic Acid Lesions of the Cerebellar Cortex on the Conditioned Nictitating Membrane Response in the Rabbit. Eur J Neurosci. 1992;4(10):966–980. doi: 10.1111/j.1460-9568.1992.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Hesslow G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. J Physiol. 1994 Apr 15;476(2):229–244. doi: 10.1113/jphysiol.1994.sp020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G. Inhibition of classically conditioned eyeblink responses by stimulation of the cerebellar cortex in the decerebrate cat. J Physiol. 1994 Apr 15;476(2):245–256. doi: 10.1113/jphysiol.1994.sp020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Neural design of the cerebellar motor control system. Brain Res. 1972 May 12;40(1):81–84. doi: 10.1016/0006-8993(72)90110-2. [DOI] [PubMed] [Google Scholar]
- Krupa D. J., Thompson J. K., Thompson R. F. Localization of a memory trace in the mammalian brain. Science. 1993 May 14;260(5110):989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Krupa D. J., Thompson R. F. Inactivation of the superior cerebellar peduncle blocks expression but not acquisition of the rabbit's classically conditioned eye-blink response. Proc Natl Acad Sci U S A. 1995 May 23;92(11):5097–5101. doi: 10.1073/pnas.92.11.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969 Jun;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink J. W., Thach W. T. Basal ganglia motor control. III. Pallidal ablation: normal reaction time, muscle cocontraction, and slow movement. J Neurophysiol. 1991 Feb;65(2):330–351. doi: 10.1152/jn.1991.65.2.330. [DOI] [PubMed] [Google Scholar]
- Nordholm A. F., Thompson J. K., Dersarkissian C., Thompson R. F. Lidocaine infusion in a critical region of cerebellum completely prevents learning of the conditioned eyeblink response. Behav Neurosci. 1993 Oct;107(5):882–886. doi: 10.1037//0735-7044.107.5.882. [DOI] [PubMed] [Google Scholar]
- Robinson F. R., Straube A., Fuchs A. F. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol. 1993 Nov;70(5):1741–1758. doi: 10.1152/jn.1993.70.5.1741. [DOI] [PubMed] [Google Scholar]
- Thompson R. F., Krupa D. J. Organization of memory traces in the mammalian brain. Annu Rev Neurosci. 1994;17:519–549. doi: 10.1146/annurev.ne.17.030194.002511. [DOI] [PubMed] [Google Scholar]
- Thompson R. F. The neurobiology of learning and memory. Science. 1986 Aug 29;233(4767):941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Welsh J. P., Harvey J. A. Cerebellar lesions and the nictitating membrane reflex: performance deficits of the conditioned and unconditioned response. J Neurosci. 1989 Jan;9(1):299–311. doi: 10.1523/JNEUROSCI.09-01-00299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J. P., Harvey J. A. Pavlovian conditioning in the rabbit during inactivation of the interpositus nucleus. J Physiol. 1991 Dec;444:459–480. doi: 10.1113/jphysiol.1991.sp018888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C. H. Cerebellum and classical conditioning of motor responses. Ann N Y Acad Sci. 1991;627:292–304. doi: 10.1111/j.1749-6632.1991.tb25933.x. [DOI] [PubMed] [Google Scholar]
- Yeo C. H., Hardiman M. J. Cerebellar cortex and eyeblink conditioning: a reexamination. Exp Brain Res. 1992;88(3):623–638. doi: 10.1007/BF00228191. [DOI] [PubMed] [Google Scholar]
- Yeo C. H., Hardiman M. J., Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. I. Lesions of the cerebellar nuclei. Exp Brain Res. 1985;60(1):87–98. doi: 10.1007/BF00237022. [DOI] [PubMed] [Google Scholar]
- Yeo C. H., Hardiman M. J., Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. III. Connections of cerebellar lobule HVI. Exp Brain Res. 1985;60(1):114–126. doi: 10.1007/BF00237024. [DOI] [PubMed] [Google Scholar]



