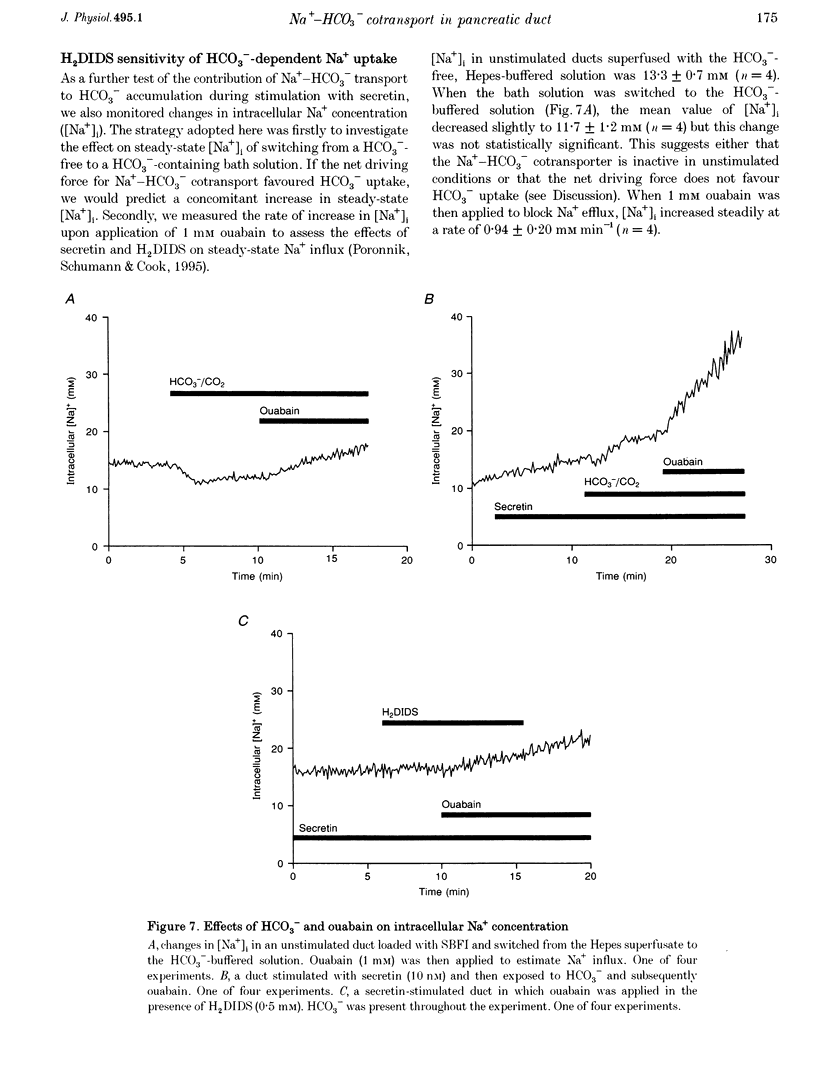
Abstract
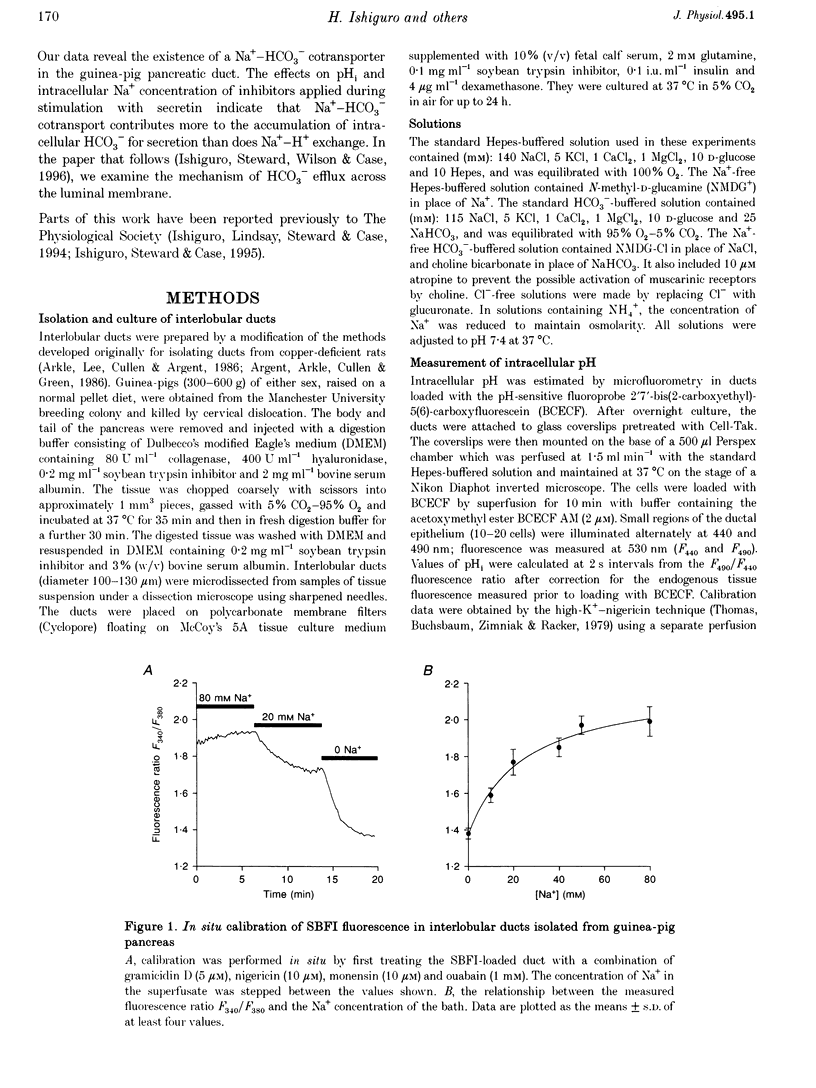
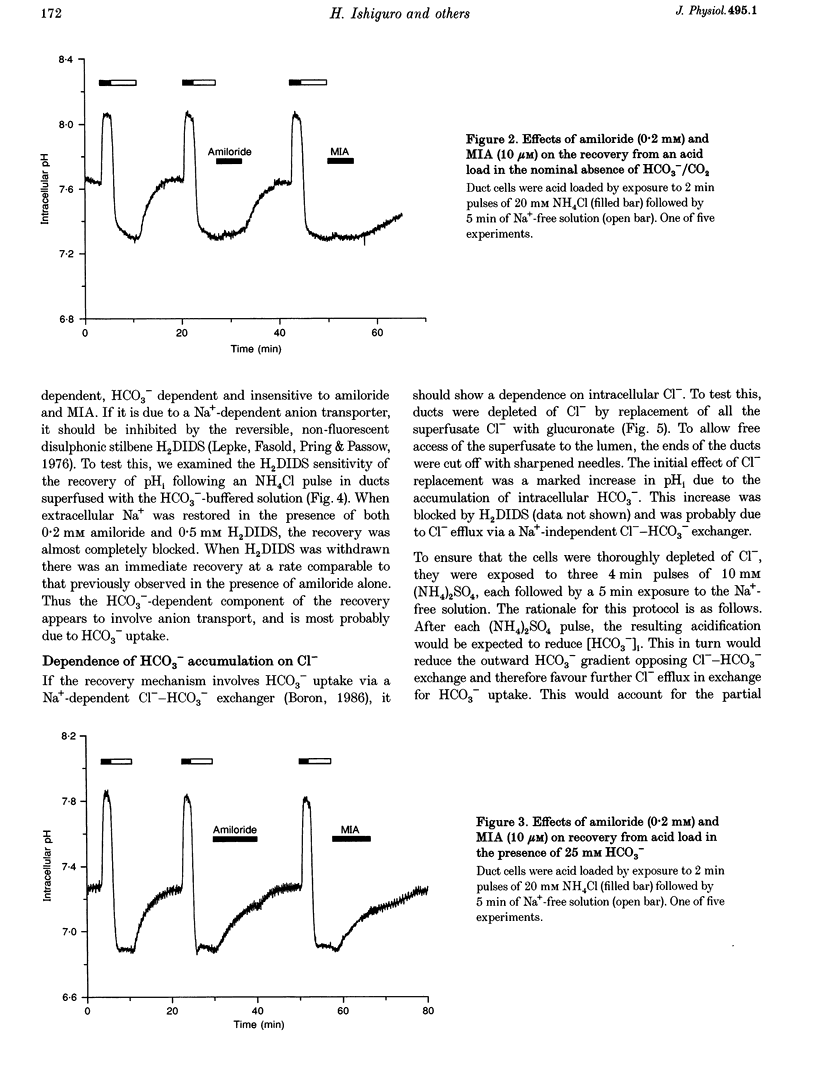
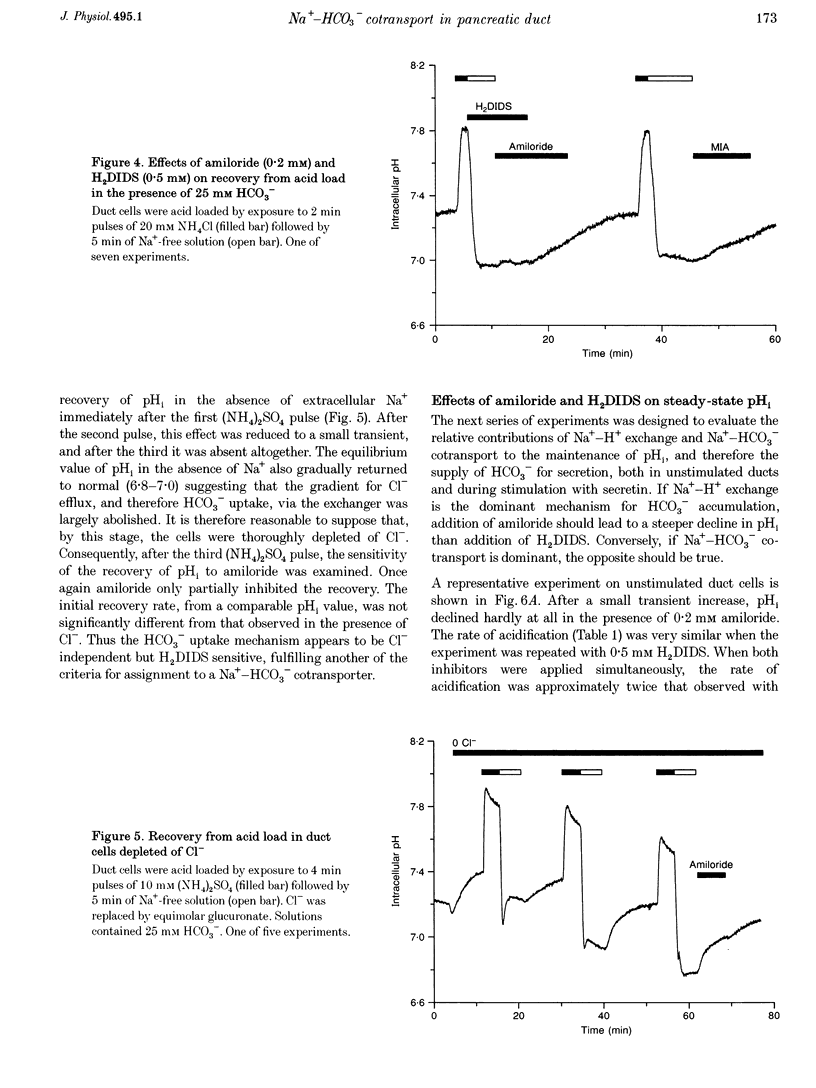
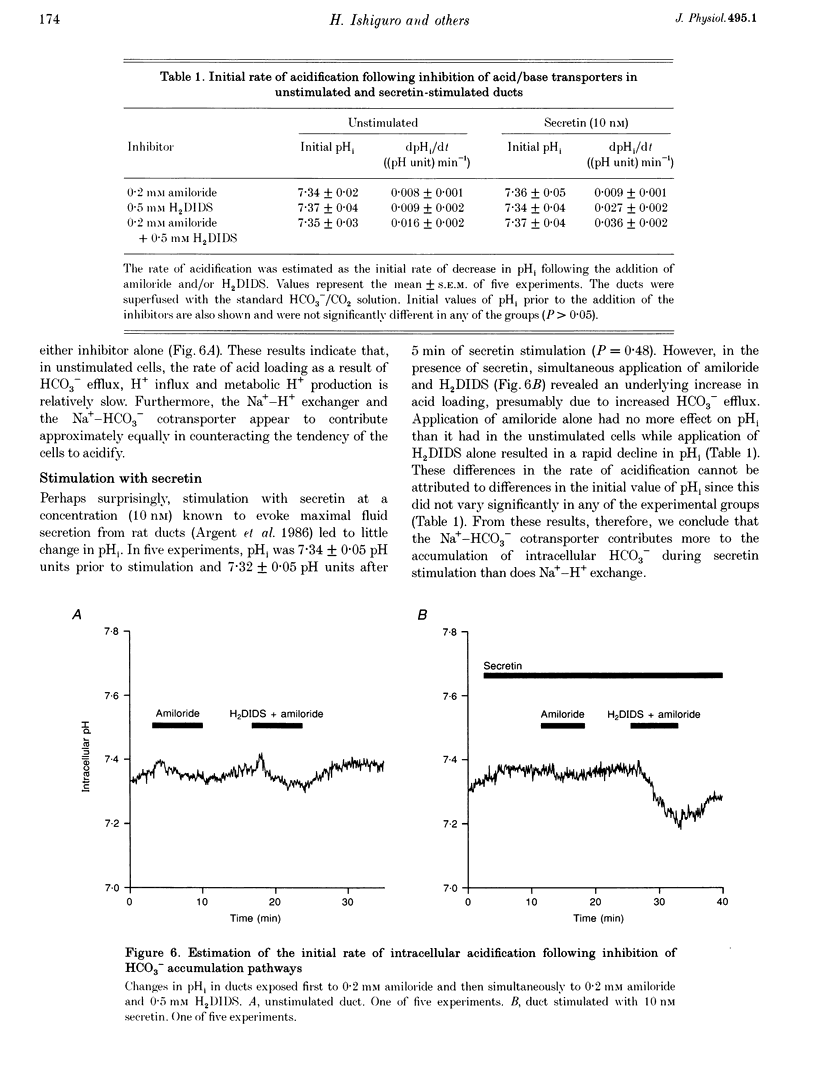
1. Short segments of interlobular duct were microdissected from guinea-pig pancreas following enzymatic digestion. After overnight culture, intracellular pH (pH1) and Na+ concentration ([Na+]i) were measured by microfluorometry in duct cells loaded with either the pH-sensitive fluoroprobe 2'7'-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) or the sodium-binding benzofuran isophthalate (SBFI). 2. The transporters responsible for maintaining pHi above equilibrium were investigated by using the NH4Cl pulse technique to acid load the cells. In the absence of HCO3-/CO2, the recovery of pH1 was Na+ dependent, abolished by 0.2 mM amiloride and by 10 microM N-methyl-N-isobutylamiloride and was therefore attributed to Na(+)-H+ exchange. 3. In the presence of HCO3-/CO2, amiloride only partially inhibited the recovery from acid loading. The amiloride-insensitive component was abolished by 0.5 mM H2DIDS and unaffected by depletion of intracellular Cl- and was therefore attributed to Na(+)-HCO3- cotransport. 4. Stimulation with 10 nM secretin did not cause a significant change in pH1 despite a significant increase in HCO3- efflux. However, in the presence of secretin, addition of 0.5 mM H2DIDS caused a decline in pH1 that was three times more rapid than that obtained with 0.2 mM amiloride. 5. In secretin-stimulated ducts, Na+ uptake increased when HCO3-/CO2 was added to the bath and this increase was strongly inhibited by 0.5 mM H2DIDS. 6. We conclude that Na(+)-HCO3- cotransport contributes approximately 75% of the HCO3- taken up by guinea-pig pancreatic duct cells during stimulation with secretin. It is proposed that electrical coupling between HCO3- efflux at the luminal membrane and electrogenic Na(+)-HCO3- cotransport at the basolateral membrane explains why secretin causes little change in pH1.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammar E. M., Hutson D., Scratcherd T. Absence of a relationship between arterial pH and pancreatic bicarbonate secretion in the isolated perfused cat pancreas. J Physiol. 1987 Jul;388:495–504. doi: 10.1113/jphysiol.1987.sp016627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argent B. E., Arkle S., Cullen M. J., Green R. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Q J Exp Physiol. 1986 Oct;71(4):633–648. doi: 10.1113/expphysiol.1986.sp003023. [DOI] [PubMed] [Google Scholar]
- Arkle S., Lee C. M., Cullen M. J., Argent B. E. Isolation of ducts from the pancreas of copper-deficient rats. Q J Exp Physiol. 1986 Apr;71(2):249–265. doi: 10.1113/expphysiol.1986.sp002982. [DOI] [PubMed] [Google Scholar]
- Boron W. F. Intracellular pH regulation in epithelial cells. Annu Rev Physiol. 1986;48:377–388. doi: 10.1146/annurev.ph.48.030186.002113. [DOI] [PubMed] [Google Scholar]
- Case R. M., Scratcherd T., Wynne R. D. The origin and secretion of pancreatic juice bicarbonate. J Physiol. 1970 Sep;210(1):1–15. doi: 10.1113/jphysiol.1970.sp009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S. J., Cohen S., Cragoe E. J., Jr, Grinstein S. Estimation of the number and turnover rate of Na+/H+ exchangers in lymphocytes. Effect of phorbol ester and osmotic shrinking. J Biol Chem. 1987 Mar 15;262(8):3626–3632. [PubMed] [Google Scholar]
- Grotmol T., Buanes T., Brørs O., Raeder M. G. Lack of effect of amiloride, furosemide, bumetanide and triamterene on pancreatic NaHCO3 secretion in pigs. Acta Physiol Scand. 1986 Apr;126(4):593–600. doi: 10.1111/j.1748-1716.1986.tb07860.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Eckert B. K., Tsien R. Y. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989 Nov 15;264(32):19458–19467. [PubMed] [Google Scholar]
- Ishiguro H., Steward M. C., Wilson R. W., Case R. M. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. J Physiol. 1996 Aug 15;495(Pt 1):179–191. doi: 10.1113/jphysiol.1996.sp021583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers G. A., Van Nooy I. G., De Pont J. J., Bonting S. L. The mechanism of fluid secretion in the rabbit pancreas studied by means of various inhibitors. Biochim Biophys Acta. 1984 Dec 5;778(2):324–331. doi: 10.1016/0005-2736(84)90376-6. [DOI] [PubMed] [Google Scholar]
- Lau K. R., Elliott A. C., Brown P. D. Acetylcholine-induced intracellular acidosis in rabbit salivary gland acinar cells. Am J Physiol. 1989 Feb;256(2 Pt 1):C288–C295. doi: 10.1152/ajpcell.1989.256.2.C288. [DOI] [PubMed] [Google Scholar]
- Lepke S., Fasold H., Pring M., Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4'-diisothiocyano stilbene-2,2'-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol. 1976 Oct 20;29(1-2):147–177. doi: 10.1007/BF01868957. [DOI] [PubMed] [Google Scholar]
- Novak I., Greger R. Electrophysiological study of transport systems in isolated perfused pancreatic ducts: properties of the basolateral membrane. Pflugers Arch. 1988 Jan;411(1):58–68. doi: 10.1007/BF00581647. [DOI] [PubMed] [Google Scholar]
- Novak I., Pahl C. Effect of secretin and inhibitors of HCO3-/H+ transport on the membrane voltage of rat pancreatic duct cells. Pflugers Arch. 1993 Nov;425(3-4):272–279. doi: 10.1007/BF00374178. [DOI] [PubMed] [Google Scholar]
- Padfield P. J., Garner A., Case R. M. Patterns of pancreatic secretion in the anaesthetised guinea pig following stimulation with secretin, cholecystokinin octapeptide, or bombesin. Pancreas. 1989;4(2):204–209. doi: 10.1097/00006676-198904000-00009. [DOI] [PubMed] [Google Scholar]
- Poronnik P., Schumann S. Y., Cook D. I. HCO3(-)-dependent ACh-activated Na+ influx in sheep parotid secretory endpieces. Pflugers Arch. 1995 Apr;429(6):852–858. doi: 10.1007/BF00374810. [DOI] [PubMed] [Google Scholar]
- Raeder M., Mo A., Aune S., Mathisen O. Relationship between plasma pH and pancreatic HCO3- secretion at different intravenous secretin infusion rates. Acta Physiol Scand. 1980 Jun;109(2):187–191. doi: 10.1111/j.1748-1716.1980.tb06585.x. [DOI] [PubMed] [Google Scholar]
- Seki G., Coppola S., Frömter E. The Na(+)-HCO3- cotransporter operates with a coupling ratio of 2 HCO3- to 1 Na+ in isolated rabbit renal proximal tubule. Pflugers Arch. 1993 Dec;425(5-6):409–416. doi: 10.1007/BF00374866. [DOI] [PubMed] [Google Scholar]
- Seo J. T., Larcombe-McDouall J. B., Case R. M., Steward M. C. Modulation of Na(+)-H+ exchange by altered cell volume in perfused rat mandibular salivary gland. J Physiol. 1995 Aug 15;487(1):185–195. doi: 10.1113/jphysiol.1995.sp020870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell W. A., Young J. A. Secretion of electrolytes by the pancreas of the anaestetized rat. J Physiol. 1975 Nov;252(2):379–396. doi: 10.1113/jphysiol.1975.sp011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Waisbren S. J., Geibel J. P., Modlin I. M., Boron W. F. Unusual permeability properties of gastric gland cells. Nature. 1994 Mar 24;368(6469):332–335. doi: 10.1038/368332a0. [DOI] [PubMed] [Google Scholar]
- Zhao H., Star R. A., Muallem S. Membrane localization of H+ and HCO3- transporters in the rat pancreatic duct. J Gen Physiol. 1994 Jul;104(1):57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]



