Abstract
The Advanced Lung Cancer Inflammation Index (ALI) represents both the inflammatory and nutritional status of the host, but its link with mortality in asthma patients is uncertain. The purpose of this study was to look at the relationship between ALI levels and all-cause and respiratory disease mortality in asthmatic patients. We conducted our research using cohort data from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018. The National Death Index was used to calculate mortality until December 31, 2019. The study employed multivariate logistic regression to look into the relationship between ALI levels and asthma prevalence. Weighted Kaplan–Meier and multivariate-adjusted Cox analyses were utilized for investigating the relationship between ALI levels and all-cause and respiratory disease mortality in individuals with asthma. A restricted cubic spline (RCS) analysis was used to assess their nonlinear relationship. Subgroup and sensitivity analyses were also performed to evaluate the robustness of the results that were obtained. We enrolled 40,497 people in our study, and 5,469 of them had asthma, representing a 14% prevalence. A median follow-up of 11.19 (9.38, 14.29) years revealed 109 fatalities from respiratory diseases and 724 deaths from all causes. After correcting for several covariates, there was no longer any link (P-trend = 0.2) between ALI levels and the prevalence of asthma. When compared to the lowest quartile, the highest quartile of ALI levels was substantially linked to a lower risk of mortality from respiratory diseases and all causes (all P-trend < 0.001). In the RCS regression model, the relationship between ALI level and both all-cause and respiratory disease mortality in asthmatic participants was nonlinear, with P for nonlinearity of 0.006 and 0.015, respectively. We also discovered that the probability of mortality from respiratory disease decreased progressively to a nadir at an ALI level of 109.13 and then increased as the ALI level increased. Multiple subgroup and sensitivity analyses revealed that ALI was consistently related to lower all-cause and respiratory disease mortality in asthma patients. Our findings suggest that ALI is associated with a reduced risk of all-cause and respiratory disease mortality in asthma patients.
Keywords: Asthma, Advanced lung cancer inflammation index, All-cause mortality, Respiratory disease mortality, National Health and Nutrition Examination Survey
Subject terms: Asthma, Prognostic markers
Introduction
Asthma is a prevalent chronic airway illness characterized by airway hyperresponsiveness, inflammatory cell infiltration, and mucus secretion that causes a large worldwide health burden1. It is one of the most common chronic airway disorders, affecting around 339 million people globally and causing over 1,000 deaths every day, with the prevalence increasing year after year2.
Numerous studies have confirmed the critical function of immune cells and the inflammatory response in the development and progression of asthma. Airway inflammation in asthma is enhanced by Th2-dependent IgE, IgE-producing B cells, mast cells, and eosinophil recruitment3. In addition to allergen-specific Th2-driven adaptive immunity, innate immune cells such as macrophages, granulocytes, epithelial cells, type 2 innate lymphocytes (ILC2), mast cells, eosinophils, dendritic cells (DCs), and natural killer cells (NKs) are thought to play a role in the allergic and nonallergic pathogenesis of asthma-extracellular acidification rates4. Neutrophils also play a significant role in the pathophysiology of asthma, acting as the first line of defense in the event of a lung infection and producing chemokines and performed granulins that attract monocytes and/or macrophages to the infection site, resulting in an immunological infiltrate5,6. However, long-term chronic inflammation can cause decreased albumin levels and weight loss7, therefore relying solely on an inflammation marker may not be sufficient to predict the prognosis of asthma patients.
ALI was developed in 2013 by Jafri et al. as a new prognostic index for patients with advanced lung cancer8. It is composed of body mass index (BMI), albumin, and neutrophil-to-lymphocyte ratio (NLR), and it can reflect both the host’s inflammatory and nutritional status. As the name implies, the ALI was initially designed to predict tumor prognosis in patients with lung cancer, and multiple studies have already proved its important predictive value for the prognosis of patients with lung cancer9–11. Furthermore, ALI has been shown to predict prognosis in a variety of disorders, including gastric cancer12, diabetes13, heart failure14, B-cell lymphoma15, and esophageal cancer16. However, rare research has investigated the relationship between ALI and the prognosis of patients with asthma.
The purpose of this study was to investigate the association between ALI and the risk of all-cause and respiratory disease mortality in asthma patients, as well as to provide some recommendations for asthma patients’ treatment and management.
Materials and methods
Study population
NHANES is a nationally representative cross-sectional survey that is conducted repeatedly in the United States by the National Center for Health Statistics17. NHANES uses a stratified multistage random sampling design. A retrospective analysis was conducted utilizing publicly accessible NHANES data from 1999 to 2018.
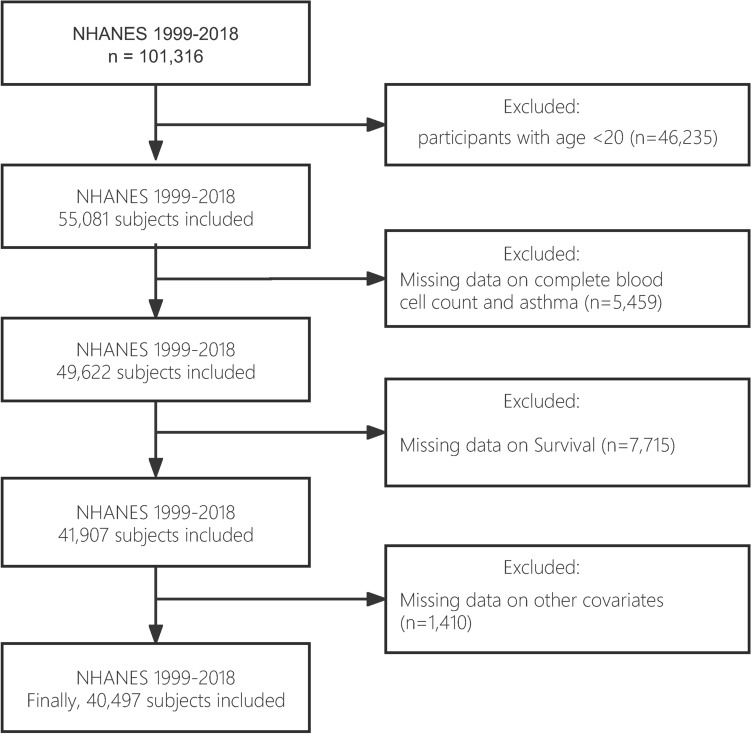
In this study, we examined NHANES data from 1999 to 2018, covering 101,316 participants. We removed all participants under the age of 20 (n = 46,235). Participants with incomplete blood cell counts and asthma were omitted from the study (n = 5,459). Participants without follow-up information were eliminated (n = 7,715). Finally, participants who were pregnant or lacking information on key variables (age, gender, race, BIM, and albumin) were eliminated (n = 1,410). As a result, our final analysis contained 40,497 individuals (Fig. 1). The NHANES study protocol has been approved by the Ethics Review Board of the National Center for Health Statistics and Research. All participants provided written informed consent authorizing the use of their data. All experiments involved in this paper are performed in accordance with relevant guidelines and regulations.
Fig. 1.
Flowchart of study design.
Calculation of ALI
ALI was calculated as follows: ALI = BMI × Alb/NLR, where BMI = weight in kilograms/(height in meters)2, Alb = serum albumin in grams per deciliter, NLR = neutrophil counts/lymphocyte counts9,18.
Assessment of asthma
NHANES collects information regarding asthma and related symptoms through a professionally self-administered questionnaire19. Patients were classified as currently having asthma if they answered yes to the following two questions: "Has a doctor or other health professional ever told you that you have asthma?" and "Do you still have asthma?" Patients who answered negatively to these two questions were classified as non-asthmatic.
Assessment of mortality
Study participants who died were identified through association with the National Death Index (NDI). As of December 31, 2019, we obtained participant records for all-cause mortality and cardiovascular disease mortality through the 2019 Linked Mortality File (LMF), which reports the most recent associations between specific NCHS surveys and the NDI. The follow-up period for the study was from the date of initial diagnosis to the date of death or the end of the study period on December 31, 2019, whichever came first.
Definitions of covariates
By considering clinical plausibility and referring to previous research, we identified relevant covariates thereby minimizing confounding bias. Information regarding participants’ baseline data was collected through questionnaires and physical examination, including age (years), sex (male or female), race/ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, or other race), educational attainment (below high school, high school, or above high school), marital status (married/cohabiting, widowed/divorced/separated, or never married) and body mass index (< 18.5, 18.5–25.0, 25.0–29.9, or > 29.9 kg/m2). Income was measured using the poverty-income ratio (PIR; the ratio of family income divided by a poverty threshold specific for family size using guidelines from the US Department of Health and Human Services) and categorized as ≤ 1.0, 1.1–3.0, and > 3.0. Never smokers were identified as those who reported smoking < 100 cigarettes throughout their lifetime. Those who smoked > 100 cigarettes in their lifetime were classified as current smokers, and those who smoked > 100 cigarettes and had quit smoking were labeled as former smokers20. Drinking status was classified as nondrinker and drinker by asking the question “Had at least 12 alcohol drinks/1 year?”. Physical activity was divided into inactive group and active group according to the presence of moderate to vigorous activity over the past 30 days. Self-reported diabetes was determined by a self-reported doctor or a glycosylated hemoglobin measurement of ≥ 6.5%. Patients with hypertension were defined as those who were taking antihypertensive medication, or by a self-reported doctor. Complete blood cell count parameters and serum albumin levels, which were necessary to calculate ALI were also extracted from the database.
Statistical analysis
We used the NHANES recommended weights to calculate the weights for specific groups. The categorical variables were expressed as percentages (%) and were compared using the chi-square test or Fisher’s exact test, as appropriate. Continuous data with a normal distribution were shown as means (± standard deviation [SD]) and were compared using an independent samples t-test. Variables with skewed distributions were presented as medians [first quantile (P25) and third quantile (P75)] and were compared using the nonparametric Wilcoxon rank sum test.
Three logistic regression models were employed to identify the adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of the association between ALI levels and the prevalence of asthma. Likewise, three Cox regression models were used to calculate adjusted hazard ratios (HRs) and 95% CIs about all-cause and respiratory disease mortality of participants with asthma. Model 1 was a crude unadjusted model of potential confounders. Model 2 was adjusted as age, sex, and race/ethnicity; Model 3 was adjusted as model 2 plus education level, family poverty income ratio, drinking status, smoking status, BMI, self-reported diabetes, and self-reported hypertension.
Kaplan–Meier analyses were conducted to investigate the connection between ALI levels and long-term mortality in asthmatic patients. In NHANES 1999–2018, a subgroup analysis was performed to investigate the relationships between quartiles of ALI levels and all-cause and respiratory disease mortality by age, gender, and smoking status among persons with asthma.
We conducted multiple sensitivity analyses to assess the robustness of the results. First, we removed people with a history of emphysema or chronic bronchitis to rule out a potential bias in the data. Second, to reduce the risk of reverse causality bias, participants who died within the first two years of follow-up were eliminated from the study. Finally, persons with a history of cancer at baseline were removed from the research. Cox regression analysis was then used to determine the link between ALI levels and mortality in asthma patients.
Results
Participant characteristics
The baseline characteristics and weighted estimates of the study population are presented in Table 1. A total of 40,497 individuals were included in our analysis, of whom 5,469 had a diagnosis of asthma and ranged in age from 20 to 85 years, with a mean age of (61.26 ± 14.30) years. Racial distribution was as follows Mexican American 4.8%, other Hispanic 5.8%, non-Hispanic white 71%, non-Hispanic black 12%, and other 6.7%. Participants with asthma were more likely to be young women, have higher levels of education, have lower incomes, be divorced, and be smokers. In addition, participants in the asthma group had higher body mass index and waist circumference, and higher rates of comorbid hypertension and diabetes. The median (P25, P75) of white blood cells, neutrophils, lymphocytes, platelets, monocytes and ALI in the asthma group were 7.20 (5.90, 8.80), 4.20 (3.20, 5.40), 2.10 (1.70, 2.50), 251 (213, 297), 0.50 (0.40, 0.70) and 62 (46, 82), higher than the non-asthmatic group. Significant differences were found in the parameters of the complete blood count and ALI levels between participants with and without asthma (P < 0.05).
Table 1.
Baseline characteristics of adults with asthma in NHANES 1999–2018.
| Characteristics | Overall, N = 40,497 (100%) | Asthma | P Value | |
|---|---|---|---|---|
| No, N = 35,028 (86%) | Yes, N = 5469 (14%) | |||
| Sex, % | < 0.001 | |||
| Female | 20,896 (52%) | 17,712 (51%) | 3,184 (59%) | |
| Male | 19,601 (48%) | 17,316 (49%) | 2,285 (41%) | |
| Age, % | < 0.001 | |||
| 20–35 years | 10,625 (28%) | 8,939 (27%) | 1,686 (33%) | |
| 35–60 years | 16,551 (48%) | 14,365 (48%) | 2,186 (45%) | |
| 60 + years | 13,321 (24%) | 11,724 (25%) | 1,597 (22%) | |
| Race/ethnicity, % | < 0.001 | |||
| Non-Hispanic White | 18,376 (69%) | 15,687 (69%) | 2,689 (71%) | |
| Non-Hispanic Black | 8,123 (11%) | 6,856 (10%) | 1,267 (12%) | |
| Mexican American | 6,965 (8.3%) | 6,427 (8.9%) | 538 (4.8%) | |
| Other Race—Including Multi-Racial | 3,633 (6.9%) | 3,175 (6.9%) | 458 (6.7%) | |
| Other Hispanic | 3,400 (5.3%) | 2,883 (5.2%) | 517 (5.8%) | |
| Education level, % | 0.001 | |||
| Below high school | 10,600 (17%) | 9,349 (17%) | 1,251 (15%) | |
| High school | 9,400 (24%) | 8,181 (24%) | 1,219 (22%) | |
| Above high school | 20,497 (59%) | 17,498 (59%) | 2,999 (62%) | |
| Marital status, % | < 0.001 | |||
| Married/cohabiting | 24,639 (64%) | 21,667 (65%) | 2,972 (58%) | |
| Widowed/divorced/separated | 8,809 (18%) | 7,485 (18%) | 1,324 (20%) | |
| Never married | 7,046 (17%) | 5,873 (17%) | 1,173 (21%) | |
| Family PIR, % | < 0.001 | |||
| ≤ 1.0 | 7,617 (14%) | 6,364 (13%) | 1,253 (18%) | |
| 1.1–3.0 | 15,745 (36%) | 13,673 (36%) | 2,072 (36%) | |
| > 3.0 | 13,986 (50%) | 12,225 (51%) | 1,761 (46%) | |
| Smoking status, % | < 0.001 | |||
| Never smoker | 21,981 (54%) | 19,288 (54%) | 2,693 (50%) | |
| Former smoker | 9,979 (25%) | 8,565 (25%) | 1,414 (26%) | |
| Current smoker | 8,537 (21%) | 7,175 (21%) | 1,362 (25%) | |
| Drinking status, % | 0.3 | |||
| Drinker | 5,245 (11%) | 4,521 (11%) | 724 (12%) | |
| Nondrinker | 35,252 (89%) | 30,507 (89%) | 4,745 (88%) | |
| Body mass index, % | < 0.001 | |||
| Underweight, kg/m2 | 647 (1.6%) | 562 (1.6%) | 85 (1.5%) | |
| Normal, kg/m2 | 11,339 (29%) | 10,036 (30%) | 1,303 (26%) | |
| Obese, kg/m2 | 14,761 (35%) | 12,293 (34%) | 2,468 (42%) | |
| Overweight, kg/m2 | 13,750 (34%) | 12,137 (34%) | 1,613 (30%) | |
| Age, years | 46.0 (33.0, 59.0) | 46.0 (33.0, 59.0) | 44.0 (30.0, 58.0) | < 0.001 |
| Family PIR | 3.00 (1.50, 5.00) | 3.05 (1.55, 5.00) | 2.70 (1.27, 4.81) | < 0.001 |
| BMI , kg/m2 | 28 (24, 32) | 28 (24, 32) | 29 (24, 34) | < 0.001 |
| Waist Circumference (cm) | 97 (87, 108) | 97 (87, 108) | 99 (87, 111) | < 0.001 |
| Activated physical activity, % | 7,777 (66%) | 6,815 (66%) | 962 (68%) | 0.10 |
| Self-reported hypertension, % | 13,925 (31%) | 11,717 (30%) | 2,208 (35%) | < 0.001 |
| Self-reported diabetes, % | 5,944 (11%) | 5,013 (10%) | 931 (12%) | 0.003 |
| White blood cell | 6.90 (5.70, 8.40) | 6.90 (5.70, 8.40) | 7.20 (5.90, 8.80) | < 0.001 |
| Neutrophils | 4.10 (3.20, 5.20) | 4.00 (3.20, 5.10) | 4.20 (3.20, 5.40) | < 0.001 |
| Monocyte | 0.50 (0.40, 0.70) | 0.50 (0.40, 0.70) | 0.50 (0.40, 0.70) | 0.004 |
| Lymphocyte | 2.00 (1.60, 2.50) | 2.00 (1.60, 2.50) | 2.10 (1.70, 2.50) | < 0.001 |
| Platelet | 246 (209, 290) | 245 (208, 289) | 251 (213, 297) | < 0.001 |
| ALI | 61 (44, 81) | 61 (44, 81) | 62 (46, 82) | 0.030 |
| ALI classifcation | 0.040 | |||
| Quartile 1 | 10,125 (24%) | 8,841 (24%) | 1,284 (23%) | |
| Quartile 2 | 10,124 (27%) | 8,796 (27%) | 1,328 (26%) | |
| Quartile 3 | 10,124 (26%) | 8,709 (26%) | 1,415 (28%) | |
| Quartile 4 | 10,124 (23%) | 8,682 (23%) | 1,442 (23%) | |
PIR, poverty income ratio; ALI, advanced lung cancer inflammation index.
Normally distributed continuous variables are described as means ± SEs, and continuous variables without a normal distribution are presented as medians [interquartile ranges]. Categorical variables are presented as numbers (percentages). N reflect the study sample while percentages reflect the survey-weighted.
ALI and asthma prevalence
Table 2 illustrates the relationship between ALI levels and asthma risk using multivariate regression modeling. Both the crude model and model 1, adjusted for age, sex, and race, showed that ALI levels were positively associated with asthma prevalence (all p < 0.05). Odds ratios (OR) and 95% confidence intervals (CI) for the highest quartile compared to the lowest quartile were OR = 1.14 (1.05–1.24), p-trend = 0.003 and OR = 1.11 (1.02–1.21), p-trend = 0.014, respectively. However, after further adjustment in Model 2, the significant relationship between ALI levels and asthma prevalence was absent (p-trend = 0.2).
Table 2.
OR (95% CIs) of the prevalence of asthma according to quartiles of ALI among adults in NHANES 1999–2018.
| Quartiles of ALI levels | P-trend | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Range | < 43.98 | 43.99–61.37 | 61.38–84.01 | > 84.02 | |
| Crude | 1.00 [Reference] | 1.04 (0.96, 1.13) | 1.12 (1.03, 1.21) | 1.14 (1.05, 1.24) | 0.003 |
| Model 1 | 1.00 [Reference] | 1.04 (0.95, 1.13) | 1.12 (1.04, 1.22) | 1.11 (1.02, 1.21) | 0.014 |
| Model 2 | 1.00 [Reference] | 1.03 (0.94, 1.12) | 1.10 (1.01, 1.19) | 1.07 (0.98, 1.16) | 0.2 |
Data are presented as OR (95% CI) unless indicated therwise; Model 1 was adjusted as age (continuous), sex (male or female), and race/ethnicity (Mexican American, Other Hispanic, NonHispanic White, Non-Hispanic Black or Other); Model 2 was adjusted as model 1 plus education level (below high school, high school, or above high school), family poverty income ratio (≤ 1.0,1.1–3.0, or > 3.0), drinking status (nondrinker, drinker), smoking status (never smoker, former smoker, or current smoker), BMI (< 18.5, 18.5- 25.0, 25.0–29.9, or > 29.9), activated physical activity (yes or no), self-reported diabetes (yes or no), and self-reported hypertension (yes or no).
ALI and mortality
During a median follow-up of 11.19 (9.38, 14.29) years, 724 (13.24%) of 5,469 participants with asthma died of all-cause mortality, and 109 (1.99%) died of respiratory disease. In Table 3, ALI was significantly associated with a reduced risk of all-cause mortality in asthma patients, both in the crude model and in the multivariable-adjusted Models 1 and 2, with hazard ratios (HRs) and 95% confidence intervals (CIs) of HR = 0.30 (0.24, 0.38), HR = 0.42 (0.31, 0.55) and HR = 0.45 (0.32, 0.63 ). Likewise in Table 4, ALI was equally significantly associated with a decreased risk of respiratory disease mortality in asthma patients in the crude and in the multivariable-adjusted Models 1 and 2, with hazard ratios (HRs) and 95% confidence intervals (CIs) of HR = 0.20 (0.10, 0.34), HR = 0.31 (0.18, 0.52), and HR = 0.31 (0.19, 0.47).
Table 3.
HRs (95% CIs) of all-cause mortality according to quartiles of ALI among asthma adults in NHANES 1999–2018.
| Quartiles of ALI levels | P-trend | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Range | < 45.13 | 45.14–62.69 | 62.70–85.86 | > 85.87 | |
| No. deaths/total | 303/1368 | 165/1367 | 147/1367 | 109/1367 | |
| Crude | 1.00 [Reference] | 0.48 (0.39, 0.59) | 0.42 (0.34, 0.52) | 0.30 (0.24, 0.38) | < 0.001 |
| Model 1 | 1.00 [Reference] | 0.63 (0.48, 0.81) | 0.58 (0.45, 0.75) | 0.42 (0.31, 0.55) | < 0.001 |
| Model 2 | 1.00 [Reference] | 0.64 (0.47, 0.87) | 0.62 (0.45, 0.84) | 0.45 (0.32, 0.63) | < 0.001 |
Data are presented as OR (95% CI) unless indicated therwise; Model 1 was adjusted as age (continuous), sex (male or female), and race/ethnicity (Mexican American, Other Hispanic, NonHispanic White, Non-Hispanic Black or Other); Model 2 was adjusted as model 1 plus education level (below high school, high school, or above high school), family poverty income ratio (≤ 1.0,1.1–3.0, or > 3.0), drinking status (nondrinker, drinker), smoking status (never smoker, former smoker, or current smoker), BMI (< 18.5, 18.5- 25.0, 25.0–29.9, or > 29.9), activated physical activity (yes or no), self-reported diabetes (yes or no), and self-reported hypertension (yes or no).
Table 4.
HRs (95% CIs) of respiratory disease mortality according to quartiles of ALI among asthma adults in NHANES 1999–2018.
| Quartiles of ALI levels | P-trend | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Range | < 45.13 | 45.14–62.69 | 62.70–85.86 | > 85.87 | |
| No. deaths/total | 64/1368 | 19/1367 | 13/1367 | 13/1367 | |
| Crude | 1.00 [Reference] | 0.29 (0.17, 0.47) | 0.20 (0.10, 0.34) | 0.20 (0.10, 0.34) | < 0.001 |
| Model 1 | 1.00 [Reference] | 0.42 (0.27, 0.63) | 0.30 (0.18, 0.48) | 0.31 (0.18, 0.52) | < 0.001 |
| Model 2 | 1.00 [Reference] | 0.43 (0.29, 0.63) | 0.32 (0.20, 0.49) | 0.31 (0.19, 0.47) | < 0.001 |
Data are presented as OR (95% CI) unless indicated therwise; Model 1 was adjusted as age (continuous), sex (male or female), and race/ethnicity (Mexican American, Other Hispanic, NonHispanic White, Non-Hispanic Black or Other); Model 2 was adjusted as model 1 plus education level (below high school, high school, or above high school), family poverty income ratio (≤ 1.0,1.1–3.0, or > 3.0), drinking status (nondrinker, drinker), smoking status (never smoker, former smoker, or current smoker), BMI (< 18.5, 18.5- 25.0, 25.0–29.9, or > 29.9), activated physical activity (yes or no), self-reported diabetes (yes or no), and self-reported hypertension (yes or no).
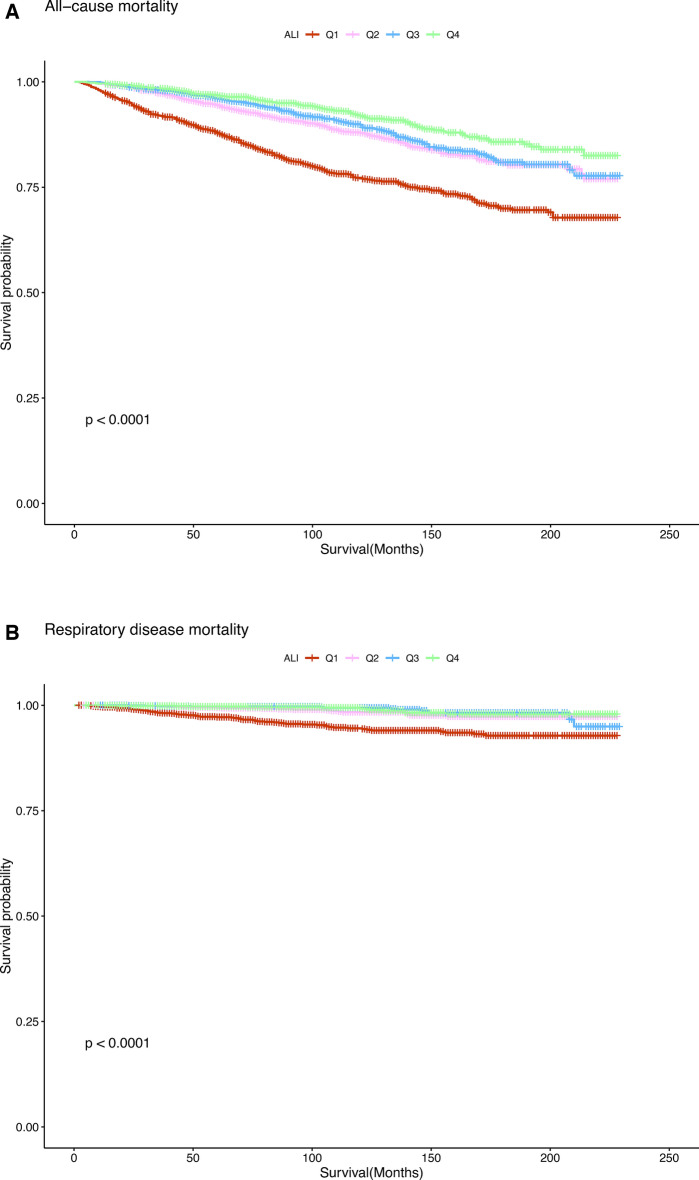
In addition, we preliminarily assessed the association between ALI levels and all-cause and respiratory disease mortality in asthma patients using Kaplan–Meier analysis. As shown in Fig. 2A, higher ALI levels were associated with lower all-cause mortality in asthma patients (P < 0.0001). Similarly, as illustrated in Fig. 2B, higher ALI levels were associated with lower respiratory disease mortality in patients with asthma (P < 0.0001).
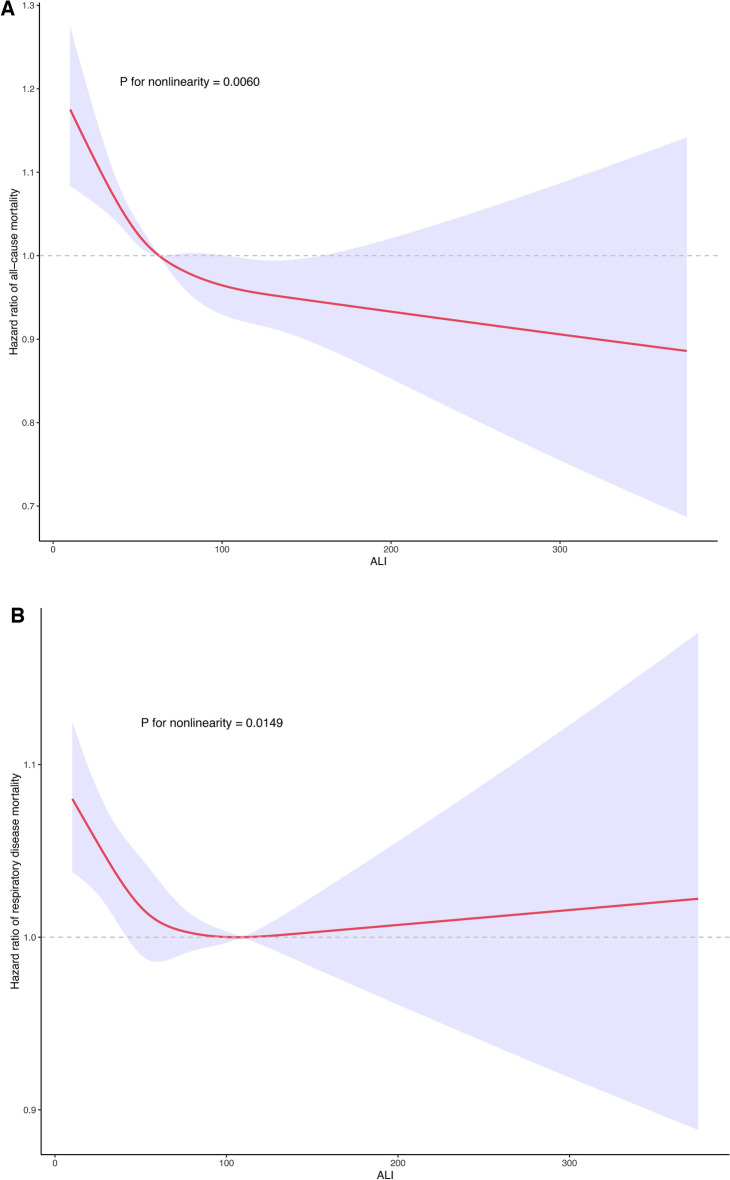
Fig. 2.
Restricted cubic spline analyses the association of ALI with all-cause (A) and respiratory disease (B) mortality in adults with asthma. Adjusted for age (continuous), sex (male or female), race/ethnicity (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black or Other), education level (below high school, high school, or above high school), family poverty income ratio (≤ 1.0, 1.1–3.0, or > 3.0), drinking status (nondrinker, drinker), smoking status (never smoker, former smoker, or current smoker), BMI (< 18.5, 18.5- 25.0, 25.0–29.9, or > 29.9), activated physical activity (yes or no), self-reported diabetes (yes or no), and self-reported hypertension (yes or no).
Restricted regression cubic spline
Using smooth curve fitting, We investigated the nonlinear relationship between ALI levels and all-cause and respiratory disease deaths after adjusting for multiple potential confounders. As shown in Fig. 3A, the results of the restricted cubic spline analysis indicated a nonlinear relationship between ALI levels and all-cause mortality in patients with asthma (P for nonlinearity = 0.0060). Low levels of ALI were associated with an increased risk of all-cause mortality in this population. More importantly, as shown in Fig. 3B, we also found a nonlinear relationship between ALI levels and respiratory disease mortality in the asthmatic population (P for nonlinearity = 0.0149). Additionally, two-piece linear regression showed that the probability of mortality from respiratory disease decreased progressively to a nadir at an ALI level of 109.13 and then increased as the ALI level increased.
Fig. 3.
Kaplan–Meier survival estimates between ALI with all-cause (A) and respiratory disease (B) mortality in adults with asthma.
Subgroup analyses and sensitivity analyses
Table S1 presents the results of a comprehensive subgroup analysis that assessed the relationship between quartiles of ALI levels and all-cause and respiratory disease mortality among participants with asthma, categorized by smoking status. Subgroup analyses showed that there was consistently a significant association between ALI levels and reduced risk of all-cause and respiratory disease mortality in both subgroups, not only in the crude model but also in multivariable-adjusted Models 1 and 2. We also stratified the association between quartiles of ALI levels and all-cause and respiratory disease mortality in patients with asthma according to gender, and the results are shown in Table S2. The results of the subgroup analysis showed that the correlation between quartiles of ALI levels and all-cause and respiratory disease mortality was not affected by gender and consistently showed a strong correlation with a reduced risk of mortality. Furthermore, we further stratified the associations between quartiles of ALI levels and all-cause mortality and respiratory disease mortality in adults with asthma according to age, and the results are shown in Table S3. The findings indicate that the associations between quartiles of ALI levels and all-cause and respiratory disease mortality were more robust at ages older than 60 years.
In sensitivity analyses, we first excluded participants with a history of cancer at baseline, and repeated Cox regressions showed that ALI levels were consistently associated with reduced all-cause and respiratory disease mortality, both in the crude model and in the multivariable-adjusted models 1 and 2 (Table S4). In addition, we further excluded the bias caused by other respiratory diseases such as emphysema and chronic bronchitis, and repeated Cox regressions presented similar results (Table S5). Finally, we excluded participants with asthma who died in the first two years of follow-up, and similar results were observed in both the crude and adjusted models (Table S6).
Discussion
We conducted a cross-sectional study to assess the association between ALI levels and asthma prevalence and long-term mortality in the US population between 1999 and 2018. After adjusting multiple confounders, the findings revealed no association between ALI levels and asthma prevalence; rather, higher ALI levels were significantly associated with lower all-cause and respiratory disease mortality in asthma patients. This connection remained persistent across multiple subgroups and sensitivity analyses. These findings indicate that ALI has the potential to be a highly useful predictor of outcomes in asthma patients.
Asthma is a chronic inflammatory airway disease in which many inflammatory cells and inflammatory factors play an important role. Airway inflammation is a major driver of the chronic intermittent nature of asthma symptoms, which ultimately leads to severe asthma attacks21,22. It has been found that up to 50% of adult asthmatics and an even higher proportion of children with asthma may experience an exacerbation of allergic airway inflammation characterized by an abundance of CD41 TH2 cells with increased interleukin (IL)−5and IL-13 production23,24. IL-5 is a strong inducer of chemotaxis and an activator of eosinophils, which are abundant in most asthmatic individuals25. A recent study found that myelopoiesis-promoting G-CSF and cytokines as the upstream-inducing factors are potential diagnostic and therapeutic targets in patients with neutrophilic asthma26.
Chronic inflammation accelerates the breakdown of proteins, leading to a decrease in albumin levels. In addition, inflammation induces insulin resistance, decreases appetite, and hinders the absorption of nutrients, ultimately leading to weight loss27–29. Furthermore, nutrition and diet also play a significant role in the development and progression of asthma. Fruit and vegetable intake may reduce the risk of developing asthma. Studies have found that high consumption of fruits and vegetables was found to be associated with a reduced risk of developing asthma in children and adults30,31, specifically for apples32,33and oranges32. In contrast, high fat intake and low fiber intake are associated with airway inflammation and worsening lung function in asthmatics. A study reported that saturated fat intake was also positively associated with a higher percentage of sputum eosinophils (p = 0.001), which correlates with asthma severity and impaired lung function34. Likewise, Kim et al35. found that a high-fat diet resulted in increased airway hyperresponsiveness via enhanced cytokine production in the lung. Therefore, relying only on a single inflammatory marker to assess the risk of death in asthma patients is not sufficient to provide accurate predictive results.
The ALI is calculated by combining serum albumin, body mass index and the inflammatory parameter NLR, which reflects both the inflammatory and nutritional status of the host. A difference between the ALI and previously reported indices or markers is that the ALI includes not only NLR and albumin, but also BMI, which is used to assess nutritional status. Low body mass index is considered one of the diagnostic criteria for malnutrition36, and preoperative low body mass index has independent predictive value as a nutritional parameter in gastric cancer patients37. In addition, it has been shown that preoperative albumin is a predictor of prognosis in patients with gastric cancer, and low albumin has the most accurate predictive ability for all short-term prognoses in patients with gastric cancer38. Therefore, ALI can achieve accurate prediction of disease prognosis by assessing inflammation and nutritional status. The prognostic predictive value of ALI in oncologic and non-oncologic diseases is validated on a large scale. However, ALI and the risk of death in patients with asthma have not been proven. Our findings suggest that higher ALI levels are associated with a reduced risk of all-cause and respiratory disease mortality in asthmatics. In addition, the results remained highly consistent when multiple subgroup analyses and multiple sensitivity analyses were performed. All of those demonstrated that ALI is a very valuable prognostic predictor for asthma patients with high robustness.
Adolescents have a high asthma prevalence of 9.9%, significantly higher than in adults (7.6%) and children (3.8%−8.1%)39. Key factors influencing asthma in adolescents include obesity, anxiety, depression, and exposure to violence40–42. In contrast, smoking history, chronic bronchitis, and COPD are more significant in older patients43,44. Although our stratified analysis showed consistent prognostic value of ALI across age groups, tailored management strategies may be necessary. In adolescents, controlling weight and promoting proper nutrition are crucial, as obesity increases asthma morbidity and disease severity. The TENOR study found that 31% of children with severe asthma were obese45. A balanced diet supports metabolism and mental health in asthmatic patients46, and controlling intake of specific dietary components—such as fat, sugar, and low-nutrient foods—can help mitigate chronic inflammation47. In elderly asthmatics, attention should be given to inflammatory and nutritional status within ALI components. As chronic bronchitis and COPD often coexist in this group, managing airway inflammation is critical. Additionally, long-term inflammation can lead to nutritional depletion, making a rational diet and optimal nutritional status vital to managing asthma and reducing mortality48.
An intriguing finding in our results was the contrasting association of ALI levels with mortality outcomes. High ALI levels were consistently linked to increased all-cause mortality. However, in the case of respiratory disease mortality, the rate initially declined with rising ALI, reaching a minimum at 109.13, before gradually increasing. We hypothesize that this difference may stem from the varied composition of all-cause and respiratory disease-related deaths in asthmatics. Common causes of death in this population include chronic ischemic heart disease, acute myocardial infarction, and COPD49, which aligns with our study, where cardiovascular deaths (367) outnumbered respiratory deaths (109). For respiratory mortality, the primary causes were COPD and pneumonia. These differences in death composition may explain the distinct RCS curves. Additionally, the limited number of respiratory disease deaths (109) in our study may introduce bias. Further research is needed to clarify the role of ALI in different mortality outcomes.
The Mediterranean Diet (MedDiet) is defined as a traditional dietary pattern of people living in the Mediterranean Basin in the 1950s and 1960s, characterized by a predominantly plant-based diet, minimally processed, and rich in monounsaturated fats derived from olive oil, but low in saturated fats, meat, and dairy products50,51. As a healthy dietary pattern, MedDiet can reduce the risk of metabolic diseases and mortality, and reduce the incidence of cardiovascular diseases, and a large number of studies have found that MedDiet has a significant impact on a wide range of diseases, such as type 2 diabetes (T2D), metabolic syndrome (MetS), obesity, cancer, cognitive decline, and cardiovascular disease mortality52–55. In recent years, the relationship between asthma and MedDiet has also gained increasing attention. A review and meta-analysis showed an inverse association between adherence to a “healthy diet” or a healthy diet rich in fruits and vegetables and the incidence of asthma in children56. Another randomized controlled trial found that a “healthy diet” supplemented with two meals of fatty fish per week may be a potential strategy to reduce airway inflammation in childhood asthma57. Another study also found that MedDiet may be a reasonable option for preventive intervention in atopic and asthma58. Therefore, in conjunction with our findings and the existing literature we strongly recommend adherence to MedDiet in patients with asthma, which may improve their health.
Our study has numerous notable advantages over earlier ones. First, our study was lengthy enough, and the sample size was large enough, that we could adequately examine the associations between ALI and all-cause and respiratory disease mortality in asthmatic patients. Second, in our study, the ALI, which represents both the inflammatory and nutritional status of patients, gives more comprehensive information than a single inflammatory indicator, making it a more helpful tool in the clinical management and treatment of adult asthma. Finally, we applied different analysis approaches, including subgroup analysis, sensitivity analysis, and Kaplan-Meyer analysis, to further identify ALI as the most powerful indicator for estimating the probability of mortality from all causes and respiratory diseases in patients with asthma.
We must recognize that there are some limitations in this study. First, asthma data in the NHANES database are self-reported and lack details on treatment and follow-up, which may have influenced our findings. A large prospective randomized controlled trial is needed to confirm these results. Second, although we adjusted for factors that could cause bias in our study, including age, sex, and smoking status, there may have been other unknown confounding factors that affected our results. Third, the ALI indicator we used was calculated based on one-time CBC parameters and serum albumin, and thus may not reflect the overall status of participants, which may also contribute to bias.
Conclusions
Through a large-scale nationally representative survey of asthma adults among US individuals, our study found that higher ALI levels were associated with a reduced risk of all-cause and respiratory disease mortality. Thus, the ALI may serve as a useful tool to identify high-risk populations of asthma patients in a simple and cost-effective way. Our findings will contribute to the potential role of ALI in predicting the prognosis of asthma patients and provide a theoretical foundation for clinical decision-making in asthma patients.
Supplementary Information
Acknowledgements
We appreciate the people who contributed to the NHANES data we studied.
Author contributions
X.J.W. and D.L.Z. designed the study and revised the manuscript. J.X.F. and Y.N.Z. were responsible for data collection, analysis, and manuscript writing. L.L. and J.Z. collected the data. All authors reviewed the manuscript.
Funding
This study was supported by the Project of medical and health technology development program of Shandong province (202204080217).
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: The National Health and Nutrition Examination Survey dataset at https://www.cdc.gov/nchs/nhanes.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The studies involving human participants were reviewed and approved by The National Center for Health Statistics (NCHS) Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xinjian Wang, Email: whchwangxj@126.com.
Dianliang Zhang, Email: qymr2014@163.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80983-1.
References
- 1.Reddel, H. K. et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J59, 10.1183/13993003.02730-2021 (2022). [DOI] [PMC free article] [PubMed]
- 2.Abdel-Aziz, M. I., Neerincx, A. H., Vijverberg, S. J., Kraneveld, A. D. & Maitland-van der Zee, A. H. Omics for the future in asthma. Semin Immunopathol42, 111–126, 10.1007/s00281-019-00776-x (2020). [DOI] [PubMed]
- 3.Holgate, S. T. Innate and adaptive immune responses in asthma. Nat Med18, 673–683. 10.1038/nm.2731 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Simpson, J. L., Brooks, C. & Douwes, J. Innate immunity in asthma. Paediatr Respir Rev9, 263–270. 10.1016/j.prrv.2008.05.007 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Nauseef, W. M. & Borregaard, N. Neutrophils at work. Nat Immunol15, 602–611. 10.1038/ni.2921 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Soehnlein, O., Weber, C. & Lindbom, L. Neutrophil granule proteins tune monocytic cell function. Trends Immunol30, 538–546. 10.1016/j.it.2009.06.006 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Lennie, T. A. Relationship of body energy status to inflammation-induced anorexia and weight loss. Physiol Behav64, 475–481. 10.1016/s0031-9384(98)00103-6 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Jafri, S. H., Shi, R. & Mills, G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer13, 158. 10.1186/1471-2407-13-158 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He, X. et al. Advanced Lung Cancer Inflammation Index, a New Prognostic Score, Predicts Outcome in Patients With Small-Cell Lung Cancer. Clin Lung Cancer16, e165-171. 10.1016/j.cllc.2015.03.005 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Hu, Z. et al. Advanced Lung Cancer Inflammation Index is a Prognostic Factor of Patients with Small-Cell Lung Cancer Following Surgical Resection. Cancer Manag Res13, 2047–2055. 10.2147/cmar.S295952 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomita, M., Ayabe, T. & Nakamura, K. The advanced lung cancer inflammation index is an independent prognostic factor after surgical resection in patients with non-small-cell lung cancer. Interact Cardiovasc Thorac Surg26, 288–292. 10.1093/icvts/ivx329 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Yin, C. et al. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: A propensity score matching analysis. Clin Nutr40, 1130–1136. 10.1016/j.clnu.2020.07.018 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Chen, Y., Guan, M., Wang, R. & Wang, X. Relationship between advanced lung cancer inflammation index and long-term all-cause, cardiovascular, and cancer mortality among type 2 diabetes mellitus patients: NHANES, 1999–2018. Front Endocrinol (Lausanne)14, 1298345, 10.3389/fendo.2023.1298345 (2023). [DOI] [PMC free article] [PubMed]
- 14.Maeda, D. et al. Prognostic impact of a novel index of nutrition and inflammation for patients with acute decompensated heart failure. Heart Vessels35, 1201–1208. 10.1007/s00380-020-01590-4 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Hu, B. L. et al. The prognostic value of lower pretreatment advanced lung cancer inflammation index (ALI) in B cell lymphoma. Zhonghua Xue Ye Xue Za Zhi39, 53–55. 10.3760/cma.j.issn.0253-2727.2018.01.012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng, J. F., Huang, Y. & Chen, Q. X. A new inflammation index is useful for patients with esophageal squamous cell carcinoma. Onco Targets Ther7, 1811–1815. 10.2147/ott.S68084 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zipf, G. et al. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat1, 1–37 (2013). [PubMed] [Google Scholar]
- 18.Shoji, F., Kozuma, Y., Toyokawa, G., Yamazaki, K. & Takeo, S. Complete Blood Cell Count-Derived Inflammatory Biomarkers in Early-Stage Non-Small-Cell Lung Cancer. Ann Thorac Cardiovasc Surg26, 248–255. 10.5761/atcs.oa.19-00315 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Z. et al. The association between ethylene oxide exposure and asthma risk: a population-based study. Environ Sci Pollut Res Int30, 24154–24167. 10.1007/s11356-022-23782-3 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Qiu, Z. et al. Associations of Serum Carotenoids With Risk of Cardiovascular Mortality Among Individuals With Type 2 Diabetes: Results From NHANES. Diabetes care45, 1453–1461. 10.2337/dc21-2371 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Koziol-White, C. J. & Panettieri, R. A. Jr. Airway smooth muscle and immunomodulation in acute exacerbations of airway disease. Immunol Rev242, 178–185. 10.1111/j.1600-065X.2011.01022.x (2011). [DOI] [PubMed] [Google Scholar]
- 22.Green, R. H. et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet360, 1715–1721. 10.1016/s0140-6736(02)11679-5 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Woodruff, P. G. et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A104, 15858–15863. 10.1073/pnas.0707413104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodruff, P. G. et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med180, 388–395. 10.1164/rccm.200903-0392OC (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell, K. J. L., Kazda, L. & Parker, G. Asthma in Adults. N Engl J Med389, 2111–2112. 10.1056/NEJMc2312345 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Kim, Y. M. et al. Airway G-CSF identifies neutrophilic inflammation and contributes to asthma progression. Eur Respir J55, 10.1183/13993003.00827-2019 (2020). [DOI] [PubMed]
- 27.Dou, L. et al. The Prognostic Significance of C-Reactive Protein to Albumin Ratio in Newly Diagnosed Acute Myeloid Leukaemia Patients. Cancer Manag Res14, 303–316. 10.2147/cmar.S343580 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aldhwayan, M. M. et al. Does Bypass of the Proximal Small Intestine Impact Food Intake, Preference, and Taste Function in Humans? An Experimental Medicine Study Using the Duodenal-Jejunal Bypass Liner. Nutrients14, 10.3390/nu14102141 (2022). [DOI] [PMC free article] [PubMed]
- 29.Merker, M. et al. Association of Baseline Inflammation With Effectiveness of Nutritional Support Among Patients With Disease-Related Malnutrition: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open3, e200663. 10.1001/jamanetworkopen.2020.0663 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seyedrezazadeh, E. et al. Fruit and vegetable intake and risk of wheezing and asthma: a systematic review and meta-analysis. Nutr Rev72, 411–428. 10.1111/nure.12121 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Hosseini, B., Berthon, B. S., Wark, P. & Wood, L. G. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients9, 10.3390/nu9040341 (2017). [DOI] [PMC free article] [PubMed]
- 32.Knekt, P. et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr76, 560–568. 10.1093/ajcn/76.3.560 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Shaheen, S. O. et al. Dietary antioxidants and asthma in adults: population-based case-control study. Am J Respir Crit Care Med164, 1823–1828. 10.1164/ajrccm.164.10.2104061 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Berthon, B. S., Macdonald-Wicks, L. K., Gibson, P. G. & Wood, L. G. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology18, 447–454. 10.1111/resp.12015 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Kim, H. Y. et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med20, 54–61. 10.1038/nm.3423 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cederholm, T. et al. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr34, 335–340. 10.1016/j.clnu.2015.03.001 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Oh, S. E. et al. Prognostic significance of perioperative nutritional parameters in patients with gastric cancer. Clin Nutr38, 870–876. 10.1016/j.clnu.2018.02.015 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Guner, A. et al. Parameters for Predicting Surgical Outcomes for Gastric Cancer Patients: Simple Is Better Than Complex. Ann Surg Oncol25, 3239–3247. 10.1245/s10434-018-6684-2 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Landeo-Gutierrez, J. & Celedón, J. C. Chronic stress and asthma in adolescents. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology125, 393–398. 10.1016/j.anai.2020.07.001 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters, U., Dixon, A. E. & Forno, E. Obesity and asthma. The Journal of allergy and clinical immunology141, 1169–1179. 10.1016/j.jaci.2018.02.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung, P. C., Cunningham, S. A., Narayan, K. M. & Kramer, M. R. Childhood Obesity Incidence in the United States: A Systematic Review. Childhood obesity (Print)12, 1–11. 10.1089/chi.2015.0055 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han, Y. Y., Forno, E. & Celedón, J. C. Health risk behaviors, violence exposure, and current asthma among adolescents in the United States. Pediatric pulmonology54, 237–244. 10.1002/ppul.24236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barger, L. W., Vollmer, W. M., Felt, R. W. & Buist, A. S. Further investigation into the recent increase in asthma death rates: a review of 41 asthma deaths in Oregon in 1982. Annals of allergy60, 31–39 (1988). [PubMed] [Google Scholar]
- 44.Braman, S. S. Asthma in the Elderly. Clinics in geriatric medicine33, 523–537. 10.1016/j.cger.2017.06.005 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Schatz, M. et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. The Journal of allergy and clinical immunology133, 1549–1556. 10.1016/j.jaci.2013.10.006 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Koliaki, C. et al. Defining the Optimal Dietary Approach for Safe, Effective and Sustainable Weight Loss in Overweight and Obese Adults. Healthcare (Basel, Switzerland)6, 10.3390/healthcare6030073 (2018). [DOI] [PMC free article] [PubMed]
- 47.Di Genova, L., Penta, L., Biscarini, A., Di Cara, G. & Esposito, S. Children with Obesity and Asthma: Which Are the Best Options for Their Management? Nutrients10, 10.3390/nu10111634 (2018). [DOI] [PMC free article] [PubMed]
- 48.Pasha, M. A., Sundquist, B. & Townley, R. Asthma pathogenesis, diagnosis, and management in the elderly. Allergy and asthma proceedings38, 184–191. 10.2500/aap.2017.38.4048 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Goldacre, M. J., Duncan, M. E. & Griffith, M. Death rates for asthma in English populations 1979–2007: comparison of underlying cause and all certified causes. Public health126, 386–393. 10.1016/j.puhe.2012.01.022 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Martínez-González, M. A., Gea, A. & Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circulation research124, 779–798. 10.1161/circresaha.118.313348 (2019). [DOI] [PubMed] [Google Scholar]
- 51.da Silva, R. et al. Worldwide variation of adherence to the Mediterranean diet, in 1961–1965 and 2000–2003. Public health nutrition12, 1676–1684. 10.1017/s1368980009990541 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Becerra-Tomás, N. et al. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Critical reviews in food science and nutrition60, 1207–1227. 10.1080/10408398.2019.1565281 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Sánchez-Sánchez, M. L. et al. Mediterranean diet and health: A systematic review of epidemiological studies and intervention trials. Maturitas136, 25–37. 10.1016/j.maturitas.2020.03.008 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Serra-Majem, L. et al. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Molecular aspects of medicine67, 1–55. 10.1016/j.mam.2019.06.001 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Dinu, M., Pagliai, G., Casini, A. & Sofi, F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. European journal of clinical nutrition72, 30–43. 10.1038/ejcn.2017.58 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Antonogeorgos, G., Kogias, C., Douros, K. & Panagiotakos, D. Greater fruit and vegetables consumption, and adherence to a Mediterranean type of diet reduces the risk for asthma in children; a systematic review and meta-analysis. International journal of food sciences and nutrition75, 4–30. 10.1080/09637486.2023.2276033 (2024). [DOI] [PubMed] [Google Scholar]
- 57.Papamichael, M. M. et al. Efficacy of a Mediterranean diet supplemented with fatty fish in ameliorating inflammation in paediatric asthma: a randomised controlled trial. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association32, 185–197. 10.1111/jhn.12609 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Vassilopoulou, E., Guibas, G. V. & Papadopoulos, N. G. Mediterranean-Type Diets as a Protective Factor for Asthma and Atopy. Nutrients14, 10.3390/nu14091825 (2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: The National Health and Nutrition Examination Survey dataset at https://www.cdc.gov/nchs/nhanes.