Abstract
Prostate cancer (PCa) is the second most common malignancy affecting men globally. Recent advances in metabolomics have highlighted significant alterations in specific amino acid (AA) metabolism linked to PCa, indicating their potential utility in diagnosis and therapy. However, no direct causal association between serum AA levels and PCa risk has been established. A total of 35 patients with PCa and 30 individuals with benign prostatic hyperplasia (BPH) were recruited for this study. Targeted metabolomic analysis was performed using ultra-high-performance liquid chromatography-tandem mass spectrometry on serum samples. Two-sample Mendelian randomization (MR) was applied to explore potential causal links between serum AA levels and PCa risk, including mediator effects using dual-phase MR and assessing reverse causality through reverse MR. Results Targeted metabolomic profiling identified six amino acids—glutamate (Glu), Ser, histidine (His), arginine (Arg), aspartic acid (Asp), and glycine (Gly)—that showed significant area under the ROC curve in differentiating between BPH and PCa cases. Notably, Glu demonstrated an inverse association with PCa risk, distinct from the other AAs identified. However, definitive evidence supporting a causal relationship between low Glu levels and increased PCa risk was not observed. Our results suggest a protective role of Glu against PCa development, which may have implications for disease prognosis. Increasing dietary Glu intake may present a potential preventive or therapeutic approach for PCa.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80986-y.
Keywords: Prostate cancer, Glutamate, Two-sample, Mendelian randomization, Protective factors
Subject terms: Cancer metabolism, Cancer therapy
Introduction
According to the American Cancer Society’s 2023 estimates, prostate cancer (PCa) is the most prevalent malignancy among men globally, ranking second in cancer-related mortality. It accounts for 29% of all new cancer diagnoses and 11% of cancer-related deaths in males worldwide1,2. PCa cells exhibit diverse behavior, ranging from indolent to highly invasive3. Current screening methods, including digital rectal examination, transrectal ultrasound, and serum prostate-specific antigen (PSA) testing4,5, suffer from limitations such as false positives and negatives, complicating the differentiation between benign and malignant PCa6. Moreover, treatment options for advanced PCa, such as anti-androgen therapy and chemotherapy, often face resistance7, underscoring the urgent need for improved diagnostic methods.
Recent advancements in metabolomics have enabled deeper exploration of metabolic alterations in PCa cells, revealing their pathophysiological mechanisms. PCa cells exhibit significant variations in amino acid (AA) metabolism and biosynthesis, which reflect their adaptation to hypoxia, oxidative stress, and heightened metabolic demands, thereby contributing to PCa progression8,9. Observational studies have identified associations between AAs and PCa risk, with case-control studies highlighting distinct AA profiles in patients compared to controls10. Emerging biomarkers for PCa have been identified, involving pathways related to ethanolamine, arginine (Arg), and branched-chain amino acids (BCAAs)11. Moreover, genetically inferred circulating alanine (Ala) levels have been linked to altered PCa risk12, and elevated aspartate (Asp) levels have been suggested as potential contributors to PCa development13. This study employs targeted metabolomics to identify unique variations in AA composition among patients with PCa. However, the findings of observational epidemiological studies are prone to biases, including confounding and reverse causation. Therefore, establishing a causal link between altered AA metabolism and PCa risk requires a more robust approach.
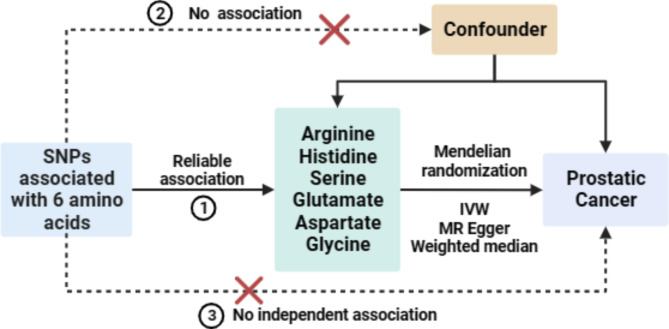
Mendelian randomization (MR) offers a powerful genetic approach to address biases inherent in traditional epidemiological studies by using genetic variants associated with exposures as instrumental variables (IVs)14. This method mitigates issues of reverse causality and confounding due to the random allocation of genotypes during gamete formation15,16. In this study, we applied a two-sample MR design to investigate the potential causal relationships between AA levels and PCa risk, aiming to elucidate the genetic determinants of AA dynamics in PCa progression.
Methods
Study participants
The retrospective cross-sectional analysis was conducted at Xuzhou No. 1 People’s Hospital and included 30 patients with benign prostatic hyperplasia (BPH) and 35 patients diagnosed with PCa. Patients were selected based on PSA screening and clinical evaluations performed by a urologist. The diagnosis of PCa was confirmed through either prostate biopsy or surgery following hospital admission. Exclusion criteria included a family history of hereditary diseases, significant hepatic or renal dysfunction, cardiovascular disorders, hematological conditions, concurrent systemic tumors, or recent use (within three months) of immunosuppressants, hormones, or lipid-lowering drugs. The study protocol was approved by the Ethical Review Committee of Xuzhou No. 1 People’s Hospital (approval number xyyll[2023]153), and informed consent was obtained from all participants. All patient samples were fully de-identified to maintain privacy and comply with ethical standards. BPH diagnoses were confirmed by negative biopsy results, excluding the presence of PCa. Further participant details are summarized in Table 1.
Table 1.
Characteristics of participants’ pathology and clinical profile.
| Parameters | BPH | PCa | P | |
|---|---|---|---|---|
| No. of subjects | 30 | 35 | ||
| Age (years) | 72.60 ± 8.58 | 74.31 ± 6.78 | 0.360a | |
| BMI (kg/m2) | 23.12 ± 3.51 | 24.58 ± 3.47 | 0.099b | |
| Smoking status, n (%) | 1(3.3%) | 2(5.7%) | 0.648c | |
| PSA (ng/mL) | 16.12 ± 17.25 | 21.70 ± 17.16 | 0.060a | |
| Gleason score | 3 + 3 | - | 4 | |
| 3 + 4 | - | 4 | ||
| 3 + 5 | - | 1 | ||
| 4 + 3 | - | 2 | ||
| 4 + 4 | - | 7 | ||
| 4 + 5 | - | 10 | ||
| 5 + 3 | - | 3 | ||
| 5 + 4 | - | 3 | ||
| 5 + 5 | - | 1 | ||
| Tumor size(cm3) | 107.21 ± 69.87 | 119.83 ± 60.83 | 0.114a |
Data were presented as Mean ± SEM; a: Mann-Whitney U test, b: unpaired Student’ s t-test, c: chi-square test, and the statistically significant P-values were highlighted in bold.
Serum sample collection
Following subject enrollment and obtaining informed consent, participant body mass index (BMI) was recorded. All participants were instructed to fast after 10 p.m. (no food or water). Venous blood samples were collected at 6 a.m. the next morning, ensuring a fasting state. Collected blood samples were immediately centrifuged at 1500 g for 10 min at 4 °C to separate the serum. The obtained serum was stored at −80 °C until subsequent analyses.
Sample preparation for UPLC-MS/MS analysis
For ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis, the stored serum samples were first thawed at 4 °C and homogenized by vortexing for 10 s. Twenty AAs and internal standards (as shown in Table 2) were obtained from Hangzhou Hanko Biotechnology Co., Ltd. Mobile phases were prepared as follows: Reagent A: 2% formic acid in acetonitrile, used to enhance ionization efficiency during the mass spectrometry process. Reagent B: 0.1% formic acid in water, used to maintain appropriate pH levels during analysis. Reagent C (Buffer): 0.5 M ammonium acetate solution, used to stabilize the pH during sample preparation. Reagent D: Acetonitrile, used as a solvent for the mobile phase preparation. Mobile phase A consisted of 500 µL of reagent D mixed with 500 mL of purified water, while mobile phase B contained 500 µL of reagent D mixed with 500 mL of acetonitrile. To precipitate proteins, 50 µL of serum, 50 µL of internal standards, and 50 µL of pre-chilled ultra-pure water (4 °C) were combined. This mixture was centrifuged at 18,000 g for 5 min at 4 °C, and 10 µL of the supernatant was transferred to a new tube. Subsequently, 70 µL of reagent C (buffer) and 20 µL of reagent A were added, followed by a second centrifugation at 21,000 g for 10 min. The resulting supernatant was mixed with 990 µL of ultra-pure water, vortexed, and 100 µL of the mixture was transferred into a vial containing a 300 µL insert for analysis.
Table 2.
20 amino acids and internal standards.
| Abbreviation | Common Name | Internal standards |
|---|---|---|
| Asn | Asparagine | L-Aspartate-d3 |
| His | Histidine | DL-Histidine-d3 |
| Arg | Arginine | L-Arginine-13C6,15N4 |
| Ser | Serine | L-13C3-Serine |
| Gly | Glycine | 15N;2-13 C-Glycine |
| Gln | Glutamine | L-Glutamine-d5 |
| Lys | Lysine | L-Lysine-d4 |
| Thr | Threonine | L-Threonine-d5 |
| Ala | Alanine | 2H4-Alanine |
| Glu | Glutamate | 2H2-Glutamate |
| Pro | Proline | DL-Proline-d3 |
| Cys | Cysteine | DL-Cystine-d6 |
| Tyr | Tyrosine | 13C6-Tyrosine |
| Val | Valine | 2H8-Valine |
| Met | Methionine | 2H4-Methionine |
| ILe | Isoleucine | 2H3-Leucine |
| Leu | Leucine | 2H3-Leucine |
| Phe | Phenylalanine | 13C6-Phenylalanine |
| Trp | Tryptophan | L-Tryptophan-d3 |
| Asp | Aspartic acid | L-Aspartate-d3 |
AA extraction and analysis using UPLC-MS/MS
The prepared sample solution was analyzed using the UPLC-MS/MS system (Waters Xevo-TQS). The setup utilized the Waters Acquity UPLC® I Class along with a CORTECS UPLC C18 column (1.6 μm, 2.1 × 150 mm). The mass spectrometer (Waters XEVO® TQS) operated in positive ion mode. Samples were loaded onto a 96-well autosampler plate in a specific sequence: blank samples, linear gradient standards, quality control samples, and test samples. Analysis began with three runs of blank samples, followed by ascending testing standards, with quality control samples analyzed every twenty test samples. Mobile phase A was replaced every two days, in conjunction with ion source cleaning for the mass spectrometry unit. Calibration standard concentrations (C1 to C6) were set using the system software according to the values in the calibration table. The AA detection showed a strong linear relationship across a concentration range of 1.0–6000.0 nmol/L, with a linear determination coefficient (R²) consistently greater than 0.99. Quantification limits for the compounds ranged between 1.0 and 10.0 nmol/L, with intra-day and inter-day precision (relative standard deviation) maintained below 15%. Additionally, the coefficient of variation for both high and low-quality control samples of AAs was kept below 15%.
Clinical statistical analysis
To compare continuous data distributions, the chi-square test was employed. Data normality was expressed as the mean ± standard error of the mean (SEM). Statistical significance for AAs, age, BMI, and PSA levels was determined using the Mann-Whitney U test and unpaired Student’s t-test, with Bonferroni correction applied for multiple comparisons. Diagnostic efficacy was assessed using receiver operating characteristic (ROC) curves, with the area under the curve (AUC) calculated in R. The Spearman method was used to analyze correlations among non-normally distributed bivariate variables. Orthogonal partial least squares discriminant analysis (OPLS-DA) was employed to improve group classification by excluding variables misaligned with the model. Key metabolites were identified by variable importance in projection (VIP) scores, with VIP values greater than 1 considered significant. Statistical significance was set at P < 0.05, with AUC values near 1 indicating high diagnostic accuracy, while values around 0.5 suggested accuracy comparable to random chance.
GWAS data sources
The genome-wide association study (GWAS) datasets for AAs and PCa were obtained from the IEU GWAS database (https://gwas.mrcieu.ac.uk/). The AA dataset included 122,776 participants, while the PCa dataset comprised 462,933 individuals. All participants were of European descent, with datasets ensuring non-overlapping independent samples. Figure 1 illustrates the overall framework of this study.
Fig. 1.
Overview of the Study Design. Schematic representation of the study workflow, encompassing serum sample collection, targeted metabolomics analysis using UPLC-MS/MS, and MR analysis to investigate causal relationships between amino acid levels and PCa risk.
Selection of instrumental variables for MR analysis
In this MR analysis, three assumptions were crucial: (1) IVs must be strongly associated with the exposure (i.e., serum AA levels); (2) IVs should not be related to confounding factors; and (3) IVs should influence the outcome only through the exposure. Single nucleotide polymorphisms (SNPs) significantly associated with serum AAs were selected at a genome-wide significance level (P < 5 × 10−8). When the number of SNPs was insufficient, the P-value threshold was adjusted to 5e-6 for Arg, serine (Ser), and threonine (Thr), and to 5 × 10−5 for glutamate (Glu) and Asp. Linkage disequilibrium was assessed using the European population from the 1000 Genomes Project, and SNPs with r² < 0.001 and a physical distance greater than 10,000 kb were retained. SNPs with minor allele frequency < 0.01 and those with an F-statistic < 10 were excluded to avoid weak instrument bias. Additionally, MR Steiger analysis verified the causal direction for each SNP to exclude those indicative of reverse causation.
Statistical analysis
The primary method for evaluating the causal relationship between exposure and outcome was the IVW approach. In addition, two supplementary MR methods, namely the weighted median method (WMM) and MR-Egger regression, each predicated on distinct modeling assumptions, were applied. The objective of utilizing these varied MR approaches was to examine the consistency and dependability of the associations across different theoretical frameworks. Within the IVW framework, the Cochran’s Q statistic was employed to probe the heterogeneity among the IVs, whereas the MR-Egger regression’s intercept term served to investigate potential horizontal pleiotropy among the IVs. To explore the possibility of a causal impact of PCa on AA levels within the body, we applied the described MR methodologies. In this reverse MR setup, PCa functioned as the exposure with Glu considered the outcome. SNPs linked to PCa, acting as IVs, were identified with genome-wide significance (P < 5 × 10−8), ensuring no LD (r2 < 0.01) with other SNPs over a 10,000 kb range. The entirety of these analyses was conducted utilizing R Language 4.2.1.
Results
Clinical attributes of participants
The study cohort included 65 subjects: 30 individuals with BPH and 35 with PCa. Clinical attributes of all participants are detailed in Table 1. Statistical analysis showed no significant differences in age, BMI, smoking status, tumor size, or PSA levels between the BPH and PCa groups. These findings highlight the limitations of PSA as a sole diagnostic marker, emphasizing the need for improved diagnostic approaches in urology.
Discriminatory disparities in AA composition among BPH and PCa patients
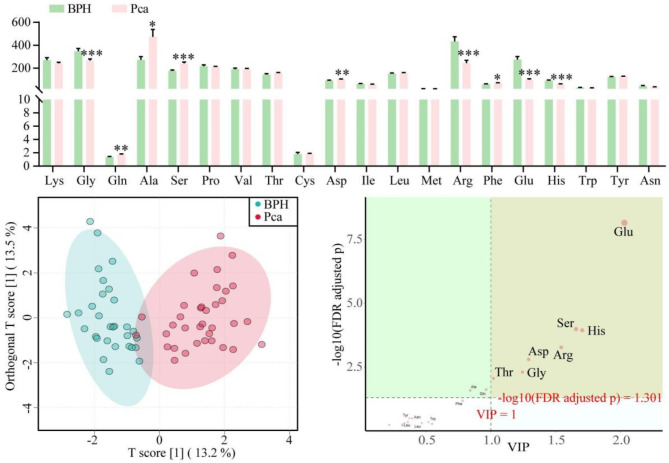
To investigate differences in serum AA profiles between BPH and PCa patients, we conducted a comparative analysis. This cross-sectional evaluation revealed that glycine (Gly), Arg, Glu, and histidine (His) levels were significantly lower in PCa patients compared to BPH controls. Conversely, higher levels of glutamine (Gln), Ala, Ser, aspartic acid (Asp), and phenylalanine (Phe) were observed in PCa patients (Fig. 2A). Using OPLS-DA, we were able to distinguish between the two groups based on these metabolic differences (Fig. 2B). Variables of VIP scores further confirmed the significance of metabolites such as Glu, Ser, His, Arg, Asp, Gly, and Thr in distinguishing BPH from PCa (Fig. 2C).
Fig. 2.
Comparison of UPLC-MS/MS-based amino acid profiles between the BPH group and the PCa group reveals significant differences. A Comparison of serum AA concentrations between the BPH group and the PCa group, analyzed using the Mann-Whitney U test. Significant differences were observed for specific AAs, as indicated by *P < 0.05, ***P < 0.001. B OPLS-DA score plot illustrating the discrimination between serum AA profiles from the BPH group (blue) and the PCa group (red). C VIP scores derived from the OPLS-DA model, showing the importance of serum AAs in distinguishing between the two groups (VIP > 1 was considered significant). Sample sizes were n = 30 for the BPH group and n = 35 for the PCa group.
Diagnostic performance of potential AA biomarkers
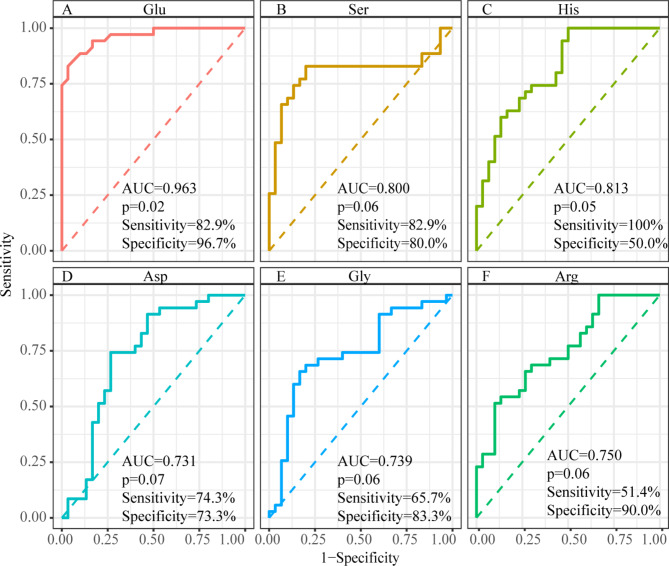
We evaluated the diagnostic performance of six AAs (Glu, Ser, His, Arg, Asp, and Gly) as potential biomarkers using ROC curve analysis. The analysis showed strong diagnostic potential, with each AA achieving an AUC value above 0.6 and a P-value below 0.05 (Fig. 3). Glu exhibited particularly high sensitivity and specificity (AUC = 0.963, sensitivity = 82.9%, specificity = 96.7%), followed by Ser (AUC = 0.800, sensitivity = 82.9%, specificity = 80.0%), His (AUC = 0.813, sensitivity = 100%, specificity = 50.0%), Arg (AUC = 0.750, sensitivity = 51.4%, specificity = 90.0%), Asp (AUC = 0.731, sensitivity = 74.3%, specificity = 73.3%), and Gly (AUC = 0.739, sensitivity = 65.7%, specificity = 83.3%).
Fig. 3.
Comparison of ROC curve analysis for selected amino acids reveals significant differences in their ability to discriminate between the BPH group and the PCa group. Evaluation of diagnostic performance for individual amino acids as potential biomarkers in distinguishing prostate cancer from benign prostatic hyperplasia. A Glutamate (Glu) (AUC = 0.963, sensitivity = 82.9%, specificity = 96.7%). B Serine (Ser) (AUC = 0.800, sensitivity = 82.9%, specificity = 80.0%). C Histidine (His) (AUC = 0.813, sensitivity = 100%, specificity = 50.0%). D Arginine (Arg) (AUC = 0.750, sensitivity = 51.4%, specificity = 90.0%). E Aspartic acid (Asp) (AUC = 0.731, sensitivity = 74.3%, specificity = 73.3%). F Glycine (Gly) (AUC = 0.739, sensitivity = 65.7%, specificity = 83.3%).
Two-sample MR analysis of AAs and PCa
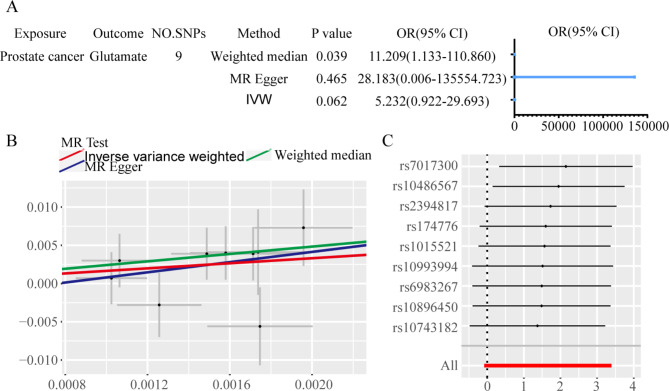
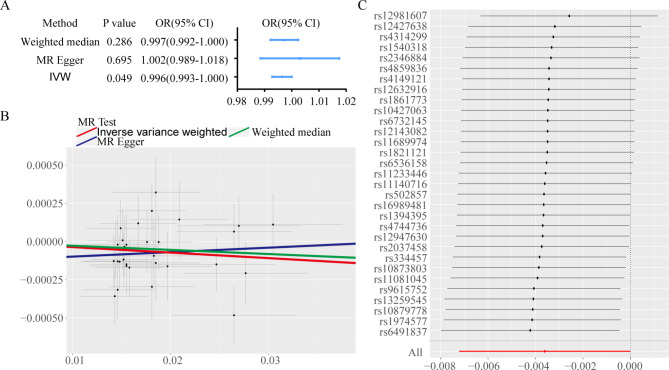
Using the IVW approach for MR analysis, we investigated the potential causal associations between serum AAs and PCa risk. The results highlighted a significant causal relationship between Glu and PCa (P < 0.05), while no significant associations were found for other AAs (P > 0.05). Detailed results are presented in Tables 3 and 4. A focused MR analysis on the relationship between Glu and PCa confirmed a significant association (P < 0.05) through the IVW method, while results from the WMM were nearly significant (P ≈ 0.05) with an odds ratio (OR) of less than 1 (Fig. 4A). An inverse correlation was observed between Glu levels and PCa risk (Fig. 4B). Leave-one-out analysis showed that no single SNP had an undue influence on the outcome (Fig. 4C). Sensitivity analyses, including the MR-Egger intercept and Cochran’s Q test, produced P-values greater than 0.05, indicating minimal bias.
Table 3.
Summary of the amino acid data sets.
| Exposure | ID | Population | Sample size | Number of SNPs | Year | Author |
|---|---|---|---|---|---|---|
| Arginine | met-a-347 | European | 7,528 | 2,545,579 | 2014 | Shin |
| Histidine | met-d-His | European | 114,895 | 12,321,875 | 2020 | Borges CM |
| Serine | met-a-464 | European | 7,796 | 2,545,555 | 2014 | Shin |
| Glutamate | met-a-466 | European | 7,804 | 2,545,537 | 2014 | Shin |
| Aspartate | met-a-388 | European | 7,721 | 2,545,425 | 2014 | Shin |
| Glycine | met-d-Gly | European | 114,972 | 12,321,875 | 2020 | Borges CM |
Table 4.
MR analysis for 6 amino acids in blood associations with prostate cancer risk.
| Exposure | ID | No. of SNPs | OR | 95% CI | P value |
|---|---|---|---|---|---|
| Arginine | met-a-347 | 7 | 0.997 | (0.987–1.008) | 0.674 |
| Histidine | met-d-His | 9 | 1.001 | (0.999–1.005) | 0.193 |
| Serine | met-a-464 | 13 | 0.996 | (0.987–1.005) | 0.415 |
| Glutamate | met-a-466 | 31 | 0.996 | (0.992–1.000) | 0.049 |
| Aspartate | met-a-388 | 16 | 1.001 | (0.996–1.005) | 0.649 |
| Glycine | met-d-Gly | 30 | 0.999 | (0.998–1.000) | 0.056 |
The statistically significant P-values were highlighted in bold.
Fig. 4.
Two-sample MR analysis results reveals the association between Glu levels and PCa risk. A Forest plot displaying MR estimates and 95% confidence intervals for the causal effect of Glu levels on PCa risk. B Scatter plot depicting the relationship between genetically predicted Glu levels and the risk of PCa using different MR methodologies (inverse-variance weighted, MR-Egger, and weighted median). C Leave-one-out sensitivity analysis indicating the stability of the association between Glu and PCa risk, demonstrating that no single SNP disproportionately influenced the results.
Reverse MR analysis of Glu and PCa
To explore a possible reverse causal relationship, we conducted an MR analysis positioning PCa as the exposure and Glu as the outcome. The results indicated no significant association between PCa and Glu levels (P > 0.05, Fig. 5A) and showed no apparent correlation (Fig. 5B). This finding was further corroborated by a leave-one-out analysis, which demonstrated the stability and robustness of the results against individual variations (Fig. 5C).
Fig. 5.
Reverse MR analysis reveals the association between PCa as exposure and Glu as outcome. A Forest plot illustrating MR estimates and corresponding 95% CI values for the causal effect of PCa as an exposure on Glu levels. B Scatter plot showing the relationship between genetically predicted PCa levels and Glu concentrations using different MR approaches. C Leave-one-out sensitivity analysis for the reverse MR analysis, confirming the robustness of the findings by demonstrating minimal influence from individual SNPs.
Discussion
Tumor cells thrive in harsh environments, including nutrient scarcity and hypoxia, while evading immune detection17. Traditional invasive biopsies, though accurate, often face challenges related to patient compliance and high costs. Non-invasive diagnostic methods, such as serum cancer embryonic antigen tests, lack sensitivity and specificity, making them unreliable for tumor detection18,19. Consequently, developing precise and broadly applicable techniques for tumor assessment remains crucial. Metabolomics, with its non-invasive nature, reliability, and cost-effectiveness, offers a promising approach by directly linking metabolic disturbances to tumor presence9,20. AAs are vital for cancer cell biosynthesis and survival21. Prior research has indicated the potential of AAs as biomarkers for enhancing PCa screening, emphasizing alterations in AA metabolism associated with PCa11–13. In this study, we analyzed serum AA profiles from patients with BPH and PCa using UPLC-MS/MS and identified significant differences in AA concentrations between the two groups. Specifically, our VIP analyses identified Glu, Ser, His, Arg, Asp, Gly, and Thr as key AAs, with ROC curve analysis highlighting Glu as a promising marker for distinguishing PCa from BPH (AUC = 0.963, sensitivity = 82.9%, specificity = 96.7%).
Glu, a nonessential AA, is crucial for metabolism, brain function, and immune response regulation22–24. Glu supplementation may offer health benefits due to its influence on the tricarboxylic acid (TCA) cycle and ATP production, impacting body weight regulation and hormone release25,26. Gln, synthesized from Glu, is abundant in blood and muscle tissues and plays an important role in cancer biology9,27. In PCa, mitochondrial DNA mutations often impair energy processes like TCA and oxidative phosphorylation, prompting tumor cells to rely on alternative metabolic pathways28. Oncogenes, such as Myc and androgen receptors, promote Gln uptake, influencing its metabolism and contributing to PCa cell proliferation29. Investigations into PCa cells have uncovered anomalies in Gln metabolism and the TCA cycle29,30. PC-3 PCa cells, characterized by high aggressiveness, exhibit increased Gln utilization, emphasizing the role of Gln in cancer progression31. In our study, patients with PCa exhibited decreased Glu and increased Gln levels, suggesting a potential protective effect of Glu against PCa. UPLC-MS/MS-based targeted metabolomics enabled the precise quantification of Glu and Gln, which are structurally similar, allowing for independent evaluation of their levels. Prior studies have linked elevated Glu levels to higher PCa mortality risk, while increased Gln may contribute to cancer progression and angiogenesis13,32. These findings support the role of Glu as a potential protective factor against PCa.
Our study employed MR to address biases and confounding factors common in traditional epidemiological research33. Previous MR studies have identified associations between genetically inferred serum levels of Ala and Asp with altered PCa risk12,13. In our study, MR analysis using the IVW approach indicated an inverse association between Glu levels and PCa risk, while no significant associations were found for the other AAs. A two-sample MR analysis, utilizing independent datasets for Glu and PCa, provided robust evidence for this association. Sensitivity analyses, including MR-Egger and Cochran’s Q test, produced P-values greater than 0.05, demonstrating minimal bias. The leave-one-out analysis confirmed that no individual SNP unduly influenced the overall findings. In a reverse MR analysis exploring PCa as the exposure and Glu as the outcome, no significant causal relationship was found, further supporting our conclusions regarding Glu’s protective effect. Early diagnosis is critical for effective PCa management, but traditional screening methods such as PSA testing are prone to overdiagnosis and overtreatment, complicating treatment decisions and affecting outcomes34,35. Although PSA has been a cornerstone of PCa diagnosis for decades, its specificity is compromised in elderly men with BPH, reducing its diagnostic accuracy36,37. In this context, we included BPH patients as the control group to identify metabolic differences that could serve as more reliable markers for distinguishing BPH from PCa. Our findings suggest that Glu could serve as an adjunct to PSA in early PCa screening and risk stratification, potentially enabling more tailored treatment strategies. However, a key limitation of this study is the lack of biopsy-confirmed BPH cases, which restricts the ability to fully explore the association between Glu levels and histopathological changes. Future studies should include pathological analyses to validate these associations.
In our MR analysis, results from the MR-Egger approach differed from those obtained using IVW and Weighted Median methods, highlighting potential issues such as pleiotropy. The MR-Egger method, while designed to estimate causal effects even with pleiotropic variants, is susceptible to biases introduced by invalid IVs or outliers. However, our findings indicated no substantial pleiotropy, as evidenced by the MR-Egger intercept (P > 0.05). This divergence underscores the complexity of genetic influences on AA levels and their association with PCa risk, emphasizing the need to consider the assumptions and limitations of each MR approach. This discussion serves to acknowledge these complexities and underscores the importance of using multiple methodologies to strengthen our conclusions. Additionally, our study did not directly measure the impact of physical activity on serum AA levels, which could be an influential factor. Although preliminary assessments showed no significant differences in activity levels between groups, incorporating more detailed measures of physical activity in future research may provide further insights into its role in cancer risk and AA metabolism.
Conclusions
Although AA dysregulation in cancer is well documented, identifying specific serum AAs as risk or protective factors has proven challenging. Our study suggests that lower serum Glu levels may be associated with a reduced risk of PCa, highlighting a potential protective role. These findings warrant further investigation and suggest that increasing Glu levels might represent a future strategy for PCa prevention and treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The feasibility of this study is indebted to the creators of the IEU GWAS database and the authors who diligently uploaded the data. Their valuable contributions have been instrumental in this research.
Abbreviations
- AA
Amino acid.
- AUC
Area under the ROC curve.
- BPH
Benign prostatic hyperplasia.
- BMI
Body Mass Index.
- GWAS
Genome-wide association study.
- MR
Mendelian randomization.
- IVs
Instrumental variables.
- IVW
Inverse Variance Weighted.
- LD
Linkage disequilibrium.
- OPLS-DA
Orthogonal partial least squares discriminant analysis.
- PCa
Prostate cancer.
- PSA
Prostate-specific antigen.
- ROC
Receiver operating characteristic.
- SNPs
Single nucleotide polymorphisms.
- TCA
Tricarboxylic acid.
- UPLC-MS/MS
Ultra-high-performance liquid chromatography-tandem mass spectrometry.
- VIP
Variable importance in the projection.
- WME
Weighted Median Method.
Author contributions
LM and QCW made equal contributions to this study. XLC and NNQ designed the study, while LM, QCW, SK and NNQ conducted experiments and analyzed data. LM, WQL, YJZ and WC collected clinical samples. LM, QCW, NNQ, and XLC finalized the manuscript. All authors listed in this manuscript contributed to data interpretation and approved the final version.
Funding
This work was supported by the Science and Technology Planning Project of Traditional Chinese Medicine, Jiangsu (YB2020050), the Science and Technology Youth Project of Health Commission, XuZhou (XWKYHT20230001), the General Project of Health Commission, XuZhou (XWKYHT20220092), and the Key Project of the Special Funds for Promoting Science and Technology Innovation, XuZhou (KC23233), and the Advanced Program of The Affiliated Hospital of Xuzhou Medical University, XuZhou (PYJH2024307).
Data availability
The published article includes all data generated or analyzed during the course of this study. The codes generated or utilized during the study are available upon request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki.The present study was approved by the ethical review committee of Xuzhou No. 1 People’s Hospital (approval number xyyll[2023]153), with all participants providing informed consent.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Long Miao and Qichao Wang contributed equally.
Contributor Information
Nienie Qi, Email: qinieys@163.com.
Xiliang Cao, Email: caoxiliang1971@sina.com.
References
- 1.Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin.73(1), 17–48 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Desai, K., McManus, J. M. & Sharifi, N. Hormonal therapy for prostate Cancer. Endocr. Rev.42(3), 354–373 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hlady, R. A. et al. Initiation of aberrant DNA methylation patterns and heterogeneity in precancerous lesions of human hepatocellular cancer. Epigenetics12(3), 215–225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekhoacha, M. et al. Prostate Cancer Review: Genetics, diagnosis, Treatment options, and alternative approaches. Molecules27(17). (2022). [DOI] [PMC free article] [PubMed]
- 5.Carlsson, S. V. & Vickers, A. J. Screening for prostate Cancer. Med. Clin. North. Am.104(6), 1051–1062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu, P. et al. Alterations of plasma exosomal proteins and motabolies are associated with the progression of castration-resistant prostate cancer. J. Transl Med.21(1), 40 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasim, S., Lee, S. Y. & Kim, J. Complexities of prostate Cancer. Int. J. Mol. Sci.23(22). (2022). [DOI] [PMC free article] [PubMed]
- 8.Cordova, R. A. et al. GCN2 eIF2 kinase promotes prostate cancer by maintaining amino acid homeostasis. Elife 11. (2022). [DOI] [PMC free article] [PubMed]
- 9.Zhang, X. et al. Identification of characteristic metabolic panels for different stages of prostate cancer by (1)H NMR-based metabolomics analysis. J. Transl Med.20(1), 275 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyagi, Y. et al. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS One. 6(9), e24143 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derezinski, P., Klupczynska, A., Sawicki, W., Palka, J. A. & Kokot, Z. J. Amino acid profiles of serum and urine in search for prostate Cancer biomarkers: a pilot study. Int. J. Med. Sci.14(1), 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang, S. et al. Genetically predicted circulating concentrations of Alanine and Alanine Aminotransferase were Associated with prostate Cancer risk. Clin. Epidemiol.14, 1255–1264 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin, Y. et al. Effects of glutamate and aspartate on prostate cancer and breast cancer: a mendelian randomization study. BMC Genom.23(1), 213 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo, B. et al. Causal associations of brain structure with bone mineral density: a large-scale genetic correlation study. Bone Res.11(1), 37 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrath, I. M., Montgomery, G. W. & Mortlock, S. Insights from mendelian randomization and genetic correlation analyses into the relationship between endometriosis and its comorbidities. Hum. Reprod. Update. 29(5), 655–674 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson, T. G., Urquijo, H., Holmes, M. V. & Davey Smith, G. Leveraging family history data to disentangle time-varying effects on disease risk using lifecourse mendelian randomization. Eur. J. Epidemiol.38(7), 765–769 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Reyes, I. & Chandel, N. S. Cancer metabolism: looking forward. Nat. Rev. Cancer. 21(10), 669–680 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Saeidi, H., Ismail, P., Samudi Raju, C., Khairul-Asri, M. G. & Bakrin, I. H. Genetic alterations in prostate cancer as diagnostic and prognostic markers. Malays J. Pathol.45(2), 149–155 (2023). [PubMed] [Google Scholar]
- 19.Kim, K. et al. Novel beta- and gamma-amino acid-derived inhibitors of prostate-specific membrane Antigen. J. Med. Chem.63(6), 3261–3273 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Kdadra, M., Hockner, S., Leung, H., Kremer, W. & Schiffer, E. Metabolomics biomarkers of prostate Cancer: a systematic review. Diagnostics (Basel)9(1). (2019). [DOI] [PMC free article] [PubMed]
- 21.Huang, J. et al. Prospective serum metabolomic profiling of lethal prostate cancer. Int. J. Cancer. 145(12), 3231–3243 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brosnan, J. T. & Brosnan, M. E. Glutamate: a truly functional amino acid. Amino Acids. 45(3), 413–418 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Schultz, J., Uddin, Z., Singh, G. & Howlader, M. M. R. Glutamate sensing in biofluids: recent advances and research challenges of electrochemical sensors. Analyst145(2), 321–347 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Gadgeel, S. et al. Updated analysis from KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for previously untreated metastatic nonsquamous non-small-cell Lung Cancer. J. Clin. Oncol.38(14), 1505–1517 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Iwatsuki, K. & Torii, K. Peripheral chemosensing system for tastants and nutrients. Curr. Opin. Endocrinol. Diabetes Obes.19(1), 19–25 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Kondoh, T. & Torii, K. MSG intake suppresses weight gain, fat deposition, and plasma leptin levels in male Sprague-Dawley rats. Physiol. Behav.95(1–2), 135–144 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Huang, C. J. et al. Randomized double-blind, placebo-controlled trial evaluating oral glutamine on radiation-induced oral mucositis and dermatitis in head and neck cancer patients. Am. J. Clin. Nutr.109(3), 606–614 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalak, K. P., Mackowska-Kedziora, A., Sobolewski, B. & Wozniak, P. Key Roles of Glutamine Pathways in Reprogramming the Cancer Metabolism. Oxid Med Cell Longev 2015:964321. (2015). [DOI] [PMC free article] [PubMed]
- 29.Beltran, H. et al. A phase II trial of the Aurora kinase A inhibitor Alisertib for patients with castration-resistant and neuroendocrine prostate Cancer: efficacy and biomarkers. Clin. Cancer Res.25(1), 43–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, B. et al. Integrated RNA and metabolite profiling of urine liquid biopsies for prostate cancer biomarker discovery. Sci. Rep.10(1), 3716 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zacharias, N. M. et al. Metabolic differences in glutamine utilization lead to metabolic vulnerabilities in prostate Cancer. Sci. Rep.7(1), 16159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang, J., Zhao, B., Weinstein, S. J., Albanes, D. & Mondul, A. M. Metabolomic profile of prostate cancer-specific survival among 1812 Finnish men. BMC Med.20(1), 362 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo, M., Zheng, Y., Zhuo, Q., Lin, L. & Han, Y. The causal effects of atopic dermatitis on the risk of skin cancers: a two-sample mendelian randomization study. J. Eur. Acad. Dermatol. Venereol. (2023). [DOI] [PubMed]
- 34.Gammel, M. C. M., Solari, E. L., Eiber, M., Rauscher, I. & Nekolla, S. G. A clinical role of PET-MRI in prostate Cancer? Semin Nucl. Med.54(1), 132–140 (2024). [DOI] [PubMed] [Google Scholar]
- 35.Hamdy, F. C. et al. Fifteen-year outcomes after monitoring, surgery, or Radiotherapy for prostate Cancer. N Engl. J. Med.388(17), 1547–1558 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Hugosson, J. et al. A 16-yr follow-up of the European randomized study of screening for prostate Cancer. Eur. Urol.76(1), 43–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giskeodegard, G. F. et al. Metabolic markers in blood can separate prostate cancer from benign prostatic hyperplasia. Br. J. Cancer. 113(12), 1712–1719 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all data generated or analyzed during the course of this study. The codes generated or utilized during the study are available upon request from the corresponding author.