Abstract
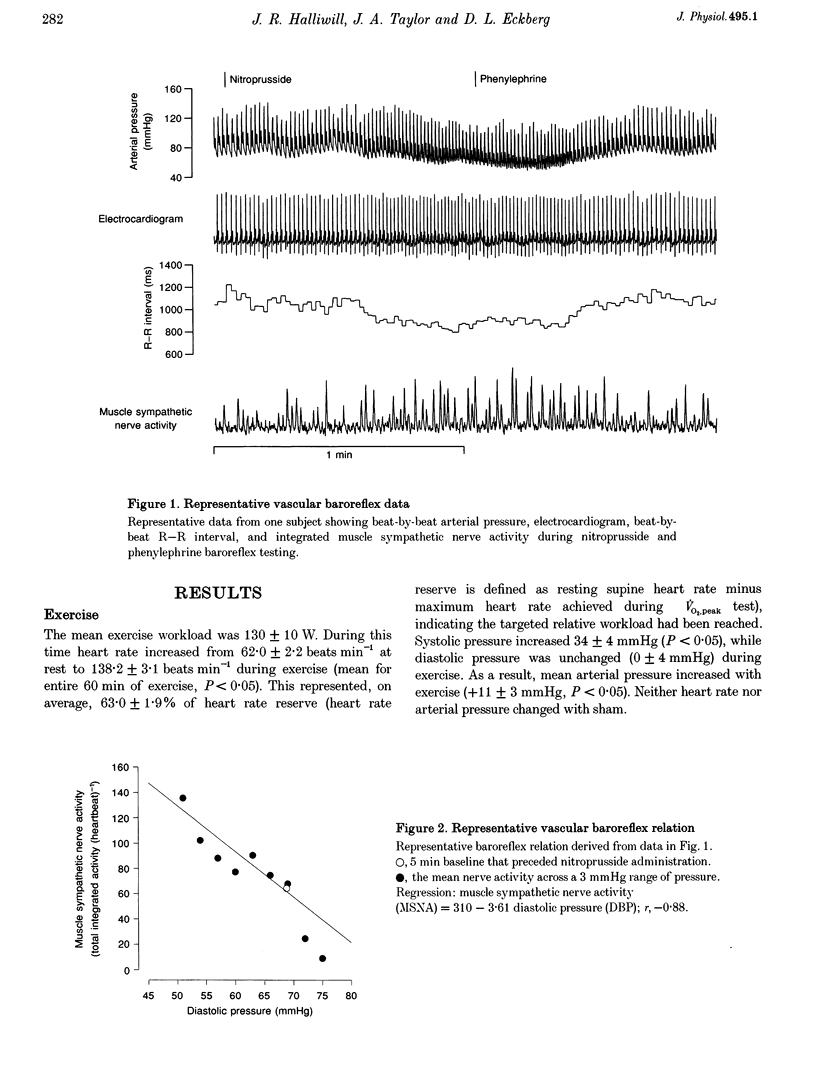
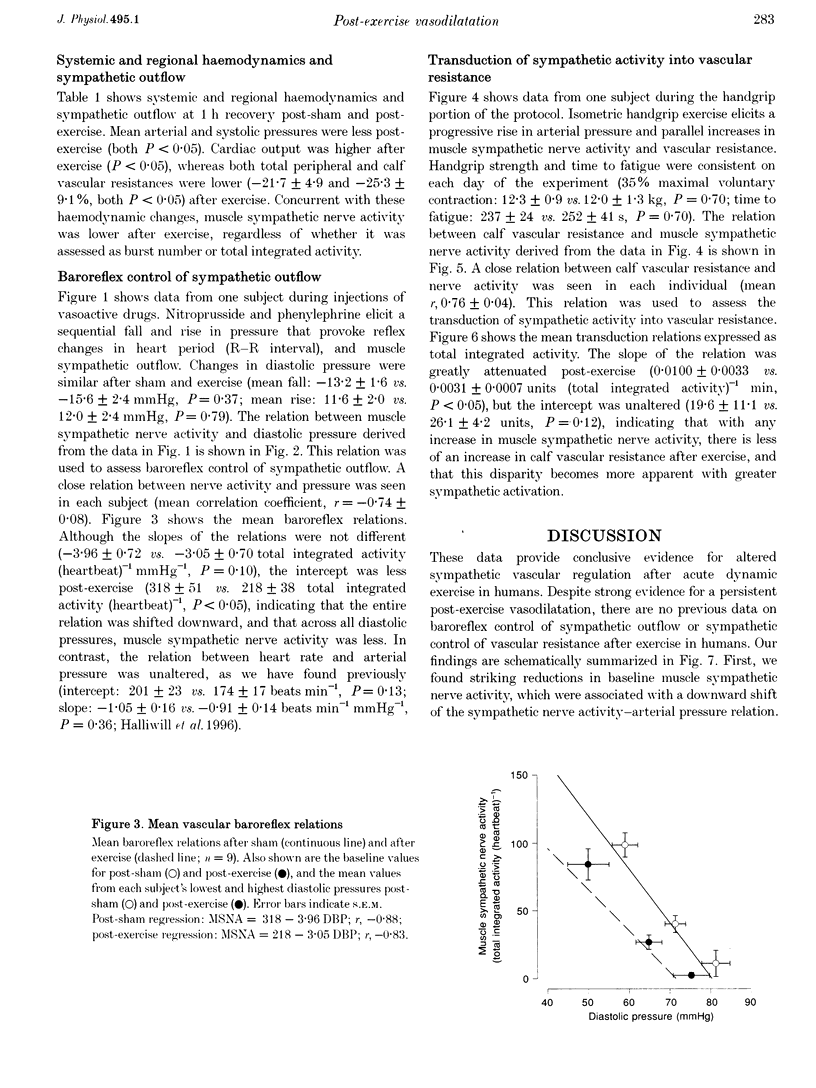
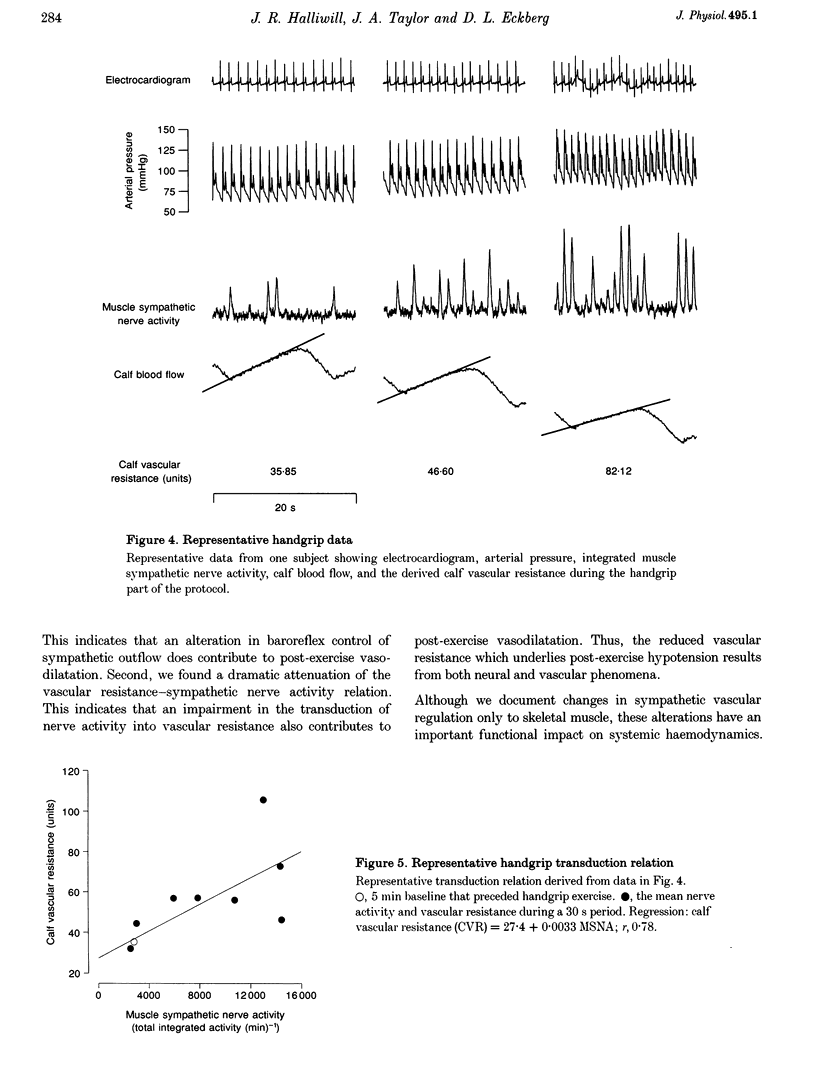
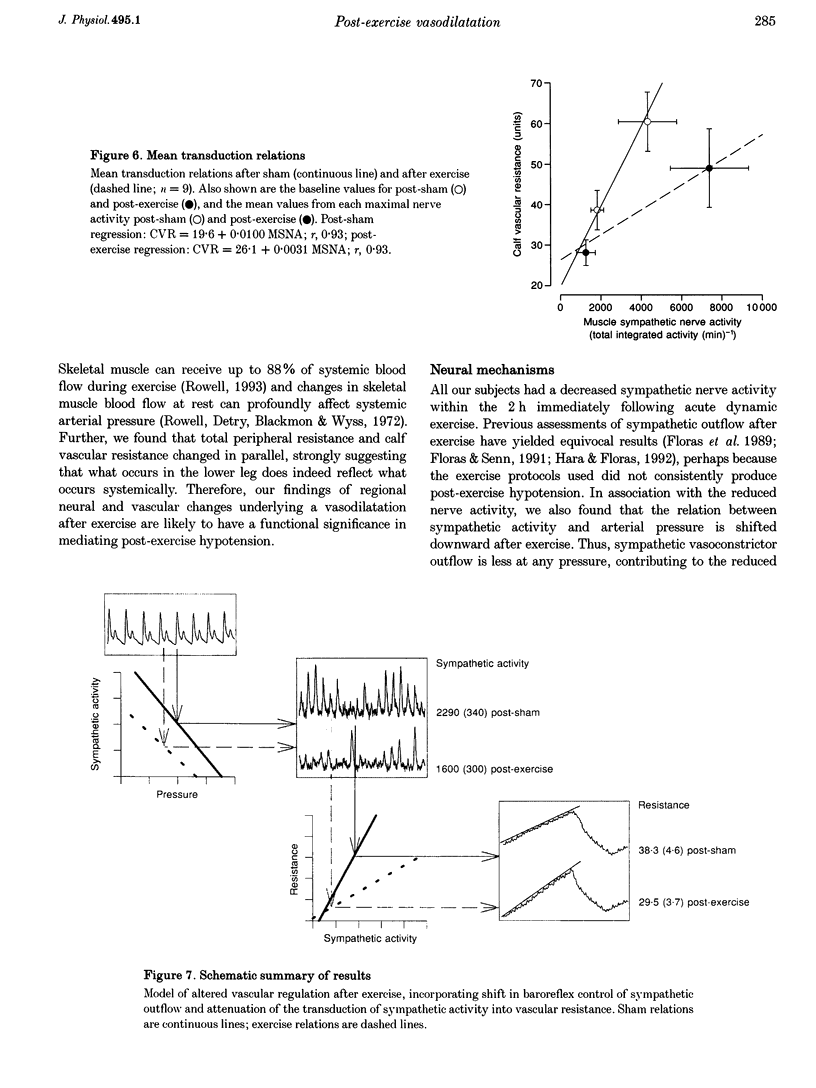
1. The reduction in vascular resistance which accompanies acute dynamic exercise does not subside immediately during recovery, resulting in a post-exercise hypotension. This sustained vasodilatation suggests that sympathetic vascular regulation is altered after exercise. 2. Therefore, we assessed the baroreflex control of sympathetic outflow in response to arterial pressure changes, and transduction of sympathetic activity into vascular resistance during a sympatho-excitatory stimulus (isometric handgrip exercise) after either exercise (60 min cycling at 60% peak aerobic power (VO2,peak)) or sham treatment (60 min seated rest) in nine healthy subjects. 3. Both muscle sympathetic nerve activity and calf vascular resistance were reduced after exercise (-29.7 +/- 8.8 and -25.3 +/- 9.1%, both P < 0.05). The baroreflex relation between diastolic pressure and sympathetic outflow was shifted downward after exercise (post-exercise intercept, 218 +/- 38 total integrated activity (heartbeat)-1; post-sham intercept, 318 +/- 51 total integrated activity (heartbeat)-1, P < 0.05), indicating less sympathetic outflow across all diastolic pressures. Further, the relation between sympathetic activity and vascular resistance was attenuated after exercise (post-exercise slope, 0.0031 +/- 0.0007 units (total integrated activity)-1 min; post-sham slope, 0.0100 +/- 0.0033 units (total integrated activity)-1 min, P < 0.05), indicating less vasoconstriction with any increase in sympathetic activity. 4. Thus, both baroreflex control of sympathetic outflow and the transduction of sympathetic activity into vascular resistance are altered after dynamic exercise. We conclude that the vasodilation which underlies post-exercise hypotension results from both neural and vascular phenomena.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett T., Wilcox R. G., Macdonald I. A. Post-exercise reduction of blood pressure in hypertensive men is not due to acute impairment of baroreflex function. Clin Sci (Lond) 1984 Jul;67(1):97–103. doi: 10.1042/cs0670097. [DOI] [PubMed] [Google Scholar]
- Boone J. B., Jr, Levine M., Flynn M. G., Pizza F. X., Kubitz E. R., Andres F. F. Opioid receptor modulation of postexercise hypotension. Med Sci Sports Exerc. 1992 Oct;24(10):1108–1113. [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–98. [PubMed] [Google Scholar]
- COLLIER C. R. Determination of mixed venous CO2 tensions by rebreathing. J Appl Physiol. 1956 Jul;9(1):25–29. doi: 10.1152/jappl.1956.9.1.25. [DOI] [PubMed] [Google Scholar]
- Cléroux J., Kouamé N., Nadeau A., Coulombe D., Lacourciere Y. Baroreflex regulation of forearm vascular resistance after exercise in hypertensive and normotensive humans. Am J Physiol. 1992 Nov;263(5 Pt 2):H1523–H1531. doi: 10.1152/ajpheart.1992.263.5.H1523. [DOI] [PubMed] [Google Scholar]
- Cléroux J., Kouamé N., Nadeau A., Coulombe D., Lacourcière Y. Aftereffects of exercise on regional and systemic hemodynamics in hypertension. Hypertension. 1992 Feb;19(2):183–191. doi: 10.1161/01.hyp.19.2.183. [DOI] [PubMed] [Google Scholar]
- Coats A. J., Conway J., Isea J. E., Pannarale G., Sleight P., Somers V. K. Systemic and forearm vascular resistance changes after upright bicycle exercise in man. J Physiol. 1989 Jun;413:289–298. doi: 10.1113/jphysiol.1989.sp017654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert T. J., Cowley A. W., Jr Baroreflex modulation of sympathetic outflow during physiological increases of vasopressin in humans. Am J Physiol. 1992 May;262(5 Pt 2):H1372–H1378. doi: 10.1152/ajpheart.1992.262.5.H1372. [DOI] [PubMed] [Google Scholar]
- Floras J. S., Senn B. L. Absence of post exercise hypotension and sympathoinhibition in normal subjects: additional evidence for increased sympathetic outflow in borderline hypertension. Can J Cardiol. 1991 Jul-Aug;7(6):253–258. [PubMed] [Google Scholar]
- Floras J. S., Sinkey C. A., Aylward P. E., Seals D. R., Thoren P. N., Mark A. L. Postexercise hypotension and sympathoinhibition in borderline hypertensive men. Hypertension. 1989 Jul;14(1):28–35. doi: 10.1161/01.hyp.14.1.28. [DOI] [PubMed] [Google Scholar]
- Gillen C. M., Lee R., Mack G. W., Tomaselli C. M., Nishiyasu T., Nadel E. R. Plasma volume expansion in humans after a single intense exercise protocol. J Appl Physiol (1985) 1991 Nov;71(5):1914–1920. doi: 10.1152/jappl.1991.71.5.1914. [DOI] [PubMed] [Google Scholar]
- Halliwill J. R., Taylor J. A., Hartwig T. D., Eckberg D. L. Augmented baroreflex heart rate gain after moderate-intensity, dynamic exercise. Am J Physiol. 1996 Feb;270(2 Pt 2):R420–R426. doi: 10.1152/ajpregu.1996.270.2.R420. [DOI] [PubMed] [Google Scholar]
- Hara K., Floras J. S. Effects of naloxone on hemodynamics and sympathetic activity after exercise. J Appl Physiol (1985) 1992 Nov;73(5):2028–2035. doi: 10.1152/jappl.1992.73.5.2028. [DOI] [PubMed] [Google Scholar]
- Howard M. G., DiCarlo S. E. Reduced vascular responsiveness after a single bout of dynamic exercise in the conscious rabbit. J Appl Physiol (1985) 1992 Dec;73(6):2662–2667. doi: 10.1152/jappl.1992.73.6.2662. [DOI] [PubMed] [Google Scholar]
- Howard M. G., DiCarlo S. E., Stallone J. N. Acute exercise attenuates phenylephrine-induced contraction of rabbit isolated aortic rings. Med Sci Sports Exerc. 1992 Oct;24(10):1102–1107. [PubMed] [Google Scholar]
- Isea J. E., Piepoli M., Adamopoulos S., Pannarale G., Sleight P., Coats A. J. Time course of haemodynamic changes after maximal exercise. Eur J Clin Invest. 1994 Dec;24(12):824–829. doi: 10.1111/j.1365-2362.1994.tb02026.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Stjarne L. Neuropeptide Y (NPY) depresses the secretion of 3H-noradrenaline and the contractile response evoked by field stimulation, in rat vas deferens. Acta Physiol Scand. 1984 Mar;120(3):477–479. doi: 10.1111/j.1748-1716.1984.tb07410.x. [DOI] [PubMed] [Google Scholar]
- Pernow J., Lundberg J. M., Kaijser L., Hjemdahl P., Theodorsson-Norheim E., Martinsson A., Pernow B. Plasma neuropeptide Y-like immunoreactivity and catecholamines during various degrees of sympathetic activation in man. Clin Physiol. 1986 Dec;6(6):561–578. doi: 10.1111/j.1475-097x.1986.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Rowell L. B., Detry J. M., Blackmon J. R., Wyss C. Importance of the splanchnic vascular bed in human blood pressure regulation. J Appl Physiol. 1972 Feb;32(2):213–220. doi: 10.1152/jappl.1972.32.2.213. [DOI] [PubMed] [Google Scholar]
- Röcker L., Kirsch K. A., Heyduck B., Altenkirch H. U. Influence of prolonged physical exercise on plasma volume, plasma proteins, electrolytes, and fluid-regulating hormones. Int J Sports Med. 1989 Aug;10(4):270–274. doi: 10.1055/s-2007-1024914. [DOI] [PubMed] [Google Scholar]
- Sallis J. F., Haskell W. L., Wood P. D., Fortmann S. P., Rogers T., Blair S. N., Paffenbarger R. S., Jr Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985 Jan;121(1):91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- Schwarz L., Kindermann W. Changes in beta-endorphin levels in response to aerobic and anaerobic exercise. Sports Med. 1992 Jan;13(1):25–36. doi: 10.2165/00007256-199213010-00003. [DOI] [PubMed] [Google Scholar]
- Seals D. R. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol (1985) 1989 May;66(5):2472–2478. doi: 10.1152/jappl.1989.66.5.2472. [DOI] [PubMed] [Google Scholar]
- Shyu B. C., Thorén P. Circulatory events following spontaneous muscle exercise in normotensive and hypertensive rats. Acta Physiol Scand. 1986 Dec;128(4):515–524. doi: 10.1111/j.1748-1716.1986.tb08007.x. [DOI] [PubMed] [Google Scholar]
- Sundlöf G., Wallin B. G. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978 Jan;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G., Wallin B. G. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977 Nov;272(2):383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. A., Halliwill J. R., Brown T. E., Hayano J., Eckberg D. L. 'Non-hypotensive' hypovolaemia reduces ascending aortic dimensions in humans. J Physiol. 1995 Feb 15;483(Pt 1):289–298. doi: 10.1113/jphysiol.1995.sp020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorén P., Floras J. S., Hoffmann P., Seals D. R. Endorphins and exercise: physiological mechanisms and clinical implications. Med Sci Sports Exerc. 1990 Aug;22(4):417–428. [PubMed] [Google Scholar]
- Vissing S. F., Scherrer U., Victor R. G. Relation between sympathetic outflow and vascular resistance in the calf during perturbations in central venous pressure. Evidence for cardiopulmonary afferent regulation of calf vascular resistance in humans. Circ Res. 1989 Dec;65(6):1710–1717. doi: 10.1161/01.res.65.6.1710. [DOI] [PubMed] [Google Scholar]
- Wong-Dusting H., Rand M. J. Inhibition of sympathetic neurotransmission by the opioid delta-receptor agonist DAMA in the pithed rat. Clin Exp Pharmacol Physiol. 1989 Nov;16(11):821–827. doi: 10.1111/j.1440-1681.1989.tb01521.x. [DOI] [PubMed] [Google Scholar]



