Abstract
Tissue-resident innate immune cells have important functions in both homeostasis and pathological states. Despite advances in the field, analyzing the metabolism of tissue-resident innate lymphocytes is still challenging. The small number of tissue-resident innate lymphocytes such as ILC, NK, iNKT and γδ T cells poses additional obstacles in their metabolic studies. In this review, we summarize the current understanding of innate lymphocyte metabolism and discuss potential pitfalls associated with the current methodology relying predominantly on in vitro cultured cells or bulk-level comparison. Meanwhile, we also summarize and advocate for the development and adoption of single-cell metabolic assays to accurately profile the metabolism of tissue-resident immune cells directly ex vivo.
Subject terms: Innate immune cells, Metabolomics, Systems analysis, Gene regulation in immune cells
Immune cells pursue metabolic adaptations to accommodate the dynamic changes of their functions. Here in this review the authors summarize immune metabolic states of various innate lymphoid cells and advocate single-cell level methods for studying the complex networks of immune metabolism.
Introduction
Innate lymphocytes, such as ILCs, NK cells, iNKT cells and γδ T cells, play pivotal roles in tissue homeostasis and immune responses, with each having an affinity for different tissue locations. Understanding their unique metabolic profiles is essential for elucidating their functions and potential therapeutic applications. This review explores the current understanding of the metabolism of tissue-resident innate lymphocytes, comprehensively examines current methodologies, and promotes the utilisation of single-cell metabolic assays directly ex vivo to capture the in vivo metabolic landscape. As little is known about the metabolism of tissue-resident lymphocytes in humans, and because some of the new cutting-edge techniques were developed in mice, this review will primarily focus on our understanding of murine innate lymphocytes. Further, it is worth noting that there are species-specific differences in immune cell metabolism, however, that are yet to be characterised for tissue-resident innate lymphocytes1,2.
Innate lymphocytes in the tissues
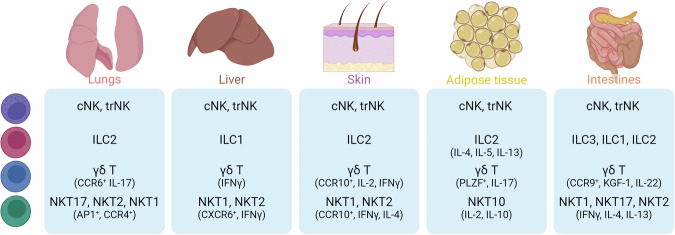
Innate lymphoid cells (ILC) comprise a heterogeneous family of tissue-resident immune cells, with functions in the defence against invading pathogens and transformed cells3. Within tissues, ILCs have critical functions in maintaining tissue balance and responding to local inflammation and injuries4,5. ILCs are found throughout the body and can be classified into three main groups based on their surface marker expression, cytokine secretion profiles, and transcription factor expression3,6 (Table 1 and Fig. 1). Group 1 ILCs, which include natural killer (NK) cells and ILC1s, can be identified through their co-expression of the surface markers NK1.1 and NKp46, the transcription factor T-bet, and the cytokine IFNγ. Further, NK cells and ILC1s can be distinguished by the expression of two transcription factors, EOMES and HOBIT, in NK and ILC1 cells, respectively; similarly, the presence of surface markers CD49b and CD200R1 also helps define NK and ILC1, respectively3,6–8. Generally, ILC1s are long-term tissue-resident cells, while NK cells recirculate through the vasculature, performing host surveillance against infections and tumours. However, there is a recent appreciation that tissue-resident NK cells exist, and they have different gene expression patterns from ILC1s9. Moreover, there is evidence that upon infection, circulating NK cells can establish residency within the infected tissue, with these tissue-resident NK cells having roles in maintaining tissue homeostasis10,11. Group 2 ILCs can be identified through their expression of the transcription factor GATA-3, and through their production of type 2 cytokines such as IL-4, IL-5 and IL-13. Group 2 ILCs contribute to the defence against helminth infection, and have been shown to be involved in the allergic response within the lungs3,12,13. Group 3 ILCs, which include ILC3s and lymphoid tissue-inducer (LTi) cells, express RORγt and produce IL-17A and IL-22, with ILC3s providing antibacterial immunity in the intestine, while LTi cells participate in the formation of secondary lymphoid structures during embryonic development3,5,14.
Table 1.
Overview of innate lymphocytes
| Immune cell subsets | Nomenclature | Surface markers | Transcription factors | Development | Tissue locations | Cytokine and effector profile |
|---|---|---|---|---|---|---|
| ILC | cNK, trNK | NK1.1, NKp46, CD49b | EOMES, T-bet | Bone marrow | Lymphoid and non-lymphoid tissues | IFNγ, TNFα, cytotoxic molecules |
| ILC1 | NK1.1, NKp46, CD49a, CD200R1 | HOBIT, T-bet | Bone marrow | Non-lymphoid tissues | IFNγ, TNFα | |
| ILC2 | ICOS, IL17Rβ | GATA-3, RORα | Bone marrow | Most abundant in the lung | IL-4, IL-5, IL-9, IL-13, AREG | |
| ILC3 | NKp46, AhR | RORγt, T-bet, | Bone marrow | Most abundant in the gut | IL-17, IL-22, GM-CSF | |
| LTi | CCR6 | RORγt | Bone marrow | Lymphoid tissues | IL-17, IL-22 | |
| γδ T cells | γδ17, γδIFN | CD27, CD45RB, CD44 | RORγt, T-bet, | Thymus | Most abundant in the intestine, skin, brain, adipose tissue | IL-17, IFNγ |
| iNKT cells | NKT1 | CD24lo, NK1.1hi, CD27hi, CXCR3+ | T-bet | Thymus | Most abundant in liver, spleen, adipose tissue | IFNγ, IL-2, IL-4, IL-10, IL-17, TNF |
| NKT2 | CD24lo, CD27hi, CD4+ | GATA-3, PLZF | ||||
| NKT17 | CD24lo, CCR6+, CD103hi, SDC-1+ | RORγt | ||||
| NKT10 | PD-1, ICOS, Nrp1 | E4BP4 |
Fig. 1. Tissue-resident innate lymphocytes.
Distribution of NK, iNKT, ILC and γδ T cells in the lungs, liver, skin, adipose tissue and intestine. For each tissue, the subsets described to be populated by these cells and their specific markers/cytokines are listed based on murine studies. This figure was created in BioRender. Finlay (2024) https://BioRender.com/e69o761.
γδ T lymphocytes have features of both innate and adaptive lymphocytes, and account for 0.5–5% of all T cells. γδ T cells encompass various subgroups based on their T cell receptor (TCR) gene usage and cellular function. Alternatively, γδ T cells may also be divided into two groups based on cytokine secretion profiles: CD27−IL-17-producing cells (γδ17) and CD27+IFNy-producing cells (γδIFN) (Table 1). Significant populations of these cells can be found in skin, intestine, brain and adipose tissue15 (Fig. 1). In the context of cancer, it is generally thought that γδIFN cells play roles in tumour surveillance and regression, while γδ17 cells promote tumour growth and metastasis16,17. The in-depth breakdown of γδ T cell subgroups has been reviewed extensively elsewhere15,18.
Natural killer T (NKT) cells are a population of unconventional αβ T lymphocytes that recognises lipids presented by the MHC-I-like molecule, CD1d. NKT cells can be subdivided in type I (or invariant NKT cells (iNKT)) and type II NKT cells. While iNKT cells have a semi-invariant TCR, type II NKT cells utilise a more diverse TCR repertoire and are less explored due to the difficulty in identifying them by specific markers19,20. Thus, for the purpose of this review, only work on iNKT cells will be discussed. iNKT cells are primarily found in the liver, spleen and adipose tissue, with lesser populations within the lungs, intestines and lymph nodes21 (Fig. 1). iNKT cells have been shown to play important roles in controlling infection and tumour development but, due to their tissue-resident nature, are also implicated in maintaining tissue homeostasis22–25. iNKT cells can display properties of NK cells, with some subsets expressing the conventional NK cell markers NK1.1 and NKG2D. While conventional T cells are activated by peptides, iNKT cells are activated by glycolipids and are characterised by the rapid production of IFNγ and IL-4. Moreover, iNKT cells have the capacity to produce a broad range of cytokines, including IL-2, IL-10, IL-13, IL-17 and TNF, and chemokines, including RANTES, MIP-1α and MIP-1β (Table 1). In-depth characterisation of the development and function of iNKT cells can be found here22.
Metabolism of innate lymphocytes
Considering that innate lymphocytes inhabit diverse tissue niches, this begs the question whether the tissue microenvironment influences the metabolism of these cells. Also, are innate lymphocytes capable of tuning their metabolism to the different tissue environments they encounter? Much about what we know on the metabolism of innate lymphocytes comes from studies of cells in culture, and as such, this data may not accurately reflect the metabolic configurations of the respective cells within the tissue niche. Regardless, innate lymphocytes at rest are metabolically inactive, relying on glycolysis-fuelled oxidative phosphorylation to support basal nutrient uptake and minimal biosynthesis. When activated, innate lymphocytes shift their metabolism to meet their energy needs, with increased metabolic rate and nutrient uptake to promote protein, lipid and nucleic acid synthesis. Metabolic configurations of these innate lymphocyte populations are outlined in Fig. 2. For the purpose of this review, murine studies will be primarily discussed unless stated otherwise. Additional considerations are required when studying other innate lymphocytes in humans such as mucosal-associated invariant T cells. Moreover, the emerging technologies discussed below for studying metabolic fluxes in single cells have largely been developed for mice, so additional challenges will be encountered when applying these techniques to human samples.
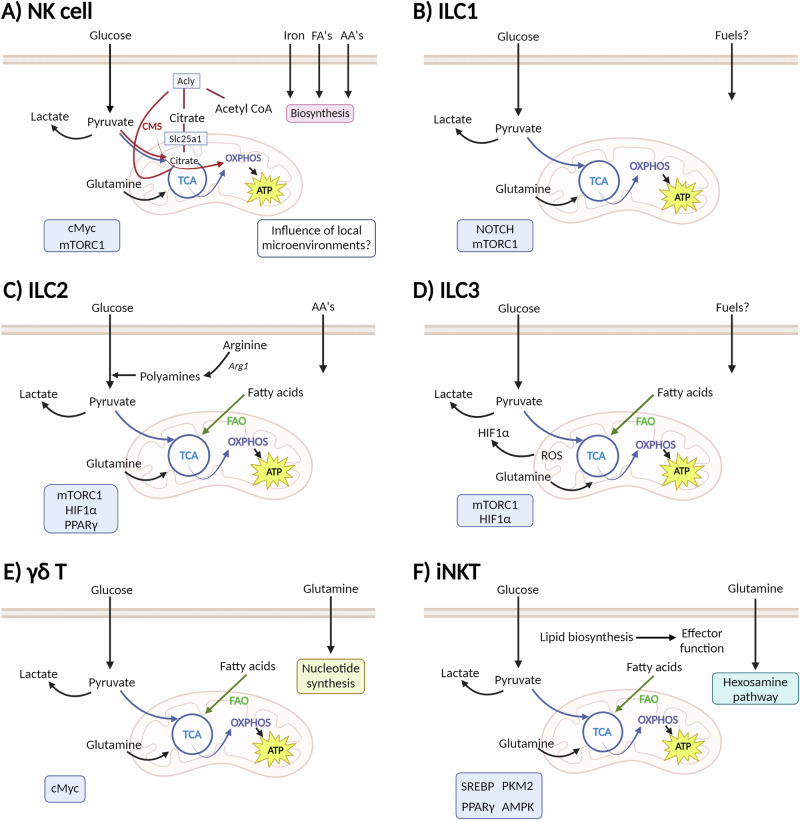
Fig. 2. Overview of NK, ILC, γδ T and iNKT cell metabolism.
Metabolic configurations described for innate lymphoid cells A NK cells, B ILC1s, C ILC2s and D ILC3. E γδ T cells present a metabolic dichotomy, with γδIFN relying on glycolytic metabolism, while γδ17 cells upon mitochondrial and lipid metabolism. F Metabolic configurations of iNKT cells are shown to be dependent on tissue location. Glycolytic metabolism is important for splenic and hepatic iNKT cells, whereas fatty acid oxidation is important for adipose tissue iNKT cells. Key signalling molecules identified in each cell type are shown in blue boxes. TCA tricarboxylic acid cycle (blue lines), OXPHOS oxidative phosphorylation, FAO fatty acid oxidation (green arrow), CMS citrate–malate shuttle (red lines). This figure created in BioRender. Finlay (2024) https://BioRender.com/c57f600.
NK cell metabolism
NK cell metabolism in the context of health and disease has been reviewed extensively elsewhere26,27. NK cells exist in a relatively quiescent state, with basal metabolic rates that increase upon activation to support the production of cytokines and cytotoxic molecules. The signalling molecules, mTORC1, cMyc and SREBP, have all been implicated in the regulation of NK cell metabolic reprogramming28–30 (Fig. 2A). Deficiency of these signalling molecules, by either pharmacological or genetic approaches, results in decreased NK cell effector functions including reduced IFNγ production and killing capacity. Mice treated with a glycolytic inhibitor, 2-DG, or a mTORC1 inhibitor, rapamycin, show decreased NK cell IFNγ production following the injection of TLR agonist Poly(I:C), or the infection with mouse cytomegalovirus (MCMV)30,31. More recently, it was demonstrated that the uptake of iron and fatty acids (FA) is important to support NK cell metabolism and function in mice challenged with retrovirus infection32,33. Iron deficiency profoundly impairs NK cell antiviral functions, resulting in increased viral loads. These findings were connected to decreased expression of the transcription factor cMyc, decreased mitochondrial function, and reduced production of cytotoxic molecules32. Moreover, upon retroviral infection, splenic NK cells increase CD36 expression and FA uptake, while pharmacological inhibition of fatty acid β-oxidation impairs NK cell cytotoxicity33. A separate study showed that carnitine palmitoyltransferase I (CPT1)-dependent β-oxidation sustains NK cell mitochondrial function, actin polarisation and proliferation in MCMV infection and tumour models34. These studies highlight how NK cells can utilise multiple fuels to support their effector functions, and may thus have implications regarding how NK cells can alter their metabolism in environments with differing levels of iron and lipids. This is likely also true for other tissue-resident lymphocytes, yet their metabolism has not been studied to the same degree as NK cells.
ILC1 metabolism
It is assumed that ILC1s share comparable metabolic features to NK cells due to their similar phenotypic characteristics (Fig. 2B), but in actuality ILC1 metabolism is sparsely described, with just one study demonstrating that ILC1s are enriched for Notch and mTOR signalling pathways35, and another showing that following Mycobacterium infection lung ILC1-like cells are primarily glycolytic with low levels of mitochondrial metabolism36.
ILC2 metabolism
There have been more studies on ILC2 metabolism compared to that on ILC1 cells (Fig. 2C). ILC2s rely on non-glucose nutrients, including fatty acids and arginine, to fuel their metabolic pathways and support their effector function8. At rest, ILC2s are highly dependent on oxidative phosphorylation and have elevated mitochondrial polarisation and mass37. Indeed, mitochondrial metabolism is crucial for ILC2 homeostasis, as individuals with mitochondrial disease have severely reduced numbers of ILC2 cells37. Surace et al. demonstrated that human ILC2s have high expression of amino acid transporters, including LAT4 (Slc3a2/Slc43a2), which transports large neutral amino acids, and Slc1a5, which transports alanine and glutamine. Metabolomic analysis confirmed that ILC2s are enriched for amino acids such as branched-chain amino acids and glutamine37. Upon activation, ILC2s continue to utilise amino acids to fuel oxidative phosphorylation and support proliferation processes which are dependent on glycolysis and mTORC1 activity37. In the intestine, ILC2s use extracellular fatty acids to fuel β-oxidation. This metabolic feature has been studied during helminth infection where inhibition of fatty acid oxidation, but not inhibition of glycolysis, reduces ILC2 cytokine production38. In addition, upon chronic activation, ILC2s increase the uptake of glucose as well as external lipids stored as lipid droplets that are subsequently converted to phospholipids to sustain ILC2 proliferation. Feeding mice a ketogenic diet, thus reducing glucose availability, reduced ILC2 infiltration and cytokine production upon papain challenge39. In parallel, the expression of the enzyme arginase 1 (Arg1) is a distinguishing feature of ILC2 cells. Arg1 metabolises arginine to generate ornithine and urea, with further metabolic reactions generating proline and polyamines important for cell growth and proliferation. Expression of Arg1 is a conserved trait of ILC2s in both human and mouse lymphoid and non-lymphoid tissues40. Pharmacological inhibition of Arg1 enzymatic activity results in reduced levels of glycolysis and polyamine biosynthesis40. Deletion of Arg1 in murine ILCs restrains ILC2 proliferation and cytokine production within the lung40. While Arg1 is also expressed in a proportion of ILC3s, it is not critical for their development or function40.
ILC3 metabolism
ILC3s have been shown to take up glucose and fatty acids at rest, although the latter to a much less extent than ILC2s38. Upon ILC3 activation there is an enrichment in pathways associated with glycolysis, including pyruvate, fructose and mannose metabolism, based on single-cell transcriptome analysis35 (Fig. 2D). Supporting the role of glycolysis in ILC3s, defective mTORC1 signalling led to reduced number of ILC3s and decreased IL-17 and IL-22 production8,41,42. As ILC3s exist primarily within the gut, they are exposed to and influenced by metabolites from food intake and the inherent hypoxic gut environment. For instance, studies have shown that vitamin A deficiency results in reduced ILC3s within the gut, decreased ILC3 cytokine production, and increased susceptibility to bacterial infection43. Following Citrobacter rodentium (C. rodentium) infection, ILC3s increase cytokine production and proliferation as induced by mTORC1-HIF1α signalling, and show heightened glycolysis and production of mitochondrial ROS for stabilising HIF1α protein levels42. Hypoxia leads to increased ILC3 numbers, activation and proliferation, and mice lacking HIF1α in RORγt-expressing cells, which includes ILC3s, had reduced ILC3 cytokine production and increased susceptibility to Clostridioides difficile infection41. In addition, deletion of acetyl-CoA carboxylase 1, which converts acetyl-CoA to malonyl-CoA, in RORγt+ ILCs results in decreased IL-22 production, intestinal barrier dysfunction and increased susceptibility to C. rodentium infection44.
γδ T cell metabolism
The metabolism of γδ T cells is poorly described compared to that of αβ T cells (Fig. 2E). One study found that the fuel preferences and metabolic pathways engaged differed between γδIFN and γδ17 cells, with γδIFN cells relying primarily on glycolytic metabolism, while γδ17 cells preferentially utilising mitochondrial and lipid metabolism45. This metabolic dichotomy was established within the thymus during development, and was maintained when mature γδ T cells reached their tissue location. In the peripheral lymph nodes, γδ17 cells have significantly higher mitochondrial mass and membrane potential, while γδIFN cells have increased glycolysis-related genes and cMyc expression. In addition to their dependence on mitochondrial metabolism, γδ17 cells have increased lipid content and increased lipid uptake compared to γδIFN cells. Increased lipids from high fat diet or the lipid-rich B16 melanoma tumour microenvironment supported the expansion of γδ17 cells but not γδIFN cells45. In humans, this dichotomy has not been studied, but glutamine metabolism was shown to be essential for the production of IL-17A by activated γδ T cells46. These findings highlight that γδ T cells adopt distinct metabolic configurations that correspond to discrete effector functions.
iNKT cell metabolism
It has been shown that the metabolic regulators, mTORC1 and cMyc, are important for iNKT cell development and proliferation within the thymus, but persistent mTORC1 activation inhibits iNKT cell development47. Glucose and glutamine are both important fuels for iNKT cell development, homeostasis and function, with glucose supporting TCR recycling and IFNγ production in splenic and hepatic iNKT cells48, and with glutamine fuelling the TCA cycle via glutaminolysis to maintain redox balance and glycosylation processes through the hexosamine biosynthesis pathway49 (Fig. 2F). Lipid biosynthesis mediated by the transcriptional regulators, PPARy, Srebf1 and PLZF, is also an important process in iNKT cells50. Furthermore, depending on their locations, tissue-resident iNKT cells exhibit specific transcriptional programmes that adapt cellular metabolism to the local environment. For instance, splenic and hepatic iNKT cells have similar glycolytic metabolic profiles driven by increased PKM2 expression, with inhibition or deletion of PKM2 impairing the responses of iNKT cells in these tissues. By contrast, adipose-resident iNKT cells rely on AMPK signalling and use FA β-oxidation to support their function and maintain adipose tissue homeostasis51. Indeed, deletion of AMPK or inhibition of β-oxidation impairs adipose iNKT cell, but not splenic iNKT cells functional outputs51. These studies highlight the importance of investigating the metabolism of iNKT cells and immune cells within their tissue niche.
Potential pitfalls when studying metabolism in cultured immune cells
Low cell number is one of the major limiting factors in analysing the metabolism of rare immune cell populations. While expansion of cells in culture is one approach to generate greater cell numbers, there is a recent appreciation that in vitro culture of immune cells can substantially alter their metabolism. Indeed, metabolic readings generated using cells cultured in vitro are very different to the metabolic features of that cell within the in vivo niche52–54. It is important to note that traditional cell culture mediums currently being used were formulated more than 50 years ago. These mediums were not developed to be physiologically relevant; instead, they were created to produce large amounts of cells with low variability. Hence, it is unsurprising that these mediums poorly represent metabolite concentrations in vivo. First established by Kaymak et al., Van Andel Institute-modified Iscove’s medium (VIM) has adjusted the concentrations of glucose and glutamine to better reflect physiological conditions. In addition, nutrients not normally found in traditional medium but present in murine serum are added to provide a more physiological method for culturing cells55. Nevertheless, even with the improvement made with VIM media, in vitro culture systems cannot fully recapitulate nutrient availability within the tissue, nor other parameters within the in vivo niche including oxygen levels and waste products.
The disparities between in vivo niches and in vitro culture conditions were first documented in tumour cells using in vivo infusion of 13C-labelled metabolites52–54. With this in mind, there has been a concerted effort to study the metabolism of immune cells in vivo using similar isotope labelling approaches. For instance, Ma et al. have compared CD8 T cells in vitro and in vivo following activation by the gram-positive bacteria Listeria monocytogenes56,57. While in vitro activated effector T cells were highly glycolytic, in vivo activated CD8 cells showed greater rates of oxidative metabolism, increased pyruvate entry into the TCA cycle as citrate, and the flow of 13C-glucose carbon was diverted to anabolic pathways such as serine and nucleotide biosynthesis56. Using 13C-labelled glutamine and acetate, it was shown that the metabolic dependencies of in vivo activated T cells change over time to support pyrimidine synthesis and ATP production, with acetate becoming a key fuel to support oxidative metabolism in T cells at later timepoints in the course of infection57. Together, these studies highlight that studying T cells metabolism within the in vivo niche is critical for truly understanding T cell function. It should be noted that several complementary methods, including proteomics and metabolomic approaches, were used in these studies in addition to stable isotope-tracing. Thus, a combination of various experimental techniques may be necessary to help create a comprehensive understanding of the metabolic needs of immune cells in vivo. However, the experimental setup for in vivo isotope-tracing experiments is complex, and this limits the scope of experimentation that can be performed and restricts these experimental approaches to specialised research groups.
Advocating for single-cell metabolic analyses
While techniques are continually improving to better probe the metabolism of rare immune cell populations, our understanding of these processes is still lacking. As outlined above, this is partly due to cell number issues and the influence of non-physiological medium. Moreover, it is important to consider the tissue-specific niches where these cells reside, as nutrient and metabolite content can be altered by locations of immune cells relative to the tissue vasculature53,58, disease states such as diabetes or obesity, or physiological states such as fasting, feeding and exercise59. Therefore, it is key that new techniques are developed to accurately measure the metabolism of rare immune cell populations ex vivo immediately after tissue digestion, or ideally within their tissue niche to avoid any potential artifacts created during the digestion process.
Single-cell analysis of metabolic features in rare immune cell populations
While technologies are currently available for, or are rapidly evolving towards, measuring metabolic features of immune cells with single-cell resolution, a number of challenges still exist due to the inaccessibility and intricacy of some single-cell methods. Thus, there is a need to develop approaches accessible-to-all to study metabolism in individual immune cells. Methodologies with such a potential are highlighted below.
Single-cell RNA sequencing
Single-cell RNA Sequencing (scRNA-seq) has revolutionised the study of rare immune cell populations by identifying gene expression profiles at the single-cell level (see ref. 60 for scRNA-seq best practices and guidelines). However, scRNA-seq has significant limitations as a technique to study the metabolism of single cells. While scRNA-seq can unveil a considerable amount of transcriptional information, there is often a poor correlation between transcriptomes and proteomes61,62. mRNA levels can provide insights into a cell’s metabolic configuration but do not accurately inform cellular metabolism, as proteins and enzymes are responsible for driving the metabolic flux, yet mRNA levels do not reliably reflect protein levels or enzymatic activity61,62.
Despite the caveats of scRNA-seq as a method to study the metabolism of single immune cells, this technique has provided some of the first datasets with insights into the metabolism of NK cells in human tissues. Netskar et al. generated a reference map of healthy blood- and tissue-derived NK cells and then incorporated data from publicly available datasets to state the differences induced by the tumour microenvironment of seven solid tumours. Interrogating these mRNA datasets revealed some interesting differences in the metabolic features of NK cells in different types of tumours that would be interesting to confirm using additional metabolic analyses63.
That said, advances in scRNA-seq technologies are increasing the value of scRNA-seq as a method to survey the metabolic configurations of tissue-resident immune cells. A modification of scRNA-seq, CITE-seq, uses oligonucleotide barcode-tagged antibodies to label surface epitopes on cells for quantification of protein levels64. Initially used to provide greater confidence in immune cell subset identification, CITE-seq could be adapted to quantify the expression of metabolic proteins, such as nutrient transporters, on the cell surface thus allowing the correlation of cellular transcriptomes with the protein expression of key metabolic machinery. However, one caveat of CITE-seq is that quantifying intracellular metabolic proteins and enzymes remains challenging as cell fixation is required, causing technical difficulties for RNA sequencing.
Additional advances are unlocking ability to analyse the transcriptome of immune cells in space and time. Until recently, scRNA-seq was limited to capturing static gene expression and lacked temporal aspects. A study from Kirschenbaum et al. defined a new technology for scRNA-seq, coined “Zman-seq”, which utilised in vivo labelling methods (“time-stamping”) to resolve scRNA-seq data for NK cells based on the duration that they were exposed to the tumour microenvironment65. These “time-stamping” techniques can be further integrated to other flow cytometry-based techniques as outlined in Figs. 3 and 4. A recent study utilised spatial CITE-seq to profile more than 180 proteins and associated single-cell transcriptomes in mouse (spleen, intestine and kidney) and human (spleen, tonsil, thymus and skin) tissue slides66. Taken together, quantifying metabolic proteins, determining the time spent within a given tissue, and understanding the spatial location of an immune cell with a tissue slice should all significantly increase the utility and versatility of scRNA-seq for studying the metabolism of tissue-resident innate lymphocytes.
Fig. 3. Overview of current flow cytometry techniques available to probe cellular metabolism.
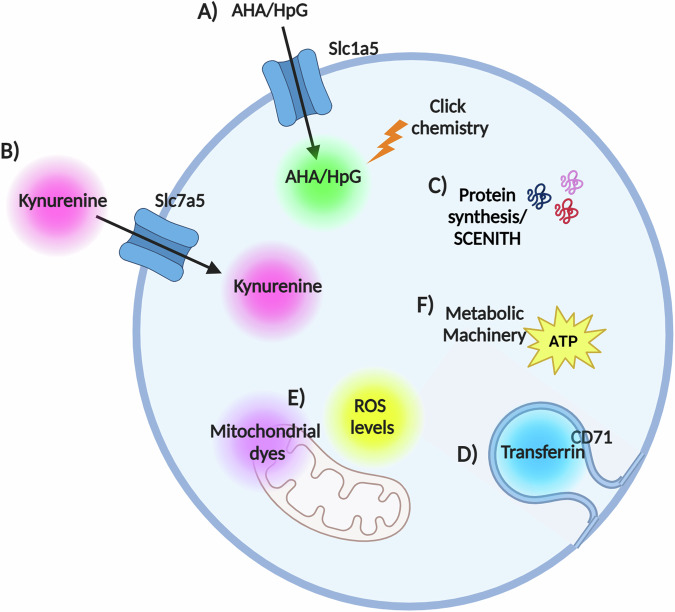
A Glutamine (Q) uptake assay with single-cell resolution (QUAS-R); bioorthogonal amino acids l-azidohomoalanine (AHA) and l-homopropargylglycine (HPG) are used to quantify amino acid transport through Slc1a5. B Kynurenine is a naturally fluorescence cargo for Large neutral amino acid (LAT1-4) transporters and is used to measure amino acid uptake through these transporters. In many lymphocytes, LAT1 (Slc7a5/Slc3a2 heterodimer) is the only LAT isoform expressed and so kynurenine uptake quantifies Slc7a5 mediated amino acid uptake. C SCENITH estimates single-cell energetic metabolism by profiling translation inhibition. Puromycin incorporation is used to quantify protein translation rates and to probe energy metabolism. D Iron–transferrin uptake is measured using a fluorescently tagged transferrin molecule. E Mitochondrial dyes can be used to measure mitochondrial mass, mitochondrial ROS and polarisation across the mitochondrial inner membrane. F METFLOW is a high-parameter flow cytometry method utilising antibodies against metabolic proteins; that probes the capacity of a cell to engage different metabolic pathways. Essential to the accurate interpretation of these assays are robust controls; for instance, competition controls are imperative for QUAS-R and Slc7a5 uptake analysis. Protocol A is compatible with protocol B, and protocol C. Further effort is being made to integrate the remaining parameters. This figure was created in BioRender. Finlay (2024) https://BioRender.com/d06l575.
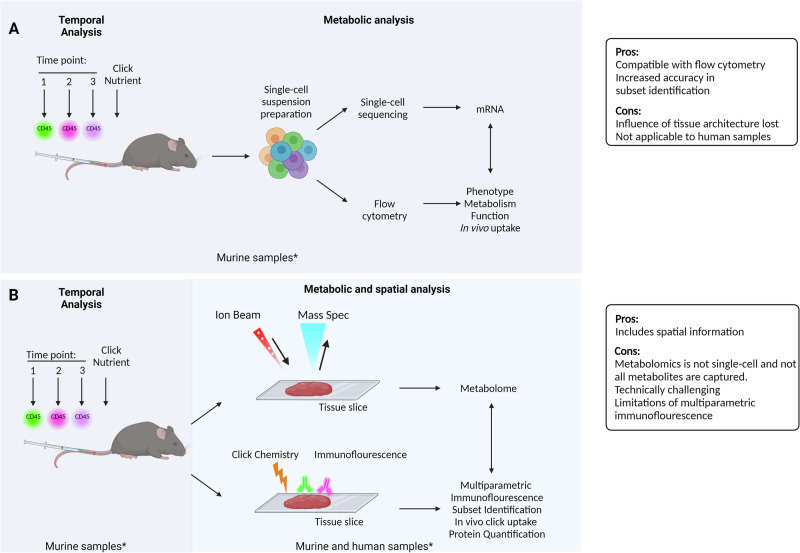
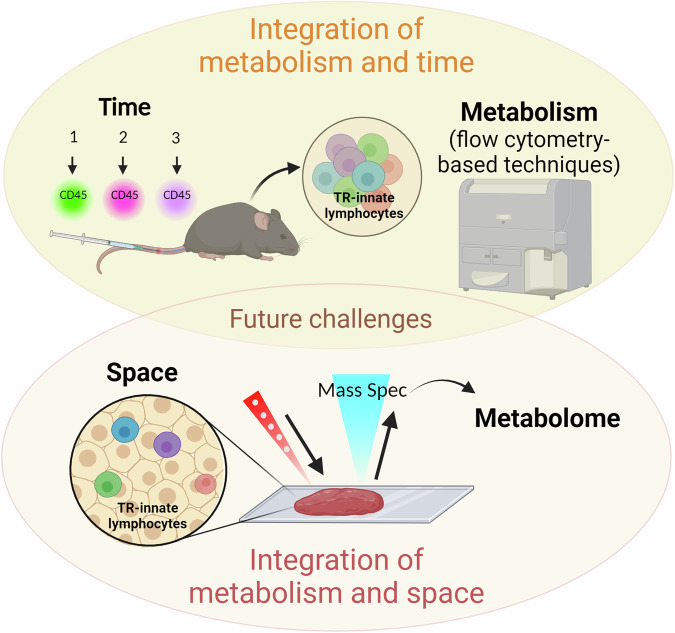
Fig. 4. Potential mechanisms for integration of techniques discussed.
A Temporal analysis of immunometabolism. In mice, time-stamping and in vivo nutrient uptake might be combined to gain understanding of how the metabolism of tissue-resident innate lymphocytes changes with the length of time they are within a tissue. With this format, flow cytometry and scRNA-seq platforms can be integrated to gain insight into the transcriptome and metabolic features, including in vivo uptake, of immune cells of interest and correlate this with cellular location at the time of sample retrieval. While spatial information is mostly lost in this approach, the in vivo uptake data will reflect whether a given cell had access to the click-nutrient and this will be impacted by the tissue architecture and competition for the click-nutrient between cells. B Spatial and temporal analysis of immunometabolism. This approach relies on the use of tissue sections where adjacent sections are processed for spatial metabolomics (top) or multiparametric immunofluorescence (bottom). In human samples this approach might be used to generate information about the metabolome of the tissue section and how spatial metabolite levels relate to the location of innate lymphocytes, identified using immunofluorescence in an adjacent tissue section. With advances in multiparametric immunofluorescence, metabolic features of innate lymphocytes could be measured using antibodies specific to metabolic machinery (nutrient transporters, enzymes) or metabolic signalling molecules (such as mTORC1 signalling components). In mice, the time-stamping and in vivo nutrient uptake approaches described in (A) could be added to the protocol, though this would add additional complexity to the multiparametric immunofluorescence. There are significant challenges involved in optimising such an experimental setup. This figure was created in BioRender. Finlay (2024) https://BioRender.com/b20j926.
Towards single-cell proteomics
Understanding protein expression data is a step closer to understanding metabolic processes within immune cells. High-resolution quantitative proteomics has been used to provide insights into the metabolism of immune cells in numerous studies, and has also been used to measure the copy numbers for greater than 7000 proteins62,67–69. Through this technique, it was demonstrated that cMyc is a key regulator in murine T cell function, controlling T cell activation and metabolic reprogramming67. Moreover, this technique was also used to describe the effect of gut microenvironment on tissue-resident intestinal intraepithelial T lymphocytes (T-IEL). Here, it was demonstrated that T-IELs have increased expression of cell surface receptors involved in epithelial interactions and upregulated cholesterol and lipid metabolic pathways in comparison to CD8 T cells isolated from lymph nodes69. In these studies ~106 cells were used per sample, numbers that cannot reasonably be isolated for most tissue-resident innate lymphocytes.
Only recently are technological advances allowing the identification and quantification of proteins by mass spectrometry in a single cell. Bennett et al. intensely reviewed the state of art for these techniques, including the challenges faced, the technological advancements, and future development for the field70. Initial studies took advantage of larger cells with abundant protein content, such as neurons and oocytes71–73, or cell lines74,75. However, immune cells, particularly lymphocytes, present a particular challenge as they are small cells with low protein content76. The work of Petelski et al. described the Single Cell Proteomic (SCoPE2) protocol in which cells are isolated by sorting into multiwell plates and require minimal sample preparation, an approach that might be applied to investigate tissue-resident cell metabolism as the sensitivity of mass spectrometers advance74. Additionally, the platform described by Keren et al., called MIBI-TOF, integrates a mass spectrometer for multiplexed ion beam imaging with orthogonal acceleration time-of-flight. With that, they could profile the subcellular expression and localisation of 36 elementally labelled antibodies to probe phenotypical and metabolic proteins (including HIF1α, IDO-1 and phosphorylated S6 ribosomal protein), as well as endogenous elements, such as iron, in human tissue biopsies77. At present, while proteomics cannot deliver on single-cell proteomes for tissue-resident innate lymphocytes, recent technology advances could provide high-resolution quantitative data for bulk-sorted immune cells using very low cell numbers such as 102–103 cells, which is within range of what can be obtained for innate lymphocytes populations from a single tissue (personal communication). This approach would allow for a robust analysis of the metabolic proteome of tissue-resident innate lymphocytes but would not capture the metabolic heterogeneity of these cells within a tissue.
Towards single-cell metabolomics
Compared to mRNA and protein abundance, quantifying metabolites levels is a more accurate indicator of the metabolic processes in a cell. High-sensitivity bulk metabolomics has proven to be a crucial technique in exploring immune cell metabolism, providing insights into alterations in metabolite levels and metabolic pathways. Through this technology, the researcher can identify metabolic signatures of immune cells both basally and in various disease states. For example, Lozada et al. revealed differences in the metabolic features of human NK cells from individuals previously infected with Cytomegalovirus (CMV), demonstrating increased intermediates of the pentose phosphate pathway30. Using stable isotopic labelling methods, it is possible to trace the flux of different atoms through metabolic pathways in immune cell populations. For example, using 13C labelled glucose, Assmann et al. demonstrated that cytokine-activated murine NK cells use a glucose-fuelled non-canonical TCA cycle called the citrate–malate shuttle to support mitochondrial energy production28. It is also important to consider that standard bulk metabolomics analysis requires pure cell populations typically achieved by procedures including magnetic bead sorting or fluorescence-activated cell sorting (FACS), but this is complicated by the fact that sheath fluid from a FACS sorting can significantly impact the cellular metabolome due to the depletion of cellular metabolites78. In addition, these approaches typically require a large number of cells, making adopting these approaches challenging, if not impossible, on rare immune populations such as tissue-resident innate lymphocytes.
Methodologies are rapidly advancing to meet the challenges of measuring the wide range of metabolite species in low sample volumes and even in single cells79. The work of Schönberger et al. described a workflow to analyse intracellular metabolites from a small number of cells by FACS-sorting them directly into the extraction buffer. They could detect six metabolites above the background level in as little as 100 B cells and up to 80 metabolites in 5000 cells80. Indeed, single-cell metabolomic platforms would be a powerful tool that would not only unlock the metabolism of tissue-resident innate lymphocytes but also reveal the metabolic heterogeneity within these immune cells populations. However, a number of challenges remain before the promise of single-cell metabolomics can be fully realised including the misidentification and annotation of metabolites. Many metabolites lack reference standards and there is an aspect of uncertainty in metabolite identification, especially in untargeted metabolomics studies, where 10s–1000s of metabolites are considered of biological significance and need to be identified81, thus making the correct identification and quantification difficult. A recent comment published in Nature Metabolism highlighted some of these issues81.
Mass spectrometry imaging using technologies including matrix-assisted laser desorption/ionisation (MALDI), desorption electrospray ionisation (DESI), and secondary ion mass spectrometry (SIMS) has the potential to be a powerful tool in studying lymphocytes within their tissue niche82. Mass spectrometry imaging can be applied to derive metabolite information with 10–100 μm resolution. Rappez et al. report a method called SpaceM that integrates MALDI-based metabolomics and fluorescence imaging to identify >100 different metabolites in individual cells83. Another method developed by Yuan et al. integrated metabolite features generated by time-of-flight secondary ion mass spectrometry (TOF-SIMS) and H&E staining with computational methods to explore the spatial metabolome and tissue anatomy at single-cell level84. However, this approach cannot identify the specific tissue-resident immune cells within a given tissue. Ganesh et al. reported a method that used an isotope-tagged antibody library to achieve cell type-specific, three-dimensional spatially resolved metabolomic profiling framework (3D-SMF), with which they identified metabolite features of immune cells in human tonsil samples85. Recently, Hu et al. reported the Single-Cell SPAtially resolved METabolic (scSpaMet) framework that combines 3D-SMF with multiplexing proteomic imaging mass spectrometry to generate a single-cell map with >200 metabolites and 25 protein markers per cell, and they showed cell type-specific local metabolic competition in lung cancer tissues and human tonsils86.
Regardless of these advances, challenges still remain for spatial mass spectrometry imaging metabolomics to generate more detailed metabolic maps with greater sensitivity and depth of coverage of diverse cellular metabolites while accurately resolving for individual immune cells within spatially crowded tissues sections. Therefore, the potential of these technologies to revolve the immunometabolism of rare tissue-resident immune cells has not yet been realised.
Flow cytometry-based metabolic analyses
Flow cytometry is arguably the most valuable tool for studying cellular metabolism of rare tissue-resident population for a number of reasons. First, flow cytometry is the preferred method used by immunologists, so flow cytometry-based approaches are essentially accessible to all researchers studying immunometabolism. Second, by allowing multiple parameters to be analysed simultaneously, including functional and phenotypical features, flow cytometry is the workhorse of those studying rare immune cell populations. Additionally, flow cytometry platforms have the capacity to provide information for immune cells ex vivo with minimal cell manipulation or processing. Advanced flow technologies including time-of-flight mass spectrometry and heavy metal-labelled antibodies (mass cytometry) and fluorescent spectral flow cytometry analysers now allow for detection of over 40 parameters87. While with less throughput, imaging flow cytometry can now provide single-cell information with subcellular spatial information. Below we describe the flow cytometry-based assays of single-cell metabolic flux that can be applied to rare immune subsets, something that cannot be achieved by scRNA-seq, proteomics or metabolomics techniques.
SCENITH
A method developed by Argüello et al.88, SCENITH adapts the fact that nearly half of a mammalian cells energy, in the form of ATP, is consumed by the process of protein translation89–91. Measuring rates of translation can thus serve as a proxy for the abundance of ATP in the cell. Puromycin is incorporated into nascent polypeptides in a way that correlates to the rates of translation. The abundance of puromycin in nascent polypeptides can then be quantified using a fluorescent anti-puromycin antibody and flow cytometric analysis. Additionally, SCENITH probes which metabolic pathways, such as glycolysis or OXPHOS, contribute to ATP production in each individual cell. In this regard, a given biological sample is divided and treated with or without metabolic inhibitors that target glycolysis (2-deoxyglucose), or mitochondrial ATP synthesis (oligomycin). The effects of individual metabolic inhibitor, or set of inhibitors, on puromycin incorporation then allows the researchers to infer which metabolic pathways are used by each cell to produce ATP. For example, Corral et al. employed SCENITH to demonstrate that the differentiation of an ILC precursor to ILC1-like cells in the lung is associated with the metabolic reprogramming towards glycolysis36. Importantly, this technique is compatible with staining for intracellular markers that are essential for the correct characterisation of some subsets of tissue-resident lymphocytes. Increasing number of studies have utilised this technique, though there is a lot to be explored45,92–94. For example, SCENITH could be adapted to use low-dose of etomoxir, an inhibitor of CPT1A to probe β-oxidation in a given cell, as showed by Schimmer et al. for NK cells33. Alternatively, an inhibitor of ACLY, such as SB204990, could be used to investigate how the citrate–malate shuttle (a non-canonical TCA cycle) contributes to ATP production. Lastly, click chemistry-compatible analogues of puromycin, such as o-propargyl-puromycin, can also be used for the SCENITH method and have potential for reducing non-specific signals on account of the highly specific nature of bioorthogonal chemical reactions95.
Nutrient uptake assays
Click chemistry is a framework for performing highly specific chemical reactions between two chemical groups, not found in nature, within the complex chemical environment of the cell. The work leading the development of this technology was awarded with the Nobel Prize in Chemistry in 2022. Click chemistry has recently been utilised to study single-cell nutrient uptake by immune cells96. The work of Pelgrom et al. demonstrated that two bioorthogonal amino acids are transported by the glutamine transporter Slc1a5. These amino acid analogues have a small modification that introduces a functional chemical group that can react with other non-native compounds by click chemistry. Fluorophores that contain the click-reactive group are commercially available and the reaction will snap the amino acid and fluorophore together. Attaching the fluorophore after the glutamine analogue has been transported into the cells provides a fluorescent signal that is quantified by flow cytometry to give Slc1a5 activity in each individual cell. The study demonstrated the applicability of the assay both ex vivo and in vivo, which makes it a valuable technique in the analysis of tissue-resident cells. Slc1a5 is the predominant glutamine transporter in many immune cells97 and analysing its role in different contexts such as tissues and diseases will be pivotal in future studies.
Similarly, the activity of another amino acid transporter Slc7a5, responsible for transporting large neutral amino acids into cells, can also be probed by flow cytometry. In 2018, Sinclair et al. demonstrated that the fluorescent properties of kynurenine could be employed to quantitatively measure the activity of Slc7a5 in T cells98. Slc7a5 is an important transporter in many immune cells through providing amino acids into the cell but also for supporting anabolic signal transduction through mTORC1 and cMyc29. This kynurenine-based uptake assay has been employed to quantify Slc7a5 activity in a wide array of immune cells and immune contexts.
Finally, these uptake assays are not limited to amino acids either. Iron exists in the body bound to transferrin and the uptake of iron-bounded transferrin is mediated by the CD71 receptor99. Through the use of a fluorescent transferrin conjugate, endocytosis through CD71 can be investigated. This technique was used to demonstrate the importance of iron uptake into NK cells during acute retroviral responses32.
Metabolic machinery
Another flow cytometry-based approach to analyse the metabolism of immune cells is to quantify the metabolic machinery. Both Ahl et al.100 and Hartmann et al.101 described methods that combine a range of antibodies against metabolite transporters, metabolic enzymes and signalling molecules to probe human immune cell metabolism101. These approaches used standard or mass cytometry, respectively, and showed dynamic metabolic states across immune cell populations. Others have then successfully applied the techniques for mouse studies102,103.
Mitochondria
A core component of the metabolic machinery within a cell are the mitochondria, and mitochondrial dynamics are crucial for sustaining immune cell function. A number of fluorescent mitochondrial dyes and transgenic mice make it possible to probe mitochondrial fitness by flow cytometry. Fluorescent mitochondrial dyes such as MitoTracker Green and tetramethylrhodamine methyl ester measure mitochondrial mass and membrane potential, respectively104. 10-N-nonyl acridine orange is another probe for mitochondrial mass because it has high affinity for cardiolipin, specific to the mitochondrial membrane105, while levels of mitochondrial reactive oxygen species can be measured using MitoSOX and similar dyes. Used in combination, these dyes can provide an overview of mitochondrial health within cells104,106. However, it should be noted that these parameters should be used with caution, as some off-target effects and toxicity have been reported for these mitochondrial dyes107. It is recommended to use appropriate controls and the lowest concentration possible when analysing these parameters.
Aside from these mitochondrial probes, a number of specialised transgenic mice have been generated to investigate mitochondrial health as well as the activity of mitophagy, the process of autophagic turnover of mitochondria. Mitophagy reporter mice express a mitochondrial-targeted pH sensitive fluorescent probe that changes its fluorescent output when the mito-autophagosome fuses with the lysosome at a late stage of mitophagy108. The PhAMfloxed mouse line facilitates the expression of a mitochondrially localised version of Dendra-2, a photo-convertible fluorescent protein109. This allows a more consistent probing of mitochondrial mass, compared to mitochondrial dyes, and has been applied by Aguiar et al. to study mitochondrial morphology in rare tissue-resident immune cells such as iNKT cells51. The photo-conversion properties of Dendra-2 also allow analysing the process of mitochondrial fusion. Lastly, Russo et al. described a transgenic mouse method called Single-cell Profiling and Imaging of Cell Energy Metabolism (SPICE-Met), which measures the intracellular ATP:ADP ratio through the expression of a fluorescent sensor110.
Cell–cell interactions
To date, studies on immunometabolism lack information on the impact of cell–cell interactions on metabolic pathways. An exciting new technique, called universal labelling immune partnerships by SorTagging intercellular contacts (uLIPSTIC), records cell–cell interactions based on the enzymatic transfer of a biotin-labelled substrate that can later be detected by flow cytometry111. The integration of this method with other flow-based metabolic assays could expand our knowledge on the importance of physical interactions between immune cell types for modulating the metabolic programming of cells.
In summary, the integration of the techniques discussed above can provide multiple dimensions of metabolic flux analysis in individual cells, especially those found in low frequency in tissues (Figs. 3 and 4). The association of temporal and metabolic analysis, for example, could be made by taking advantage of injecting labelled antibodies in mice at different timepoints and later analysing the metabolism of immune cells by flow cytometry-based techniques, such as SCENITH, nutrient uptake and/or mitochondrial probes. This would provide valuable information in different disease models but also on how immune cells adapt their metabolism to different tissues under homeostatic conditions. In this regard, the integration of spatial and metabolic analysis has already been demonstrated77, but still faces challenges on the range of metabolites that can be analysed, and may require adaptations to integrate some of the nutrient uptake analyses to be detected in tissue slides. Thus, flow cytometry-based metabolic analysis have the potential to generate a multidimensional ex vivo metabolic footprint of individual immune cells and reveal new insights into the immune heterogeneity within and between tissues, in homeostasis and disease states.
Conclusions and future perspectives
While studying tissue-resident immune cell metabolism is now achievable, there are several challenges remaining. Glucose metabolism is important for most immune cells, but there are no accurate flow cytometry-based techniques to define changes in glucose uptake and use within immune cells. This challenge is compounded by the continued erroneous use of fluorescent glucose analogue, 2-NBDG, as a measure of glucose uptake. Sinclair et al. provide conclusive proof that 2-NBDG is not transported by the glucose transporters expressed by the majority of immune cells, Slc2a1 and Slc2a3112, likely due to the attachment of large fluorophores changing the physical and chemical properties of a nutrient and unsurprisingly the transport characteristics of that nutrient. Similar artifacts are true for BODIPY-associated lipids that are commonly used to analyse lipid uptake. A recent study, however, provides the promise of measuring glucose uptake by flow cytometry using a bioorthogonal glucose probe, though this approach still requires careful testing in immune cells113.
Species-specific differences on the study of immunometabolism present several challenges due to variations in physiology, genetics, developmental timing and immune system function114. There are notable differences in metabolic pathways and immune responses that can impact the relevance of findings in mice to human health. For instance, certain immune cell populations, such as macrophages, behave differently in mice compared to humans1, leading to divergent outcomes in disease studies. Additionally, the metabolic environments in which immune cells function can vary significantly between the two species, thereby affecting how immune responses are regulated. Some of the techniques described here, such as the Zman-seq, involves injecting anti-CD45 antibodies at different timepoints, and therefore cannot be directly translated to human studies. All together, these differences necessitate careful consideration when extrapolating mouse data to human conditions, or vice versa.
In summary, tissue-resident immune cells play an important role in maintaining tissue homeostasis and repair or influencing the development of diseases. The metabolic reprogramming of these cells is tightly connected to their function, but accurately measuring the metabolic parameters of these rare subsets remains extremely challenging. Therefore, it is imperative that we develop new strategies to explore the metabolic profile of these immune cells. In this review, we provided a comprehensive examination of current methodologies employed for the analysis of rare immune cell populations. Ideally, in the future, we will be able to integrate functional, metabolic, spatial and temporal data in the analysis of tissue-resident immune cells and advance the knowledge to develop strategies to manipulate these cells as a therapeutic approach (Fig. 5).
Fig. 5. Integration of single-cell metabolic assays.
Measuring immune cell metabolism with single-cell resolution will be crucial in unlocking the metabolic features of rare tissue-resident lymphocytes. Achieving this while retaining information about space, the tissue location of the cell, and probing the time spent in a given tissue will be another step towards understanding the true metabolism of these cells in situ within tissues. This figure was created in BioRender. Finlay (2024) https://BioRender.com/q29i709.
Acknowledgements
C.F.A. was supported by the European Commission via a MSCA Postdoctoral fellowship (Project 101108116). C.C. is funded by a Research Ireland PhD studentship (GOIPG/2022/081). D.K.F. is supported by Research Ireland (22/FFP-A/10326, IRCLA/2023/1402).
Author contributions
C.C. and C.F.A. wrote the manuscript and designed the figures. D.K.F. wrote and reviewed the manuscript. All authors revised and finalised the manuscript for publication.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Carrie Corkish, Cristhiane Favero Aguiar.
References
- 1.Vijayan, V. et al. Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide – a divergent role for glycolysis. Redox Biol.22, 101147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabogal-Guáqueta, A. M. et al. Species-specific metabolic reprogramming in human and mouse microglia during inflammatory pathway induction. Nat. Commun.14, 6454 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell174, 1054–1066 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Weizman, O.-E. et al. ILC1 confer early host protection at initial sites of viral infection. Cell171, 795–808.e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasteiger, G., Fan, X., Dikiy, S., Lee, S. Y. & Rudensky, A. Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science350, 981–985 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riggan, L., Freud, A. G. & O’Sullivan, T. E. True detective: unraveling group 1 innate lymphocyte heterogeneity. Trends Immunol.40, 909–921 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meininger, I. et al. Tissue-specific features of innate lymphoid cells. Trends Immunol.41, 902–917 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Pelletier, A. & Stockmann, C. The metabolic basis of ILC plasticity. Front. Immunol.13, 858051 (2022). [DOI] [PMC free article] [PubMed]
- 9.McFarland, A. P. et al. Multi-tissue single-cell analysis deconstructs the complex programs of mouse natural killer and type 1 innate lymphoid cells in tissues and circulation. Immunity54, 1320–1337.e4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster, I. S. et al. Infection induces tissue-resident memory NK cells that safeguard tissue health. Immunity56, 531–546.e6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torcellan, T. et al. Circulating NK cells establish tissue residency upon acute infection of skin and mediate accelerated effector responses to secondary infection. Immunity57, 124–140.e7 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbert, D. R., Douglas, B. & Zullo, K. Group 2 innate lymphoid cells (ILC2): type 2 immunity and Helminth immunity. Int. J. Mol. Sci.20, 2276 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartemes, K. R. & Kita, H. Roles of innate lymphoid cells (ILCs) in allergic diseases: the 10-year anniversary for ILC2s. J. Allergy Clin. Immunol.147, 1531–1547 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valle-Noguera, A., Ochoa-Ramos, A., Gomez-Sánchez, M. J. & Cruz-Adalia, A. Type 3 innate lymphoid cells as regulators of the host-pathogen interaction. Front. Immunol.12, 748851 (2021). [DOI] [PMC free article] [PubMed]
- 15.Ribot, J. C., Lopes, N. & Silva-Santos, B. γδ T cells in tissue physiology and surveillance. Nat. Rev. Immunol.21, 221–232 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Silva-Santos, B., Mensurado, S. & Coffelt, S. B. T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat. Rev. Cancer19, 392–404 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva-Santos, B., Serre, K. & Norell, H. γδ T cells in cancer. Nat. Rev. Immunol.15, 683–691 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Hu, Y. et al. γδ T cells: origin and fate, subsets, diseases and immunotherapy. Signal Transduct. Target. Ther.8, 1–38 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godfrey, D. I., Stankovic, S. & Baxter, A. G. Raising the NKT cell family. Nat. Immunol.11, 197–206 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Singh, A. K., Tripathi, P. & Cardell, S. L. Type II NKT cells: an elusive population with immunoregulatory properties. Front. Immunol. 9, 1969 (2018). [DOI] [PMC free article] [PubMed]
- 21.Crosby, C. M. & Kronenberg, M. Tissue-specific functions of invariant natural killer T cells. Nat. Rev. Immunol.18, 559–574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krovi, S. H. & Gapin, L. Invariant natural killer T cell subsets—more than just developmental intermediates. Front. Immunol.9, 1393 (2018). [DOI] [PMC free article] [PubMed]
- 23.LaMarche, N. M. et al. Distinct iNKT cell populations use IFNγ or ER stress-induced IL-10 to control adipose tissue homeostasis. Cell Metab.32, 243–258.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opasawatchai, A. & Matangkasombut, P. iNKT cells and their potential lipid ligands during viral infection. Front. Immunol.6, 378 (2015). [DOI] [PMC free article] [PubMed]
- 25.Krijgsman, D., Hokland, M. & Kuppen, P. J. K. The role of natural killer T cells in cancer—a phenotypical and functional approach. Front. Immunol.9, 367 (2018). [DOI] [PMC free article] [PubMed]
- 26.O’Brien, K. L. & Finlay, D. K. Immunometabolism and natural killer cell responses. Nat. Rev. Immunol.19, 282–290 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Sohn, H. & Cooper, M. A. Metabolic regulation of NK cell function: implications for immunotherapy. Immunometabolism5, e00020 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assmann, N. et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat. Immunol.18, 1197–1206 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Loftus, R. M. et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun.9, 2341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozada, J. R., Zhang, B., Miller, J. S. & Cichocki, F. NK cells from human cytomegalovirus–seropositive individuals have a distinct metabolic profile that correlates with elevated mTOR signaling. J. Immunol.211, 539–550 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mah, A. Y. et al. Glycolytic requirement for NK cell cytotoxicity and cytomegalovirus control. JCI Insight2, e95128 (2017). [DOI] [PMC free article] [PubMed]
- 32.Littwitz-Salomon, E. et al. Metabolic requirements of NK cells during the acute response against retroviral infection. Nat. Commun.12, 5376 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schimmer, S. et al. Fatty acids are crucial to fuel NK cells upon acute retrovirus infection. Front. Immunol.14, 1296355 (2023). [DOI] [PMC free article] [PubMed]
- 34.Sheppard, S. et al. Fatty acid oxidation fuels natural killer cell responses against infection and cancer. Proc. Natl. Acad. Sci. USA121, e2319254121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gury-BenAri, M. et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell166, 1231–1246.e13 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Corral, D. et al. ILC precursors differentiate into metabolically distinct ILC1-like cells during mycobacterium tuberculosis infection. Cell Rep.39, 110715 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surace, L. et al. Dichotomous metabolic networks govern human ILC2 proliferation and function. Nat. Immunol.22, 1367–1374 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilhelm, C. et al. Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. J. Exp. Med.213, 1409–1418 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karagiannis, F. et al. Lipid-droplet formation drives pathogenic group 2 innate lymphoid cells in airway inflammation. Immunity52, 620–634.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Monticelli, L. A. et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat. Immunol.17, 656–665 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fachi, J. L. et al. Hypoxia enhances ILC3 responses through HIF-1α-dependent mechanism. Mucosal Immunol.14, 828–841 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Luccia, B., Gilfillan, S., Cella, M., Colonna, M. & Huang, S. C.-C. ILC3s integrate glycolysis and mitochondrial production of reactive oxygen species to fulfill activation demands. J. Exp. Med.216, 2231–2241 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goverse, G. et al. Vitamin A controls the presence of RORγ+ innate lymphoid cells and lymphoid tissue in the small intestine. J. Immunol.196, 5148–5155 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Mamareli, P. et al. Targeting cellular fatty acid synthesis limits T helper and innate lymphoid cell function during intestinal inflammation and infection. Mucosal Immunol.14, 164–176 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Lopes, N. et al. Distinct metabolic programs established in the thymus control effector functions of γδ T cell subsets in tumor microenvironments. Nat. Immunol.22, 179–192 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li, G., Liu, L., Yin, Z., Ye, Z. & Shen, N. Glutamine metabolism is essential for the production of IL-17A in γδ T cells and skin inflammation. Tissue Cell71, 101569 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Yarosz, E. L., Chang, C.-H. & Kumar, A. Metabolism in invariant natural killer T cells: an overview. Immunometabolism3, e210010 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu, S. et al. Immunometabolism regulates TCR recycling and iNKT cell functions. Sci. Signal.12, eaau1788 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Kumar, A. et al. NKT cells adopt a glutamine-addicted phenotype to regulate their homeostasis and function. Cell Rep.41, 111516 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu, S. et al. Impaired lipid biosynthesis hinders anti-tumor efficacy of intratumoral iNKT cells. Nat. Commun.11, 438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aguiar, C. F. et al. Tissue-specific metabolic profile drives iNKT cell function during obesity and liver injury. Cell Rep.42, 112035 (2023). [DOI] [PubMed] [Google Scholar]
- 52.Davidson, S. M. et al. Environment impacts the metabolic dependencies of ras-driven non-small cell lung cancer. Cell Metab.23, 517–528 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell164, 681–694 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartman, C. R. et al. Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature614, 349–357 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaymak, I. et al. Carbon source availability drives nutrient utilization in CD8+ T cells. Cell Metab.34, 1298–1311.e6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma, E. H. et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8+ T cells. Immunity51, 856–870.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Ma, E. H. et al. 13C metabolite tracing reveals glutamine and acetate as critical in vivo fuels for CD8 T cells. Sci. Adv.10, eadj1431 (2024). [DOI] [PMC free article] [PubMed]
- 58.Lane, A. N., Higashi, R. M. & Fan, T. W.-M. Metabolic reprogramming in tumors: contributions of the tumor microenvironment. Genes Dis.7, 185–198 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaspers, R. T. et al. Exercise, fasting, and mimetics: toward beneficial combinations? FASEB J.31, 14–28 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Heumos, L. et al. Best practices for single-cell analysis across modalities. Nat. Rev. Genet.24, 550–572 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cuadrado, E. et al. Proteomic analyses of human regulatory T cells reveal adaptations in signaling pathways that protect cellular identity. Immunity48, 1046–1059.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Hukelmann, J. L. et al. The cytotoxic T cell proteome and its shaping by the kinase mTOR. Nat. Immunol.17, 104–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Netskar, H. et al. Pan-cancer profiling of tumor-infiltrating natural killer cells through transcriptional reference mapping. Nat. Immunol.25, 1445–1459 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods14, 865–868 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirschenbaum, D. et al. Time-resolved single-cell transcriptomics defines immune trajectories in glioblastoma. Cell187, 149–165.e23 (2024). [DOI] [PubMed] [Google Scholar]
- 66.Liu, Y. et al. High-plex protein and whole transcriptome co-mapping at cellular resolution with spatial CITE-seq. Nat. Biotechnol.41, 1405–1409 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marchingo, J. M., Sinclair, L. V., Howden, A. J. & Cantrell, D. A. Quantitative analysis of how Myc controls T cell proteomes and metabolic pathways during T cell activation. Elife9, e53725 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howden, A. J. M. et al. Quantitative analysis of T cell proteomes and environmental sensors during T cell differentiation. Nat. Immunol.20, 1542–1554 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brenes, A. J. et al. Tissue environment, not ontogeny, defines murine intestinal intraepithelial T lymphocytes. Elife10, e70055 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bennett, H. M., Stephenson, W., Rose, C. M. & Darmanis, S. Single-cell proteomics enabled by next-generation sequencing or mass spectrometry. Nat. Methods20, 363–374 (2023). [DOI] [PubMed] [Google Scholar]
- 71.Nemes, P., Rubakhin, S. S., Aerts, J. T. & Sweedler, J. V. Qualitative and quantitative metabolomic investigation of single neurons by capillary electrophoresis electrospray ionization mass spectrometry. Nat. Protoc.8, 783–799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Virant-Klun, I., Leicht, S., Hughes, C. & Krijgsveld, J. Identification of maturation-specific proteins by single-cell proteomics of human oocytes. Mol. Cell. Proteom.15, 2616–2627 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubakhin, S. S., Romanova, E. V., Nemes, P. & Sweedler, J. V. Profiling metabolites and peptides in single cells. Nat. Methods8, S20–S29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petelski, A. A. et al. Multiplexed single-cell proteomics using SCoPE2. Nat. Protoc.16, 5398–5425 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Specht, H. et al. Single-cell proteomic and transcriptomic analysis of macrophage heterogeneity using SCoPE2. Genome Biol.22, 50 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lanz, M. C., Fuentes Valenzuela, L., Elias, J. E. & Skotheim, J. M. Cell size contributes to single-cell proteome variation. J. Proteome Res.22, 3773–3779 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keren, L. et al. MIBI-TOF: a multiplexed imaging platform relates cellular phenotypes and tissue structure. Sci. Adv.5, eaax5851 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryan, K., Rose, R. E., Jones, D. R. & Lopez, P. A. Sheath fluid impacts the depletion of cellular metabolites in cells afflicted by sorting induced cellular stress (SICS). Cytom. Part A99, 921–929 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lanekoff, I., Sharma, V. V. & Marques, C. Single-cell metabolomics: where are we and where are we going? Curr. Opin. Biotechnol.75, 102693 (2022). [DOI] [PubMed] [Google Scholar]
- 80.Schönberger, K. et al. LC-MS-based targeted metabolomics for FACS-purified rare cells. Anal. Chem.95, 4325–4334 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koistinen, V. et al. Towards a Rosetta stone for metabolomics: recommendations to overcome inconsistent metabolite nomenclature. Nat. Metab.5, 351–354 (2023). [DOI] [PubMed] [Google Scholar]
- 82.Vickerman, J. C. Molecular imaging and depth profiling by mass spectrometry-SIMS, MALDI or DESI? Analyst136, 2199–2217 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Rappez, L. et al. SpaceM reveals metabolic states of single cells. Nat. Methods18, 799–805 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuan, Z. et al. SEAM is a spatial single nuclear metabolomics method for dissecting tissue microenvironment. Nat. Methods18, 1223–1232 (2021). [DOI] [PubMed] [Google Scholar]
- 85.Ganesh, S. et al. Spatially resolved 3D metabolomic profiling in tissues. Sci. Adv.7, eabd0957 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu, T. et al. Single-cell spatial metabolomics with cell-type specific protein profiling for tissue systems biology. Nat. Commun.14, 8260 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sahir, F., Mateo, J. M., Steinhoff, M. & Siveen, K. S. Development of a 43 color panel for the characterization of conventional and unconventional T-cell subsets, B cells, NK cells, monocytes, dendritic cells, and innate lymphoid cells using spectral flow cytometry. Cytom. Part A10.1002/cyto.a.24288. (2020). [DOI] [PMC free article] [PubMed]
- 88.Argüello, R. J. et al. SCENITH: a flow cytometry based method to functionally profile energy metabolism with single cell resolution. Cell Metab.32, 1063–1075.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buttgereit, F. & Brand, M. D. A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J.312, 163–167 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schimmel, P. GTP hydrolysis in protein synthesis: two for Tu? Science259, 1264–1265 (1993). [DOI] [PubMed] [Google Scholar]
- 91.Lindqvist, L. M., Tandoc, K., Topisirovic, I. & Furic, L. Cross-talk between protein synthesis, energy metabolism and autophagy in cancer. Curr. Opin. Genet. Dev.48, 104–111 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tagliatti, E. et al. Trem2 expression in microglia is required to maintain normal neuronal bioenergetics during development. Immunity57, 86–105.e9 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ibusuki, A., Kawai, K., Nitahara-Takeuchi, A., Argüello, R. J. & Kanekura, T. TCR signaling and cellular metabolism regulate the capacity of murine epidermal γδ T cells to rapidly produce IL-13 but not IFN-γ. Front. Immunol. 15, 1361139 (2024). [DOI] [PMC free article] [PubMed]
- 94.Adamik, J. et al. Distinct metabolic states guide maturation of inflammatory and tolerogenic dendritic cells. Nat. Commun.13, 5184 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scinto, S. L. et al. Bioorthogonal chemistry. Nat. Rev. Methods Prim.1, 30 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pelgrom, L. R. et al. QUAS-R: an SLC1A5-mediated glutamine uptake assay with single-cell resolution reveals metabolic heterogeneity with immune populations. Cell Rep.42, 112828 (2023). [DOI] [PubMed] [Google Scholar]
- 97.Brenes, A. J., Lamond, A. I. & Cantrell, D. A. The immunological proteome resource. Nat. Immunol.24, 731–731 (2023). [DOI] [PubMed] [Google Scholar]
- 98.Sinclair, L. V., Neyens, D., Ramsay, G., Taylor, P. M. & Cantrell, D. A. Single cell analysis of kynurenine and system L amino acid transport in T cells. Nat. Commun.9, 1981 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hentze, M. W., Muckenthaler, M. U. & Andrews, N. C. Balancing acts: molecular control of mammalian iron metabolism. Cell117, 285–297 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Ahl, P. J. et al. Met-Flow, a strategy for single-cell metabolic analysis highlights dynamic changes in immune subpopulations. Commun. Biol.3, 305 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hartmann, F. J. et al. Single-cell metabolic profiling of human cytotoxic T cells. Nat. Biotechnol.39, 186–197 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heieis, G. A. et al. Metabolic heterogeneity of tissue-resident macrophages in homeostasis and during helminth infection. Nat. Commun.14, 5627 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liang, H. et al. Elucidating the cell metabolic heterogeneity during hematopoietic lineage differentiation based on Met-Flow. Int. Immunopharmacol.121, 110443 (2023). [DOI] [PubMed] [Google Scholar]
- 104.Monteiro, L., de, B., Davanzo, G. G., de Aguiar, C. F. & Moraes-Vieira, P. M. M. Using flow cytometry for mitochondrial assays. MethodsX7, 100938 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodriguez, M. E. et al. Targeting of mitochondria by 10-N-alkyl acridine orange analogues: role of alkyl chain length in determining cellular uptake and localization. Mitochondrion8, 237–246 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kauffman, M. E. et al. MitoSOX-based flow cytometry for detecting mitochondrial ROS. React. Oxyg. Species2, 361–370 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kowaltowski, A. J. & Abdulkader, F. How and when to measure mitochondrial inner membrane potentials. Biophys. J.10.1016/j.bpj.2024.03.011 (2024). [DOI] [PubMed]
- 108.McWilliams, T. G. et al. mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J. Cell Biol.214, 333–345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pham, A. H., McCaffery, J. M. & Chan, D. C. Mouse lines with photo-activatable mitochondria to study mitochondrial dynamics. Genesis50, 833–843 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Russo, E. et al. SPICE-Met: profiling and imaging energy metabolism at the single-cell level using a fluorescent reporter mouse. EMBO J.41, e111528 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakandakari-Higa, S. et al. Universal recording of immune cell interactions in vivo. Nature627, 399–406 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sinclair, L. V., Barthelemy, C. & Cantrell, D. A. Single cell glucose uptake assays: a cautionary tale. Immunometabolism2, e200029 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsuchiya, M., Tachibana, N. & Hamachi, I. Post-click labeling enables highly accurate single cell analyses of glucose uptake ex vivo and in vivo. Commun. Biol.7, 459 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iwata, R. & Vanderhaeghen, P. Metabolic mechanisms of species-specific developmental tempo. Dev. Cell59, 1628–1639 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]