Abstract
Background:
Spontaneous coronary artery dissection (SCAD) is a rare and often underdiagnosed cause of acute coronary syndrome (ACS), predominantly affecting younger women without traditional cardiovascular risk factors. The management of SCAD remains a subject of debate, likely secondary to inconclusive evidence. This study aims to compare the clinical outcomes of SCAD patients treated with optimal medical therapy (OMT) versus those who underwent percutaneous coronary intervention (PCI) using a national population-based cohort.
Methods:
We conducted a retrospective analysis using the National Inpatient Sample (NIS) database from 2016 to 2020. The study included patients identified with SCAD using the ICD-10-CM (the International Classification of Diseases, Tenth Revision, Clinical Modification) code I25.42. We excluded individuals who did not receive PCI or coronary angiography, those who underwent coronary artery bypass grafting, and patients with incomplete records. The primary outcome was in-hospital mortality, while secondary outcomes included acute kidney injury, cardiac arrest, cardiogenic shock, use of temporary mechanical circulatory support, cost of hospitalization, and length of stay. National estimates were obtained using discharge weights, and statistical comparisons were performed using chi-square tests and linear regression. Multivariate logistic regression was employed to identify predictors of mortality and other outcomes.
Results:
A total of 31,105 SCAD patients were included in the study, with 10,480 receiving OMT and 20,625 undergoing PCI. Patients in the PCI group were older (mean age 64 vs. 54 years) and had higher comorbidities compared to those in the OMT group. The proportion of SCAD patients receiving PCI declined from 72% in 2016 to 60% in 2020. In multivariable analysis, PCI was associated with increased in-hospital mortality (odds ratio (OR) 1.89, 95% confidence interval (CI) 1.24–2.90, p = 0.0003), cardiogenic shock (OR 2.29, 95% CI 1.71–3.07, p < 0.0001), use of a left ventricular assist device (LVAD) (OR 3.97, 95% CI 2.42–6.53, p < 0.0001), and an intra-aortic balloon pump (IABP) (OR 2.24, 95% CI 1.63–3.09, p < 0.0001). Trends also suggested an association between PCI and cardiac arrest, extracorporeal membrane oxygenation (ECMO), and acute kidney injury (AKI). The PCI group had significantly higher hospitalization costs and longer lengths of stay compared to the OMT group (both p < 0.001).
Conclusions:
In this large, national cohort study, SCAD patients who underwent PCI had significantly higher risks of adverse in-hospital outcomes, including mortality, compared to those treated with OMT. These findings underscore the importance of careful patient selection and the potential advantages of conservative management in SCAD, particularly in patients without severe or unstable presentations. Further research is needed to develop evidence-based guidelines for the optimal management of SCAD.
Keywords: spontaneous coronary artery dissection, PCI, acute coronary syndrome
1. Introduction
Spontaneous coronary artery dissection (SCAD) is a rare but increasingly recognized cause of acute coronary syndrome (ACS), accounting for a small yet significant proportion of ACS cases, particularly in younger women without traditional cardiovascular risk factors [1, 2, 3]. SCAD is characterized by the separation of the coronary artery wall layers, which leads to the formation of a false lumen and subsequent compromise of blood flow, potentially resulting in myocardial ischemia, infarction, and even sudden cardiac death. The incidence of SCAD is reported to be between 0.1% and 1.1% of all cases of ACS, though it is likely underdiagnosed due to its variable presentation and the challenges in detection using conventional coronary angiography. The pathophysiology of SCAD is distinct from atherosclerotic coronary artery disease, as it is not associated with plaque rupture or thrombosis. Instead, the dissection typically occurs within the intima or media of the coronary artery, creating an intramural hematoma that compresses the true lumen [4, 5, 6]. This unique mechanism of ischemia poses significant challenges in the management of SCAD, as traditional interventional strategies, such as percutaneous coronary intervention (PCI), which are effective in atherosclerotic ACS, but may not be appropriate or effective in SCAD.
The management of SCAD remains a subject of debate due to the lack of randomized controlled trials (RCTs) specifically addressing the optimal treatment strategy. The most commonly employed strategy is conservative management with optimal medical therapy (OMT), which may include antiplatelet agents, beta-blockers, and angiotensin-converting enzyme (ACE) inhibitors [7]. This approach is often preferred due to the potential for spontaneous healing of the dissection and the high risk of procedural complications associated with PCI in SCAD patients [8]. Despite the preference for conservative management, there are circumstances where revascularization may be considered, particularly in patients with ongoing ischemia, left main or proximal artery involvement, or hemodynamic instability. In such cases, the decision to pursue PCI must be made cautiously, weighing the risks of the procedure against the potential benefits.
Given the complexities and risks associated with SCAD management, there is a pressing need for more robust data to guide treatment decisions. To address this gap, we performed analyses using a national population-based cohort to evaluate the clinical outcomes of SCAD patients managed with OMT versus those who underwent PCI.
2. Methods
We performed a retrospective study using the National Inpatient Sample (NIS) database from 2016 to 2020. NIS is one of the largest national databases that contains information from approximately 7 million hospital stays annually in its unweighted form. When weighted, it could project up to 35 million hospitalizations across the nation each year. The data contained in this database is deidentified, thus, the approval from the Institutional Review Board (IRB) was not required.
2.1 Study Population
In this study, we identified hospital admissions for SCAD by using the ICD-10-CM (the International Classification of Diseases, Tenth Revision, Clinical Modification) code I25.42. In line with previous analyses concerning SCAD patient populations, we excluded individuals who did not receive PCI or coronary angiography to maintain diagnostic precision. Additionally, we excluded patients who underwent concurrent coronary artery bypass grafting and those with a diagnosis of accidental puncture to preserve the homogeneity of our study cohort. We also omitted data from patients with incomplete or missing records pertaining to age, gender, or mortality.
2.2 Outcomes
Our primary outcome was in-hospital mortality. Secondary outcomes included acute kidney injury, cardiac arrest, cardiogenic shock, use of temporary mechanical circulatory support, cost of hospitalization and length of stay.
2.3 Statistical Analysis
We obtained the national estimates using the discharge weight provided within the database. We described dichotomous variables using frequencies and/or percentages and compared them using the chi-square test. Non-dichotomous variables were described in mean and standard deviation and comparison was made using linear regression. Hospitalization trends were demonstrated using a bar chart. We described the raw unadjusted outcomes and subsequently performed multivariate logistic regression analysis, using variables that were significant on univariate analysis with a threshold of 0.05. To control for confounding variables and improve the comparability between treatment and control groups, we employed propensity score matching using the caliper method. We then performed nearest neighbor matching with a caliper width set to 0.2 of the standard deviation of the logit of the propensity score to reduce bias. In assessing the outcomes using the matched data, we constructed 2 models. Model 1 was analyzed using only the matched data, while Model 2 expanded upon Model 1 by additionally adjusting for covariates that remained significant in the univariate analysis. We further assessed the predictors of mortality among those who received PCI and those who received OMT respectively using the similar regression approach as described earlier. All statistical analyses were conducted using STATA version 17.0 (StataCorp, College Station, TX, USA).
3. Results
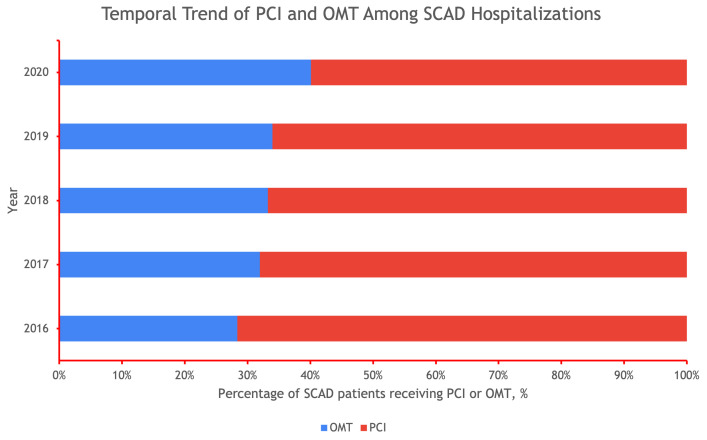
We analyzed 31,105 patients with SCAD, 10,480 of which received OMT and 20,625 of which had PCI. Of the patients with SCAD who had PCI, the mean age was 64 years with 48% of the patients being female and most patients being Caucasian. In comparison, of the patients with SCAD who received optimal medical therapy, the mean age was 54 years with 78% of the patients being female and most patients being Caucasian. In the SCAD receiving PCI compared to the SCAD receiving OMT, the SCAD receiving PCI population had significantly higher comorbidities including cardiac arrhythmias, congestive heart failure, valvular heart disorders, peripheral valvular disease, hypertension, chronic lung disease, diabetes mellitus, fluid disorders, chronic kidney disease (CKD), smoking, and prior stroke (Table 1). The rate of PCI has declined yearly from 72% in 2016 to 60% in 2020 (Fig. 1).
Table 1.
Baseline characteristics of SCAD patients between OMT versus PCI.
| Variables | OMT | PCI | Total | p-value | |||
| n | % | n | % | ||||
| Number of patients, n | 10,480 | 20,625 | 31,105 | ||||
| Age | 54.37 13.61 | 64.01 13.64 | 0.001 | ||||
| Female | 8195 | 78.20 | 9920 | 48.10 | 18,115 | 0.001 | |
| Race | 0.001 | ||||||
| White | 6985 | 66.65 | 15,095 | 73.19 | 22,080 | ||
| Black | 1550 | 14.79 | 1820 | 8.82 | 3370 | ||
| Hispanic | 1025 | 9.78 | 1650 | 8.00 | 2675 | ||
| Asian or Pacific Islander | 220 | 2.10 | 470 | 2.28 | 690 | ||
| Native American | 40 | 0.38 | 90 | 0.44 | 130 | ||
| Other | 225 | 2.15 | 555 | 2.69 | 780 | ||
| Hospital bed size | 0.024 | ||||||
| Small | 1375 | 13.12 | 2975 | 14.42 | 4350 | ||
| Medium | 2685 | 25.62 | 5800 | 28.12 | 8485 | ||
| Large | 6420 | 61.26 | 11,850 | 57.45 | 18,270 | ||
| Hospital teaching status | 0.001 | ||||||
| Rural | 340 | 3.24 | 1075 | 5.21 | 1415 | ||
| Urban non-teaching | 1615 | 15.41 | 3710 | 17.99 | 5325 | ||
| Urban teaching | 8525 | 81.35 | 15,840 | 76.80 | 24,365 | ||
| Admission | |||||||
| Elective | 760 | 7.25 | 3735 | 18.11 | 4495 | ||
| Primary payment coverage | 0.001 | ||||||
| Medicare | 2425 | 23.14 | 10,560 | 51.20 | 12,985 | ||
| Medicaid | 1325 | 12.64 | 2035 | 9.87 | 3360 | ||
| Private insurance | 5840 | 55.73 | 6445 | 31.25 | 12,285 | ||
| Self-pay | 520 | 4.96 | 895 | 4.34 | 1415 | ||
| No charge | 30 | 0.29 | 90 | 0.44 | 120 | ||
| Other | 330 | 3.15 | 580 | 2.81 | 910 | ||
| Median household income, $ | 0.001 | ||||||
| 1–28,999 | 2260 | 21.56 | 5545 | 26.88 | 7805 | ||
| 29,000–35,999 | 2370 | 22.61 | 5150 | 24.97 | 7520 | ||
| 36,000–46,999 | 2875 | 27.43 | 5380 | 26.08 | 8255 | ||
| 47,000+ | 2825 | 26.96 | 4220 | 20.46 | 7045 | ||
| Hospital region | 0.001 | ||||||
| Northeast | 2110 | 20.13 | 3675 | 17.82 | 5785 | ||
| Midwest | 2675 | 25.52 | 5000 | 24.24 | 7675 | ||
| South | 3105 | 29.63 | 8110 | 39.32 | 11,215 | ||
| West | 2590 | 24.71 | 3840 | 18.62 | 6430 | ||
| Comorbidities | |||||||
| Congestive heart failure | 2450 | 23.38 | 7315 | 35.47 | 9765 | 0.001 | |
| Cardiac arrhythmias | 2800 | 26.72 | 8025 | 38.91 | 10,825 | 0.001 | |
| Valvular heart diseases | 1085 | 10.35 | 2730 | 13.24 | 3815 | 0.001 | |
| Pulmonary circulatory disorders | 395 | 3.77 | 1025 | 4.97 | 1420 | 0.029 | |
| Peripheral vascular disease | 830 | 7.92 | 2865 | 13.89 | 3695 | 0.001 | |
| Hypertension | 6265 | 59.78 | 16,295 | 79.01 | 22,560 | 0.001 | |
| Paralysis | 55 | 0.52 | 160 | 0.78 | 215 | 0.2602 | |
| Other neurologic disorders | 470 | 4.48 | 1425 | 6.91 | 1895 | 0.0002 | |
| Chronic lung disease | 1615 | 15.41 | 4125 | 20.00 | 5740 | 0.001 | |
| Diabetes mellitus | 1370 | 13.07 | 6760 | 32.78 | 8130 | 0.001 | |
| Hypothyroidism | 1315 | 12.55 | 2400 | 11.64 | 3715 | 0.3015 | |
| CKD | 730 | 6.97 | 3440 | 16.68 | 4170 | 0.001 | |
| Liver disease | 320 | 3.05 | 865 | 4.19 | 1185 | 0.0275 | |
| AIDS | 15 | 0.14 | 60 | 0.29 | 75 | 0.2628 | |
| Cancer | 140 | 1.34 | 440 | 2.13 | 580 | 0.0261 | |
| Rheumatologic disorders | 310 | 2.96 | 555 | 2.69 | 865 | 0.5489 | |
| Coagulopathy | 445 | 4.25 | 1125 | 5.45 | 1570 | 0.0402 | |
| Obesity | 2285 | 21.80 | 4305 | 20.87 | 6590 | 0.4003 | |
| Weight loss | 185 | 1.77 | 490 | 2.38 | 675 | 0.112 | |
| Fluid and electrolyte disorders | 1770 | 16.89 | 4485 | 21.75 | 6255 | 0.001 | |
| Anemia | 430 | 4.10 | 695 | 3.37 | 1125 | 0.1563 | |
| Alcohol abuse | 190 | 1.81 | 525 | 2.55 | 715 | 0.0657 | |
| Drug abuse | 400 | 3.82 | 755 | 3.66 | 1155 | 0.763 | |
| Psychoses | 45 | 0.43 | 50 | 0.24 | 95 | 0.2062 | |
| Depression | 1405 | 13.41 | 2015 | 9.77 | 3420 | 0.001 | |
| FMD | 255 | 2.43 | 30 | 0.15 | 285 | 0.001 | |
| Smoking | 1835 | 17.51 | 4865 | 23.59 | 6700 | 0.001 | |
| Prior MI | 1225 | 11.69 | 3305 | 16.02 | 4530 | 0.001 | |
| Prior PCI | 85 | 0.81 | 245 | 1.19 | 330 | 0.1514 | |
| Prior CABG | 300 | 2.86 | 1570 | 7.61 | 1870 | 0.001 | |
| Prior stroke | 410 | 3.91 | 1420 | 6.88 | 1830 | 0.001 | |
| AMI | 8000 | 76.34 | 14,555 | 70.57 | 22,555 | 0.001 | |
AIDS, acquired immunodeficiency syndrome; AMI, acute myocardial infarction; CABG, coronary artery bypass graft surgery; CKD, chronic kidney disease; FMD, fibromuscular dysplasia; MI, myocardial infarction; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection.
Variables with less than 10 in any of the cells are not reported according to Agency for Healthcare Research and Quality’s data use agreement.
Fig. 1.
Temporal Trend of Percutaneous Coronary Intervention (PCI) and Optimal Medical Therapy (OMT) Among Spontaneous Coronary Artery Dissection (SCAD) Hospitalizations.
Using the multivariable regression model, we found that SCAD patients who underwent PCI were associated with in-hospital mortality (odds ratio (OR) 1.89, 95% confidence interval (CI) (1.24–2.90), p = 0.0003), cardiogenic shock (OR 2.29, 95% CI (1.71–3.07), p 0.0001), use of a left ventricular assist device (LVAD) (OR 3.97, 95% CI (2.42–6.53), p 0.0001), or use of an intra-aortic balloon pump (IABP) (OR 2.24, 95% CI (1.63–3.09), p 0.0001). There were trends that SCAD patients who underwent PCI were associated with cardiac arrests, extracorporeal membrane oxygenation (ECMO) and development of AKI. The cost of hospitalization was higher in the PCI group (p-value 0.001) and so was the length of stay (p-value 0.001) (Table 2).
Table 2.
Outcomes of SCAD patients between PCI and OMT.
| Outcomes | OMT | PCI | p-value | Adjusted OR | Lower limit | Upper limit | p-value | ||
| n | % | n | % | ||||||
| Mortality | 170 | 1.62 | 1170 | 5.67 | 0.001 | 1.89 | 1.24 | 2.90 | 0.003 |
| Cardiac arrest | 340 | 3.24 | 895 | 4.34 | 0.04 | 1.12 | 0.78 | 1.61 | 0.521 |
| Cardiogenic shock | 400 | 3.82 | 2295 | 11.13 | 0.001 | 2.29 | 1.71 | 3.07 | 0.001 |
| Use of MCS | |||||||||
| LVAD | 105 | 1 | 1110 | 5.38 | 0.001 | 3.97 | 2.42 | 6.53 | 0.001 |
| IABP | 300 | 2.86 | 1855 | 8.99 | 0.001 | 2.24 | 1.63 | 3.09 | 0.001 |
| ECMO | 40 | 0.38 | 100 | 0.48 | 0.570 | 0.79 | 0.20 | 3.07 | 0.736 |
| AKI | 760 | 7.25 | 3085 | 14.96 | 0.001 | 1.14 | 0.89 | 1.45 | 0.307 |
| Cost of hospitalization, USD | 16,408 21,948 | 33,880 29,774 | 0.001 | ||||||
| Length of stay, days | 3.49 3.63 | 4.49 5.75 | 0.001 | ||||||
AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; MCS, mechanical circulatory support; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection; OR, odds ratio.
OMT as reference category.
We further conducted a secondary analysis using propensity-score matching between patients receiving PCI and OMT. Baseline characteristics of this cohort of patients are shown in Table 3. Overall, we observed a balanced cohort of 7170 pairs of SCAD patients. As a result, we measured the outcomes using two separate models. Model 1 incorporated the propensity-score matched data while Model 2 was further adjusted for the characteristics that were significant on univariate analysis. The results of both models are shown in Table 4. As observed in the maximally adjusted Model 2, the PCI cohort was associated with a higher risk of in-hospital mortality compared to the OMT cohort, but marginally missed the statistical significance threshold (OR 1.58, 95% CI (1.00–2.50), p = 0.05) (Table 4). Results of the other outcomes remained largely aligned with primary analysis, whereby the risk of cardiogenic shock, use of IABP and LVAD remained significantly higher in the PCI group compared to the OMT group. Table 5 showed predictor mortality of SCAD patients who underwent PCI while Table 6 showed the predicted mortality of SCAD patients who were treated with OMT.
Table 3.
Baseline characteristics of SCAD patients between OMT versus PCI using propensity-score matched data.
| Variables | OMT | PCI | Total | p-value | |||
| Number of patients, n | 7170 | 7170 | 14,340 | ||||
| Age | 58.58 14.67 | 57.74 13.45 | 0.11 | ||||
| Female | 5090 | 70.99 | 4585 | 63.95 | 9675 | 0.00 | |
| Race | 0.82 | ||||||
| White | 5155 | 71.90 | 5245 | 73.15 | 10,400 | ||
| Black | 1005 | 14.02 | 905 | 12.62 | 1910 | ||
| Hispanic | 645 | 9.00 | 620 | 8.65 | 1265 | ||
| Asian or Pacific Islander | 160 | 2.23 | 160 | 2.23 | 320 | ||
| Native American | 25 | 0.35 | 20 | 0.28 | 45 | ||
| Other | 180 | 2.51 | 220 | 3.07 | 400 | ||
| Hospital bed size | 0.39 | ||||||
| Small | 945 | 13.18 | 1045 | 14.57 | 1990 | ||
| Medium | 1925 | 26.85 | 1995 | 27.82 | 3920 | ||
| Large | 4300 | 59.97 | 4130 | 57.60 | 8430 | ||
| Hospital teaching status | 0.78 | ||||||
| Rural | 290 | 4.04 | 325 | 4.53 | 615 | ||
| Urban non-teaching | 1155 | 16.11 | 1180 | 16.46 | 2335 | ||
| Urban teaching | 5725 | 79.85 | 5665 | 79.01 | 11,390 | ||
| Admission | |||||||
| Elective | 685 | 9.55 | 890 | 12.41 | 1575 | 0.02 | |
| Primary payment coverage | 0.24 | ||||||
| Medicare | 2240 | 31.24 | 2515 | 35.08 | 4755 | ||
| Medicaid | 885 | 12.34 | 830 | 11.58 | 1715 | ||
| Private insurance | 3420 | 47.70 | 3275 | 45.68 | 6695 | ||
| Self-pay | 380 | 5.30 | 315 | 4.39 | 695 | ||
| No charge | 30 | 0.42 | 15 | 0.21 | 45 | ||
| Other | 215 | 3.00 | 220 | 3.07 | 435 | ||
| Median household income, $ | 0.84 | ||||||
| 1–28,999 | 1755 | 24.48 | 1665 | 23.22 | 3420 | ||
| 29,000–35,999 | 1650 | 23.01 | 1705 | 23.78 | 3355 | ||
| 36,000–46,999 | 2000 | 27.89 | 1980 | 27.62 | 3980 | ||
| 47,000+ | 1765 | 24.62 | 1820 | 25.38 | 3585 | ||
| Hospital region | 0.58 | ||||||
| Northeast | 1435 | 20.01 | 1430 | 19.94 | 2865 | ||
| Midwest | 1825 | 25.45 | 1690 | 23.57 | 3515 | ||
| South | 2365 | 32.98 | 2520 | 35.15 | 4885 | ||
| West | 1545 | 21.55 | 1530 | 21.34 | 3075 | ||
| Comorbidities | |||||||
| Congestive heart failure | 1900 | 26.50 | 2095 | 29.22 | 3995 | 0.10 | |
| Cardiac arrhythmias | 2140 | 29.85 | 2445 | 34.10 | 4585 | 0.01 | |
| Valvular heart diseases | 805 | 11.23 | 875 | 12.20 | 1680 | 0.43 | |
| Pulmonary circulatory disorders | 335 | 4.67 | 335 | 4.67 | 670 | 1.00 | |
| Peripheral vascular disease | 660 | 9.21 | 790 | 11.02 | 1450 | 0.11 | |
| Hypertension | 4820 | 67.22 | 4915 | 68.55 | 9735 | 0.44 | |
| Paralysis | 55 | 0.77 | 55 | 0.77 | 110 | 1.00 | |
| Other neurologic disorders | 365 | 5.09 | 390 | 5.44 | 755 | 0.68 | |
| Chronic lung disease | 1245 | 17.36 | 1235 | 17.22 | 2480 | 0.92 | |
| Diabetes | 1270 | 17.71 | 1605 | 22.38 | 2875 | 0.00 | |
| Hypothyroidism | 880 | 12.27 | 855 | 11.92 | 1735 | 0.78 | |
| CKD | 645 | 9.00 | 895 | 12.48 | 1540 | 0.00 | |
| Liver disease | 265 | 3.70 | 275 | 3.84 | 540 | 0.85 | |
| AIDS | 15 | 0.21 | 15 | 0.21 | 30 | 1.00 | |
| Cancer | 125 | 1.74 | 145 | 2.02 | 270 | 0.58 | |
| Rheumatologic disorders | 230 | 3.21 | 195 | 2.72 | 425 | 0.44 | |
| Coagulopathy | 290 | 4.04 | 335 | 4.67 | 625 | 0.41 | |
| Obesity | 1630 | 22.73 | 1655 | 23.08 | 3285 | 0.83 | |
| Weight loss | 150 | 2.09 | 155 | 2.16 | 305 | 0.90 | |
| Fluid and electrolyte disorders | 1330 | 18.55 | 1355 | 18.90 | 2685 | 0.81 | |
| Anemia | 265 | 3.70 | 265 | 3.70 | 530 | 1.00 | |
| Alcohol abuse | 160 | 2.23 | 175 | 2.44 | 335 | 0.71 | |
| Drug abuse | 280 | 3.91 | 315 | 4.39 | 595 | 0.51 | |
| Psychoses | 35 | 0.49 | 25 | 0.35 | 60 | 0.56 | |
| Depression | 915 | 12.76 | 875 | 12.20 | 1790 | 0.65 | |
| FMD | 30 | 0.42 | 30 | 0.42 | 60 | 1.00 | |
| Smoking | 1475 | 20.57 | 1510 | 21.06 | 2985 | 0.75 | |
| Prior MI | 930 | 12.97 | 975 | 13.60 | 1905 | 0.61 | |
| Prior PCI | 65 | 0.91 | 75 | 1.05 | 140 | 0.69 | |
| Prior CABG | 250 | 3.49 | 445 | 6.21 | 695 | 0.00 | |
| Prior stroke | 350 | 4.88 | 395 | 5.51 | 745 | 0.63 | |
| AMI | 5350 | 74.62 | 5295 | 73.85 | 10,645 | 0.63 | |
AIDS, acquired immunodeficiency syndrome; AMI, acute myocardial infarction; CABG, coronary artery bypass graft surgery; CKD, chronic kidney disease; FMD, fibromuscular dysplasia; MI, myocardial infarction; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection.
Table 4.
Outcomes of SCAD patients between OMT versus PCI using propensity-score matched data.
| Outcomes | Model 1 | Model 2 | ||||||
| Adjusted OR | Lower limit | Upper limit | p-value | Adjusted OR | Lower limit | Upper limit | p-value | |
| Mortality | 1.87 | 1.19 | 2.92 | 0.006 | 1.58 | 1.00 | 2.50 | 0.051 |
| Cardiac arrest | 1.16 | 0.79 | 1.70 | 0.443 | 1.04 | 0.69 | 1.56 | 0.844 |
| Cardiogenic shock | 2.11 | 1.55 | 2.87 | 0.001 | 1.91 | 1.39 | 2.62 | 0.001 |
| Use of MCS | ||||||||
| LVAD | 3.49 | 1.94 | 6.27 | 0.001 | 3.11 | 1.72 | 5.64 | 0.001 |
| IABP | 2.36 | 1.69 | 3.31 | 0.001 | 2.16 | 1.54 | 3.03 | 0.001 |
| ECMO | 1.60 | 0.52 | 4.97 | 0.413 | 1.26 | 0.42 | 3.82 | 0.678 |
| AKI | 1.38 | 1.09 | 1.76 | 0.009 | 1.12 | 0.85 | 1.47 | 0.409 |
AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; MCS, mechanical circulatory support; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection; OR, odds ratio.
OMT as reference category.
Model 1: using propensity-score matched data.
Model 2: Model 1 + adjusted for imbalance covariates including gender, elective admission, cardiac arrhythmias and chronic kidney disease.
Table 5.
Predicted mortality of SCAD patients who underwent PCI.
| Variables | Odds ratio | Lower limit | Upper limit | p-value |
| Cardiogenic shock | 10.14 | 6.86 | 14.99 | 0.001 |
| Age | 1.05 | 1.03 | 1.06 | 0.001 |
| Female | 1.59 | 1.15 | 2.18 | 0.005 |
| Smoking | 0.89 | 0.58 | 1.37 | 0.600 |
| Fluid and electrolyte disorders | 1.68 | 1.19 | 2.36 | 0.003 |
| Weight loss | 1.15 | 0.50 | 2.66 | 0.740 |
| Obesity | 0.65 | 0.43 | 1.00 | 0.050 |
| Coagulopathy | 1.34 | 0.77 | 2.31 | 0.298 |
| Liver disease | 0.88 | 0.60 | 1.30 | 0.519 |
| CKD | 1.75 | 1.01 | 3.03 | 0.045 |
| Other neurological disorders | 2.24 | 1.45 | 3.47 | 0.001 |
| Diabetes mellitus | 1.82 | 1.31 | 2.53 | 0.001 |
| Peripheral vascular disease | 1.24 | 0.85 | 1.80 | 0.272 |
| Pulmonary circulatory disorders | 1.16 | 0.67 | 2.00 | 0.598 |
| Valvular heart diseases | 0.78 | 0.51 | 1.20 | 0.265 |
| Cardiac arrhythmia | 1.52 | 1.09 | 2.10 | 0.013 |
| Congestive heart failure | 1.13 | 0.81 | 1.57 | 0.483 |
| Primary payment coverage | ||||
| Medicare | Ref | |||
| Medicaid | 0.67 | 0.32 | 1.41 | 0.290 |
| Private insurance | 0.82 | 0.51 | 1.33 | 0.428 |
| Self-pay | 0.97 | 0.37 | 2.60 | 0.959 |
| No charge | N/A | N/A | N/A | N/A |
| Other | 0.88 | 0.28 | 2.72 | 0.823 |
CKD, chronic kidney disease; PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection.
Variables with less than 10 in any of the cells are not reported according to Agency for Healthcare Research and Quality’s data use agreement and are marked as N/A.
Table 6.
Predicted mortality of SCAD patients who were treated with OMT.
| Variables | Odds ratio | Lower limit | Upper limit | p-value |
| Cardiogenic shock | 8.96 | 2.86 | 28.07 | 0.001 |
| Age | 1.07 | 1.04 | 1.11 | 0.001 |
| Female | 0.50 | 0.22 | 1.17 | 0.111 |
| Fluid and electrolyte disorders | 2.65 | 1.06 | 6.59 | 0.036 |
| Weight loss | 1.36 | 0.33 | 5.54 | 0.672 |
| Rheumatologic disorders | 3.32 | 0.71 | 15.48 | 0.126 |
| Liver disease | 1.73 | 0.68 | 4.40 | 0.249 |
| CKD | 1.22 | 0.30 | 5.03 | 0.778 |
| Other neurological disorders | 5.68 | 1.78 | 18.15 | 0.003 |
| Pulmonary circulatory disorders | 1.27 | 0.43 | 3.73 | 0.659 |
| Peripheral vascular disease | 2.18 | 0.45 | 10.52 | 0.330 |
| Cardiac arrhythmia | 1.25 | 0.50 | 3.12 | 0.637 |
| Congestive heart failure | 0.47 | 0.19 | 1.18 | 0.107 |
| Elective admission | 1.68 | 0.55 | 5.19 | 0.365 |
| Primary payment coverage | ||||
| Medicare | Ref | |||
| Medicaid | 2.96 | 0.70 | 12.51 | 0.140 |
| Private insurance | 0.71 | 0.21 | 2.42 | 0.580 |
| Self-pay | 3.65 | 0.65 | 20.60 | 0.143 |
| No charge | N/A | N/A | N/A | N/A |
| Other | N/A | N/A | N/A | N/A |
CKD, chronic kidney disease; OMT, optimal medical therapy; SCAD, spontaneous coronary artery dissection.
Variables with less than 10 in any of the cells are not reported according to Agency for Healthcare Research and Quality’s data use agreement and are marked as N/A.
4. Discussion
In our national study, there were 3 main findings. First, the temporal trend of PCI and OMT among SCAD had shifted, with a yearly decrease in the percentage of patients receiving PCI, for the years 2016–2020. This is likely due to more data on SCAD management leaning towards medical therapy and a more conservative approach. Most importantly, both the American Heart Association (AHA) scientific statement and the European society of cardiology Expert opinion recommend conservative management of SCAD in stable cases, as SCAD is known to heal with the resorption of intramural hematoma overtime unlike ischemia secondary to atherosclerotic plaque [7, 9]. This recommendation is consistent with our prior meta-analysis, which showed no difference in terms of long-term mortality and recurrent SCAD among patients with SCAD treated with medical therapy compared with those treated with PCI [10].
Second, we found SCAD patients who underwent PCI were associated with in-hospital mortality, cardiogenic shock, LVAD, and IABP. This finding suggests that the baseline of SCAD patients who underwent PCI were much sicker compared to SCAD patients who were treated with medical therapy. SCAD patients with comorbidities (e.g., hypertension, diabetes mellitus, CKD, heart failure, shock) may be considered as a high-risk SCAD phenotype and may require intervention rather than conservative management. PCI and medical management have both been used in both case series and retrospective studies looking at SCAD management in inpatients [11, 12, 13]. The choice of which management to choose has been guided in these cases by the degree of coronary artery obstruction, severity of symptoms at presentation, whether the patient has acute coronary syndrome at presentation or not, and their coronary artery anatomy. SCAD patients with comorbidity or high-risk features probably underwent PCI rather than medical therapy.
Third, the mortality predictors of SCAD patients who underwent PCI were cardiac arrhythmia or acutely decompensated heart failure. SCAD patients with ventricular arrhythmia are likely to get treated with PCI rather than medical therapy and mortality is higher. There are technical challenges for PCI and SCAD patients. A study has reported variable success rates, with PCI success rates reports ranging from 29 to 92% [12]. With procedural failure and recurrence, a possibility. There are some potential risks to having PCI during SCAD, and these are thought to be the drivers of the failure rates. These risks include possible iatrogenic secondary dissection, where the guide wire engages with the false lumen which is then enlarged during ballon dilation [12].
As the intervention is offered based on clinical decision and patient presentation, patients with SCAD and other co-morbidities may present initially more unstable with vital sign or laboratory abnormalities, and need urgent intervention, such as cardiac catheterization, which leads to PCI placement. However, this is not yet clearly understood in the literature. There is no data from RCTs comparing medical therapy and PCI. We previously discussed that revascularization is associated with suboptimal procedural success rates and high rates of complications despite preserved coronary flow [14]. Long term follow up is recommended to ensure management is working, and that further interventions are not necessary for symptom management. More research is needed to understand optimal interventional guidelines and medical management to be implemented.
The current study has certain limitations that should be taken into consideration while interpreting the results. The major limitation was inherent to the database itself. While the NIS database has a strength in its huge sample size and ability to extrapolate to the US population, the lack of detailed clinical information, such as specific indications for PCI and comprehensive angiographic findings, including coronary flow, limits our ability to assess procedural outcomes and patient selection fully. Furthermore, key clinical data, such as patient presentation, laboratory results, imaging or echocardiographic findings, and medication use before, during, and after SCAD diagnosis, were not readily available within the database.
5. Conclusions
In this retrospective study looking at the NIS data base over four years we saw SCAD patients who underwent PCI are likely to be much sicker and have more comorbidities and higher rate of mortality, compared to SCAD patients who were treated with medical therapy. SCAD patients with heart failure and ventricular arrhythmia who underwent PCI were associated with higher mortality.
Availability of Data and Materials
The data sets generated and/or analyzed during the current study are not publicly available due to HCUP data policy but are available from the corresponding author on reasonable request.
Acknowledgment
Not applicable.
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
CK, BCR, SPA, YKQ, ZW, MA, SS, HJ wrote original article. CK, BCR, SPA, YKQ, ZW, MA, SS, HJ performed analysis. CK, BCR, SPA, YKQ, ZW, MA, SS, HJ reviewed and edited original article. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Ethical approval is waived from local institutional review board given this is a retrospective analysis of national database containing deidentified data. The Patient’s informed consent is not required since the data was deidentified.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest. Hani Jneid is serving as one of the Editorial Board members of this journal. We declare that Hani Jneid had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Dimitris Tousoulis.
References
- [1].Tweet MS, Gulati R, Hayes SN. Spontaneous Coronary Artery Dissection. Current Cardiology Reports . 2016;18:60. doi: 10.1007/s11886-016-0737-6. [DOI] [PubMed] [Google Scholar]
- [2].Hayes SN, Tweet MS, Adlam D, Kim ESH, Gulati R, Price JE, et al. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. Journal of the American College of Cardiology . 2020;76:961–984. doi: 10.1016/j.jacc.2020.05.084. [DOI] [PubMed] [Google Scholar]
- [3].Kok SN, Hayes SN, Cutrer FM, Raphael CE, Gulati R, Best PJM, et al. Prevalence and Clinical Factors of Migraine in Patients With Spontaneous Coronary Artery Dissection. Journal of the American Heart Association . 2018;7:e010140. doi: 10.1161/JAHA.118.010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Di Fusco SA, Rossini R, Zilio F, Pollarolo L, di Uccio FS, Iorio A, et al. Spontaneous coronary artery dissection: Overview of pathophysiology. Trends in Cardiovascular Medicine . 2022;32:92–100. doi: 10.1016/j.tcm.2021.01.002. [DOI] [PubMed] [Google Scholar]
- [5].Waterbury TM, Tweet MS, Hayes SN, Eleid MF, Bell MR, Lerman A, et al. Early Natural History of Spontaneous Coronary Artery Dissection. Circulation. Cardiovascular Interventions . 2018;11:e006772. doi: 10.1161/CIRCINTERVENTIONS.118.006772. [DOI] [PubMed] [Google Scholar]
- [6].Waterbury TM, Tarantini G, Vogel B, Mehran R, Gersh BJ, Gulati R. Non-atherosclerotic causes of acute coronary syndromes. Nature Reviews. Cardiology . 2020;17:229–241. doi: 10.1038/s41569-019-0273-3. [DOI] [PubMed] [Google Scholar]
- [7].Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation . 2018;137:e523–e557. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tweet MS, Eleid MF, Best PJM, Lennon RJ, Lerman A, Rihal CS, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circulation. Cardiovascular Interventions . 2014;7:777–786. doi: 10.1161/CIRCINTERVENTIONS.114.001659. [DOI] [PubMed] [Google Scholar]
- [9].Adlam D, Alfonso F, Maas A, Vrints C, Writing Committee. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. European Heart Journal . 2018;39:3353–3368. doi: 10.1093/eurheartj/ehy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Krittanawong C, Nazir S, Hassan Virk H, Hahn J, Wang Z, Fogg SE, et al. Long-Term Outcomes Comparing Medical Therapy versus Revascularization for Spontaneous Coronary Artery Dissection. The American Journal of Medicine . 2021;134:e403–e408. doi: 10.1016/j.amjmed.2021.02.011. [DOI] [PubMed] [Google Scholar]
- [11].Maeder M, Ammann P, Angehrn W, Rickli H. Idiopathic spontaneous coronary artery dissection: incidence, diagnosis and treatment. International Journal of Cardiology . 2005;101:363–369. doi: 10.1016/j.ijcard.2004.03.045. [DOI] [PubMed] [Google Scholar]
- [12].Velagapudi P, Kirtane AJ, Saw J. Spontaneous Coronary Artery Dissection Causing Acute Myocardial Infarction: Is Revascularization the Best Course of Action? JACC. Cardiovascular Interventions . 2023;16:1870–1872. doi: 10.1016/j.jcin.2023.06.032. [DOI] [PubMed] [Google Scholar]
- [13].Al Emam ARA, Almomani A, Gilani SA, Khalife WI. Spontaneous Coronary Artery Dissection: One Disease, Variable Presentations, and Different Management Approaches. The International Journal of Angiology: Official Publication of the International College of Angiology, Inc . 2016;25:139–147. doi: 10.1055/s-0035-1563604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Krittanawong C, Gulati R, Eitzman D, Jneid H. Revascularization in Patients With Spontaneous Coronary Artery Dissection: Where Are We Now? Journal of the American Heart Association . 2021;10:e018551. doi: 10.1161/JAHA.120.018551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are not publicly available due to HCUP data policy but are available from the corresponding author on reasonable request.