Abstract
Extracellular vesicles (EVs) are nanoscale vesicles released by cells, which play an important role in intercellular communication by transporting proteins, lipids, nucleic acids, and other molecules. Different intensities of exercise can induce the release of EVs from cells and tissues, such as endothelial cells, skeletal muscle and adipose tissue, hepatocytes, immune cells, and neuronal cells. Exercise-induced EVs exert cardiovascular protective effects such as anti-inflammatory and anti-oxidative by altering their contents. This paper reviews the cell and tissue sources of EVs induced by exercise of different intensities, the regulatory effects of different exercise intensities on EVs, and their mechanisms of action in cardiovascular diseases. The aim is to provide new insights for the treatment of cardiovascular diseases and offer scientific evidence for the construction of engineered EVs mimicking the effects of exercise.
Keywords: EVs, exercise, cardiovascular diseases, miRNA
1. Introduction
The prevalence of cardiovascular diseases is continually increasing, which has become the primary cause of disease-related mortality [1]. Therefore, the prevention and treatment of cardiovascular diseases are of urgent importance. As a primary strategy for non-invasive proactive health maintenance and cardiovascular disease prevention, exercise stimulates the release of extracellular vesicles (EVs) from various tissues and cells, including endothelial cells, skeletal muscle, and adipose tissue [2, 3]. EVs are small vesicles with a lipid bilayer membrane structure, containing contents such as proteins, lipids, nucleic acids, and metabolites, which allow for intercellular communication [4, 5]. At present, EVs are considered to be important mediators of communication in regulating cardiovascular health through exercise [6]. Studies have shown that exercise of appropriate intensity can regulate the contents of EVs, exerting anti-inflammatory and anti-oxidative effects, enhancing angiogenesis, alleviating myocardial injury, and improving endothelial function. These effects contribute to the effective prevention and treatment of cardiovascular diseases [7, 8, 9]. Conversely, EVs generated from inappropriate exercise intensity may induce inflammatory and oxidative stress reactions, thus compromising cardiovascular function [10, 11, 12]. This review provides a comprehensive discussion of the influence of exercise of varying intensities on the secretion of EVs as well as the regulatory effects and mechanisms of EVs secreted by different intensity exercises on cardiovascular diseases. The aim is to provide a theoretical basis for the promotion of cardiovascular health through exercise.
2. EVs
EVs are tiny vesicles released by cells into the microenvironment, with diameters ranging from approximately 35 nm to 1 µm. Based on their origin and biogenesis, EVs can be classified into microvesicles, exomeres, exosomes, and apoptotic bodies [5, 13]. Microvesicles, also referred to as microparticles, are anucleate vesicular clusters originating from the plasma membrane ranging from 50 nm to 1 µm. These microparticles can be shed directly from the cell surface through mechanisms involving calcium influx, membrane reorganization, and cytoskeletal remodeling. They perform functions by conveying specific information to target cells [5, 14, 15, 16]. Exomeres are non-vesicular nanoparticles with a diameter of approximately 35 nm, composed of various lipids and rich in metabolic enzymes, participating in multiple metabolic processes within the body [17, 18]. Exosomes are lipid bilayer vesicles that originate from endosomes, with a diameter ranging from approximately 40 nm to 160 nm (average about 100 nm). During the formation of exosomes, the cytoplasmic membrane undergoes an initial invagination to form early-sorting endosomes (ESEs). Following maturation, ESEs develop into late-sorting endosomes (LSEs), which ultimately evolve into multivesicular bodies (MVBs). MVBs release exosomes into the extracellular environment by fusing with the cytoplasmic membrane [19, 20, 21, 22]. Apoptotic bodies are defined as vesicular structures, which derive from apoptotic cells through two distinct mechanisms: germination shedding and autophagosome formation. Based on their diameter, apoptotic bodies can be categorized into two distinct groups: small apoptotic bodies (100 nm–1 µm) and large apoptotic bodies (1 µm–5 µm) [23]. Increasing evidence indicates that exercise, as a critical physical stimulus, can promote the release of EVs into the circulatory system and then regulate cardiovascular function.
3. Cell and Tissue Sources of Exercise-Modulated EVs Release
3.1 Endothelial Cells
Endothelial cells are situated on the innermost layer of the vascular wall. They can detect mechanical force alterations induced by exercise, such as blood flow shear stress and wall tension through membrane sensors or receptors, and then regulate the release of exosomes [24, 25]. A study of mice carried out 4 weeks of swimming and athletes underwent 1 year of rowing indicated that exercise promoted the release of exosomes enriched with miR-342-5p from endothelial cells. These exosomes enhanced the phosphorylation of protein kinase B (Akt) in cardiomyocytes by inhibiting the apoptotic pathways of caspase-9 and c-Jun N-terminal kinase 2 (Jnk2) and targeting protein phosphatase 1F (PPM1F). This process ultimately increased the anti-apoptotic capability of cardiomyocytes and then protected the heart from myocardial ischemic injury [26]. Additionally, exercise can regulate cardiovascular function by stimulating the release of EVs from endothelial progenitor cells (EPCs). EPCs are precursor cells of endothelial cells, which can differentiate into mature endothelial cells to promote the repair of damaged blood vessels. Ma et al. [27] found that exosomes released from EPCs after 4 weeks of treadmill exercise in mice protected vascular endothelial cells from impairment caused by high glucose and hypoxic conditions. Moreover, 4 weeks of treadmill exercise before stroke modeling in mice accelerated post-stroke recovery via promoting the release of a large number of exosomes from EPCs [28].
3.2 Skeletal Muscle
Skeletal muscle, the primary executor of physical activity, can release EVs containing various signaling regulators into the circulatory system. Appropriate exercise can induce changes in the content of EVs released from skeletal muscle cells, thereby modulating inflammatory and oxidative stress responses in the cardiovascular system, as well as systemic glucose and lipid metabolism. These ultimately reduce the incidence and progression of cardiovascular disease [11, 29, 30, 31, 32]. Guescini et al. [4] conducted a study in which they analyzed blood samples from healthy individuals who had just completed an acute exercise session. Their findings revealed that exercise stimulated skeletal muscle to secrete -sarcoglycan-positive EVs carrying miR-181a-5p, which in turn inhibited myocardial inflammation and oxidative stress, thereby preventing the onset of cardiovascular diseases [33]. Barone et al. [34] observed that mice subjected to 6 weeks of endurance exercise demonstrated enhanced rapid secretion of exosomes containing heat shock protein 60 (HSP60) from skeletal muscle into the circulatory system, thus increasing mitochondrial activity in cardiomyocytes and providing a protective effect on these cells. Moreover, research demonstrated that 16 weeks of swimming training stimulated the secretion of EVs from skeletal muscle, which improved glucose tolerance in obese mice and apolipoprotein E knock out (ApoE-/-) mice, reduced visceral lipid accumulation, alleviated liver damage, as well as inhibited the development of atherosclerosis [35]. Furthermore, exercise has been demonstrated to decelerate vascular aging in mice by stimulating the release of EVs from skeletal muscle, which are enriched with fibronectin type III domain-containing protein 5 (FNDC5)/irisin. This process concurrently reduces oxidative stress, inflammation, and endothelial dysfunction induced by angiotensin II, thereby exerting a protective effect on the cardiovascular system [36, 37].
3.3 Adipose Tissue
Adipose tissue, which encompasses both white and brown adipose tissues, represents the body’s largest endocrine organ. It regulates cardiovascular homeostasis through the secretion of a series of cytokines [8]. Dysfunction of white adipose tissue can result in the onset of type II diabetes and cardiovascular complications. Conversely, brown adipose tissue plays a positive role in the physiological regulation of the cardiovascular system [8]. Research indicated that exercise promoted the browning of white adipose tissue and increased the volume of brown adipose tissue [38]. Concurrently, exercise can exert anti-apoptotic effects on cardiomyocytes by altering the content of EVs secreted by adipose tissue. The 4 weeks of swimming exercise promoted the secretion of EVs enriched with miR-125b-5p, miR-128-3p, and miR-30d-5p from brown adipose tissue in mice to inhibit the pro-apoptotic mitogen-activated protein kinase (MAPK) signaling pathway and then protect the heart from myocardial ischemia-reperfusion (MI/R) injury [8].
3.4 Hepatocytes
The liver is responsible for the synthesis, metabolism, and redistribution of nutrients within the human body, playing a crucial role in the overall metabolic process [39]. Li et al. [40] and Zhao et al. [41] have demonstrated that EVs secreted by hepatocytes containing let-7b-5p can reduce mitochondrial oxidative phosphorylation and inhibit the conversion of white adipose tissue to brown adipose tissue, thereby promoting the development of obesity. Furthermore, research has indicated that EVs synthesized and secreted by the liver play a pivotal role in the regulation of cardiovascular diseases, including atherosclerosis, coronary artery disease, thrombosis, and myocardial infarction [42, 43]. In a murine model of nonalcoholic fatty liver disease, EVs derived from steatotic hepatocytes have been observed to enhance coronary microvascular permeability by modulating the novel miR-7/lysosomal associated membrane protein 1 (LAMP1)/Cathepsin B/NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome pathway which in turn disrupts the integrity of the microvascular endothelial barrier, leading to the occurrence of abnormal coronary blood flow reserve [44, 45]. Additionally, Lou et al. [46] found that exercise promoted the enrichment of miR-122-5p in liver-derived EVs. By targeting endothelial cells through 1-acyl-sn-glycerol-3-phosphate acyltransferase (AGPAT1), miR-122-5p enhances the utilization of fatty acids by endothelial cells, thereby increasing angiogenesis. In conclusion, the release of liver-derived EVs is subject to regulation by exercise, and these EVs play a role in the regulation of metabolism and the protection of the cardiovascular system [40, 41, 42, 43, 44, 45, 46, 47].
3.5 Immune Cells
Immune cells, including lymphocytes, macrophages, monocytes, and antigen-presenting cells, have the capacity to regulate the immune system and modulate cardiovascular function modulation through the release of EVs [48]. Xiong et al. [49] discovered that following an acute myocardial infarction, EVs released by T lymphocytes, macrophages, dendritic cells, and mast cells were involved in post-infarction immunomodulation and acted as part of myocardial repair. Moreover, following incremental cycling exercise, EVs secreted by leukocytes, lymphocytes, and antigen-presenting cells contribute to the enhancement of vascular function and immune regulation, including adaptive immunity [50]. In a study conducted by Highton et al. [51], renal transplant patients were observed after engaging in moderate-to-high intensity exercise. The findings indicated that the percentage of microparticles produced by intermediate monocytes decreased following exercise, which contributed to anti-thrombotic effects and protected vascular function in the transplanted kidneys. The aforementioned studies suggest that exercise can promote exercise adaptation and improve cardiovascular function by regulating the release of EVs from immune cells.
3.6 Neuronal Cells
Neuronal cells are a vital component of the nervous system [52]. Neurons that release EVs facilitate intercellular communication, process components of unwanted neuronal activity, and propagate pathological factors in neurodegenerative disease [53, 54]. In a study conducted by Zumkehr et al. [55], it was found that exosomes secreted by neuronal cells containing miR-124a can be absorbed by primary astrocytes, resulting in elevated intracellular levels of miR-124a and enhanced expression of glutamate transporter 1. This mechanism assists in maintaining synaptic glutamate homeostasis and prevents neuronal excitotoxicity. Meanwhile, Luo et al. [56] discovered that miR-150-3p-rich exosomes secreted by neural stem cells could promote neuronal proliferation by inhibiting the caspase 2 (CASP2) signaling pathway, thus preventing brain injury. Furthermore, EVs secreted by neurons have been demonstrated to modulate the cardiovascular system. Wang et al. [57] demonstrated that under conditions of oxygen-glucose deprivation, neurons are capable of secreting exosomes containing lncRNA H19. These exosomes have been demonstrated to increase endothelial cell permeability through the miR-18a/vascular endothelial growth factor (VEGF) pathway, thereby disrupting the integrity of the blood-brain barrier. Katsur et al. [58] found that exosomes secreted by neural stem cells can delay the opening of mitochondrial permeability transition pores (mPTP) mediated by reactive oxygen species (ROS) in cardiomyocytes through the gp130/Janus tyrosine Kinase (JAK)/signal transducer and activator of transcription (STAT) pathway. This mechanism serves to safeguard cardiomyocytes from oxidative stress and to diminish the infarct size in myocardial infarction. In addition, Delgado-Peraza et al. [59] showed that exercise stimulates neurons to secrete EVs rich in brain-derived neurotrophic factor (BDNF), proBDNF, and humanin. This secretion has been demonstrated to enhance cognitive function in patients diagnosed with Alzheimer’s disease (AD). Indeed, EVs secreted by neural cells play an important role in neuroprotection and the regulation of the cardiovascular system.
In addition to the aforementioned tissues and cells, mesenchymal stem cell, cardiomyocytes, platelets, neurons, and antigen-presenting cells can also secrete exercise-induced EVs [8, 46, 47, 50, 59, 60, 61]. Although efforts are being made, the exact tissues and cells sources of exercise-induced EVs are not entirely clear. Endothelial cells and skeletal muscle may be the predominant sources of circulating EVs [26].
4. Regulation of EVs Secretion by Exercise of Different Intensities
4.1 Classification of Exercise Intensity Levels
According to the guidelines of the American college of sports medicine (ACSM), the intensity levels are typically classified into low, moderate, and high based on absolute and relative exercise intensity indicators in human physical activity (Table 1) [62, 63]. Absolute indicator primarily refers to metabolic equivalents (METs), which accounts for the influence of age on the classification of exercise intensity levels. Relative indicators contain percentages of maximal oxygen uptake (VO2max), maximal heart rate (HRmax), heart rate reserve (HRR), and percentage of one repetition maximum (1 RM) [62, 63]. The classification of exercise intensity levels based on relative indicators does not consider the factors of age and sex. In fact, the potential influence of age and sex cannot be ignored. Females have a higher heart rate (HR) and oxygen consumption (VO2) than in males and older persons possess higher HR and VO2 than in younger individuals when they carry out the same intensity exercise [64]. Therefore, the current classification of exercise intensity levels may result in excessive exercise intensity for females and older people, who can choose the low value in the range of exercise intensity indicators. In animal experiments, the exercise intensity is usually set with reference to the Bedford exercise protocol [65], with specific grading of exercise intensity levels as referenced in Table 1.
Table 1.
Grading of different intensities exercise.
| Human | Animal | |||||||||
| Absolute exercise intensity (METs) in adults (age in years) | Relative exercise intensity | Mouse | Rat | |||||||
| Classification | 20–39 yr | 40–64 yr | 65–79 yr | 80+ yr | %VO2max | %HRmax | %HRR | %1 RM | Running speed (m/min) | |
| Low intensity | 2.4–4.7 | 2.0–3.9 | 1.6–3.1 | 1.1–1.9 | 40 | 35–54 | 20–39 | 30–50 | 10 | 16 |
| Moderate intensity | 4.8–7.1 | 4.0–5.9 | 3.2–4.7 | 2.0–2.9 | 40–60 | 55–69 | 40–59 | 51–70 | 10–20 | 16–24 |
| High intensity | 7.2–10.1 | 6.0–8.4 | 4.8–6.7 | 3.0–4.25 | 60 | 70–89 | 60–84 | 71–85 | 20 | 24 |
METs, metabolic equivalents; yr, year; VO2max, maximal oxygen uptake; HRmax, maximal heart rate; HRR, heart rate reserve; 1 RM, one repetition maximum.
4.2 Modulation of EVs Secretion by Low-Intensity Exercise
A series of studies have demonstrated that both acute and chronic low-intensity exercise effectively increase the concentration of EVs in the serum of various populations or mice, but do not significantly affect the diameter of EVs [27, 66]. Just et al. [32] observed that following the performance of five sets of knee joint extension exercises at an intensity of 30% 1 RM with blood flow restriction in 9 healthy men, an increase in EV concentration was evident as early as 5 min post-exercise. Xhuti et al. [67] observed a significant increase in the plasma levels of tumor susceptibility gene (TSG101) in elderly individuals and young adults after 12 weeks of thrice-weekly home-based resistance training. TSG101 serves as a marker for extracellular vesicle biogenesis, indicating that 12 weeks of low-intensity resistance training augmented the concentration of EVs. Furthermore, following a single session of acute treadmill exercise intervention consisting of 40 min at a speed of 14–16 m/min in 5 Wistar rats, it was observed that low-intensity exercise did not alter the diameter of EVs in the rat serum. However, the median concentration of EVs in serum was increased from 1.1 109/mL in sedentary rats to 3 109/mL post-exercise [66]. Ma et al. [27] found that after 4 weeks of low-intensity treadmill exercise, consisting of 5 sessions per week lasting 60 min each, the number of exosomes isolated from the plasma of C57BL/6J mice was higher in the exercise group compared to the sedentary group. Nevertheless, the diameters of the exosomes isolated from the plasma of exercise and sedentary mice were 110 8 nm and 112 5 nm, respectively, showing no significant difference between the two groups. An 8-week regimen of low-intensity treadmill exercise in type II diabetes model db/db mice revealed that exercise promoted the release of miR-445-rich exosomes. This process downregulated matrix metallopeptidase 9 (MMP9), thus preventing myocardial fibrosis and exerting a cardioprotective effect [60].
4.3 Modulation of EVs Secretion by Moderate-Intensity Exercise
As with low-intensity exercise, both acute and chronic moderate-intensity exercise can also induce the generation of EVs in serum from various populations or rodents. However, they do not exert a significant impact on the diameter of exosomes. Warnier et al. [68] observed that the number of exosome-like vesicles (ELVs) in the plasma significantly increased in healthy subjects following 60 min of cycling exercise at an intensity of 55% VO2max. In another study, healthy female subjects aged 18–40 showed no change in the size of EVs in their plasma but did exhibit a significant increase in EVs quantity after 30 min of running at 59% HRR [69]. In animal experiments, following a single acute running exercise session of 40 min at a speed of 20–22 m/min, the average diameter of EVs in the serum of rats was 91.5 nm, which was not significantly different compared to the sedentary and low-intensity exercise groups. However, the concentration of EVs in the serum were significantly higher than that in the sedentary group [66]. Barcellos et al. [70] found that after a 12-week treadmill exercise program at 60% VO2max intensity in elderly rats, there was a significant increase in the content of the EV surface marker CD63 in plasma, indicating that exercise promotes the secretion of EVs. Additionally, 4 weeks of moderate-intensity treadmill exercise prior to stroke modeling in mice promoted the secretion of EVs derived from EPCs [28].
4.4 Modulation of EVs Secretion by High Intensity Exercise
Currently, it is widely believed that high intensity exercise promotes the secretion of EVs in the serum/plasma of healthy individuals. After a single bout of 20 min of cycling exercise at 70% VO2max intensity in healthy subjects, it was observed that the size of EVs remained unchanged, with measurements of 88 4.7 nm before exercise and 89 4.9 nm post-exercise. However, there was a significant increase in the concentration of EVs in their blood [71]. Meanwhile, Chong et al. [72] reached a consistent conclusion in healthy men subjected to the same duration and intensity of cycling exercise. Following a single 60-minute acute exercise session involving 11 healthy subjects, with an exercise intervention consisting of 30 min at 55% VO2max, 20 min at 70% VO2max, and 10 min at 80% VO2max, a significant increase in plasma EVs was observed four hours post-exercise [73].
However, the regulation of EV secretion in cardiovascular disease patients or individuals at risk for cardiovascular disease following high intensity exercise yields different results. Apostolopoulou et al. [74] conducted a 12-week high intensity interval training program (three times per week, consisting of five rounds of 4 min at 90% HRmax followed by 3 min at 70% HRmax) in 20 male subjects with type 2 diabetes, 12 sedentary insulin-sensitive non-diabetic subjects, and 11 insulin-resistant non-diabetic subjects. The researchers observed that exercise significantly increased serum EV concentrations in both type 2 diabetic subjects and insulin-resistant non-diabetic subjects. However, they did not find a significant effect of exercise on the EV concentration in sedentary insulin-sensitive non-diabetic subjects, nor did they observe any effect on the size of the EVs. Dimassi et al. [75] demonstrated that an 8-week high intensity interval training program (three times per week, consisting of 15 min of warm-up, three bouts of 10 min at 60–80% HRmax, with 5 min of active recovery) significantly increased endothelial microparticle levels in both normal weight and obese populations. However, a 12-week high intensity interval training regimen (three times per week, intensity at 90–95% HRmax, with 3-minute intervals of rest, totaling 38 min of exercise and rest cycles) was found to have no effect on the levels of endothelial microparticles in stable coronary artery disease patients [76]. The differential results may be attributed to the types of disease among participants and the maximum exercise intensity in the study. It has been demonstrated that exercise at a certain intensity range can promote the generation of EVs. However, exceeding the threshold intensity may not affect EV secretion. Further investigation is required to determine the intensity threshold for exercise concerning different diseases.
In conclusion, low and moderate-intensity exercise can stimulate the secretion of EVs across different populations, while high intensity exercise can enhance EV secretion in healthy individuals. Nevertheless, for individuals with cardiovascular diseases or cardiovascular risk factors, differential outcomes may arise due to variations in disease type or the maximal exercise intensity employed. Maybe, the current classification of exercise intensity levels may result in excessive exercise intensity for individuals with cardiovascular diseases or cardiovascular risk factors in the different exercise intensity levels. At present, the majority of research is focused on the regulation of EV secretion by individual low, moderate, and high intensity exercises. The evidence regarding the regulatory patterns of EV secretion by different intensity exercises is limited and inconsistent. Oliveira et al. [66] found that acute low, moderate, and high intensity exercises significantly increased the concentration of EVs in rat serum, with no significant differences observed between the various intensity levels. However, Ma et al. [27] observed that following a 4-week treadmill exercise intervention in mice, exercise intensity exhibited a dose-dependent effect on EV secretion. Moderate-intensity exercise was found to significantly enhance EV secretion compared to low-intensity exercise. Consequently, further investigation is required to elucidate the regulation rule of EV secretion by exercise at different intensities.
Furthermore, it is noteworthy that the release of EVs by exercise exhibits a temporal effect. In healthy subjects, during 30 min of cycling exercise at 55% VO2max, the quantity of ELVs increased by 313% compared to pre-exercise levels, but decreased by 53% when the exercise duration was extended to 60 min [68]. Concurrently, after a single running session in mice, an immediate increase in extracellular vesicle quantity was observed in both serum and brain tissue, which returned to pre-exercise levels within 90 min post-exercise [77]. In healthy males, following an exercise regimen consisting of eight sets of cycling at 140% VO2max for 20 s with 10 s rest intervals, a significant elevation in the levels of EVs in serum was observed immediately post-exercise. However, there was a noticeable decline in EV quantity at 30 min and 120 min post-exercise [78]. EVs are considered to be an exercise factor, and the increase in their release is thought to reflect an adaptive response of the body to physical activity. Consequently, in order to maintain elevated levels of exosome release to facilitate the organism’s adaptation to exercise and to exert cardioprotective effects, sportspeople should pay attention to the duration of each exercise session and also maintain a long-term habit of physical activity. Furthermore, the regulation of cardiovascular diseases through exercise-mediated EVs is associated not only with the quantity of their release but also with the types of constituents they contain. The following text will elucidate the regulatory effects and mechanisms of EVs secreted during exercise of varying intensities on cardiovascular diseases.
5. Regulation of Cardiovascular Disease by EVs Secreted by Exercise of Different Intensities
5.1 Ischemic Stroke
Stroke is the second leading risk factor for disability and mortality in humans [79, 80]. Among these, ischemic stroke constitutes 87% of stroke incidence. Physical activity exerts both preventive and ameliorative effects on ischemic stroke by releasing EVs and altering their contents. Wang et al. [28] demonstrated that four weeks of moderate-intensity treadmill exercise before mice stroke modeling significantly reduced brain infarct volume, apoptotic cell rate, and caspase-3 clearance capability, while also increasing cerebral microvascular density. Further research indicated that exercise promoted the secretion of EVs derived from EPCs and the enrichment of miR-126 within these exosomes, which protected against ischemic injury in mice. Concurrently, additional research demonstrated that miR-126-3p within EVs mediated the recovery from ischemic stroke facilitated by a single session of high intensity interval cycling exercise [11, 81]. A 4-week treadmill exercise intervention in rats with ischemic stroke revealed a significant decrease in miR-338 levels in the serum following analysis of the contents of exosomes. The reduction in miR-338 expression regulated the hypoxia-inducible factor alpha (HIF-) pathway, thereby protecting cerebral microvascular endothelial cells from the damage induced by ischemic stroke [82]. Eight weeks of moderate-intensity exercise increased the levels of miR-27a in the EVs secreted by EPCs, which alleviated oxidative stress by regulating mitochondrial membrane potential and then protecting cerebral neuroblastoma cells in hypertensive mice from damage caused by angiotensin II and hypoxic conditions [83]. Barcellos et al. [70] demonstrated that elderly rats subjected to 12 weeks of treadmill exercise had enhanced neurological recovery following stroke, as evidenced by elevated BDNF expression in plasma EVs. Study demonstrated that 8 weeks of high intensity interval training results in an enrichment of miR-223 in the EVs of obese individuals [75]. MiR-223 not only protected the brain from ischemia-induced neuronal death following a stroke by inhibiting calcium influx in hippocampal neurons through the suppression of glutamate receptor 2 (GluR2) and N-methyl-D-aspartate receptor 2B (NR2B), but also mitigated inflammation by targeting the NLRP3 inflammasome [80, 84]. The analysis of exosomes immediately extracted from the plasma of healthy subjects after a single bout of high intensity interval training revealed significant increases in the levels of miR-1-3p, miR-16-5p, miR-222-3p, miR-23a-3p, miR-208a-3p, and miR-150-5p [11]. Nevertheless, the elevated expression of miR-1-3p and miR-16-5p may represent a risk factor for the development of secondary cardiovascular diseases, while high levels of miR-222-3p have a detrimental impact on post-stroke recovery [10, 12]. The preceding analysis indicates that moderate and high intensity interval exercise primarily promote the recovery of ischemic stroke by regulating the expression of microRNA (miRNA) in EVs. However, high intensity continuous exercise may have adverse effects on the cerebrovascular systems. For example, Doncheva et al. [85] found that after a single 45-minute acute exercise session at 70% VO2max in adult males, the levels of miR-222-3p in plasma EVs significantly increased. MiR-222-3p can induce the injury of human brain microvascular endothelial cells and increased expression of miR-222-3p is detrimental to recovery following a stroke [10].
5.2 Atherosclerosis
Atherosclerosis (AS) is the pathophysiological basis of cardiovascular diseases, and damage to the endothelium is an important early event leading to AS [35, 86, 87]. It is of paramount importance to maintain endothelial cell homeostasis as well as endothelial integrity in order to prevent the development of cardiovascular diseases such as AS. The study revealed that EVs derived from mouse skeletal muscle post-exercise are enriched with proteins involved in mitochondrial biogenesis and fatty acid -oxidation. These EVs can be absorbed by metabolic tissue cells, such as adipocytes and hepatocytes, which regulate metabolic disorders and alleviate the occurrence and development of AS [35]. Low-intensity exercise has been demonstrated to stimulate the release of EVs containing miR-126 from EPCs [27]. MiR-126 can induce the recruitment of EPCs to injury sites through the stromal cell-derived factor-1 (SDF-1) in rats, thereby maintaining endothelial integrity [81]. Lou et al. [46] found that 9 days of moderate-intensity treadmill exercise promoted the aggregation of miR-122-5p in mouse hepatogenic EVs, and miR-122-5p could facilitate endothelial cell migration, thus aiding in the repair of damaged endothelium and other tissues. Moreover, patients with chronic coronary syndrome (CCS) exhibited a restored endothelialization capacity of EVs following 4 weeks of high intensity interval exercise, indicating that exercise may ameliorate CCS caused by AS through EVs [88]. Guescini et al. [4] demonstrated that exercise increased miR-181a-5p levels in plasma EVs of male students after a single 80% VO2max high intensity running exercise intervention. It has been demonstrated that miR-181a-5p can mitigate endothelial activation and alleviate vascular inflammation through the inhibition of nuclear factor B (NF-B), thereby exerting anti-atherosclerotic effects [89]. The aforementioned studies indicate that EVs generated by exercise of varying intensities exert anti-atherosclerotic effects through mechanisms such as the inhibition of endothelial cell inflammation and the maintenance of endothelial integrity, with miRNAs playing a crucial mediating role therein.
5.3 MI/R Injury
MI/R can inflict serious damage to the structure and function of cardiomyocytes. Prevention of MI/R injury is particularly important for the prognosis of patients with myocardial infarction. Following a single moderate-intensity cycling intervention in both young and elderly individuals, there was an increase in nicotinamide phosphoribosyltransferase (Nampt) levels in their EVs, with a particularly significant elevation observed in the young population [71]. Nampt is a rate-limiting enzyme, the expression of which is significantly reduced when MI/R injury occurs, and up-regulation of Nampt enhances nicotinamide adenine dinucleotide (NAD+) and adenosine triphosphate (ATP) to inhibit reperfusion injury during myocardial ischemia [90]. The EVs generated by acute moderate-intensity endurance exercise induced nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) and phosphorylation of heat shock protein 27 (HSP27) in cardiomyocytes, exerting antioxidant effects, thereby conferring protective effects on the heart [91]. Moreover, studies have demonstrated that high intensity exercise induces a substantial increase in brown adipose tissue in mice [92, 93, 94]. This process protects the heart from MI/R injury by secreting EVs enriched in microRNAs, including miR-125b-5p, miR-128-3p, and miR-30d-5p to inhibit the pro-apoptotic MAPK pathway [8]. Elevated levels of catalase (CAT) in serum EVs were found in young men following an acute high intensity interval exercise intervention [78], and specific overexpression of CAT in the heart has a cardioprotective effect on the heart by modulating autophagy, iron death, and oxidative stress in cardiomyocytes [95]. In conclusion, exercise of different intensities can play a role in cardioprotection and prevention of myocardial ischemia/reperfusion injury by regulating the expression of proteins and miRNAs in EVs.
6. Clinical Applications of Exercise-Induced EVs in Cardiovascular Protection
6.1 EVs-Containing miRNAs as Potential Biomarkers for Diagnosis of Cardiovascular Diseases
MiRNAs are one of the most widely studied cargos of EVs in exercise regulating cardiovascular function. The expression of miRNAs can be modulated by cardiovascular diseases. Altered miRNA expression have been found in stroke, acute myocardial infarction, heart failure and other cardiovascular diseases [96]. In stroke patients, miR-145 and miR-21 levels were significantly higher compared with that in healthy controls [97]. MiR-208, miR-499 and miR-1 expressions were remarkably upregulated in acute myocardial infraction patients [98, 99, 100]. MiR-29b and miR-455-1 levels were positively correlated with the myocardial fibrosis and myocyte uncoupling [60]. The cardiovascular system is extremely sensitive to the changes in miRNAs levels [96]. Meanwhile, EVs are important sources and major transportation vehicles of miRNAs. Therefore, EVs containing miRNAs might become potential biomarkers for cardiovascular diseases. In addition, exercise exerts cardiovascular protection through increasing the expression of miR342-5p, miR126, miRNA-125b-5p, miR-445, miR-122-5p and other miRNAs [8, 26, 27, 46, 60]. These novel miRNAs might serve as biomarkers of exercise effectiveness in the exercise rehabilitation of cardiovascular diseases. In brief, EVs containing miRNAs are expected to be potential biomarkers for the predication, diagnosis, and therapy of cardiovascular diseases.
6.2 Exercise-Induced EVs as a Potential Strategy for the Therapies of Cardiovascular Diseases
Exercise improve cardiovascular function through altering the contents of EVs (Table 2, Ref. [8, 11, 25, 26, 28, 35, 46, 60, 70, 71, 78, 81, 82, 83, 91]), suggesting the potential therapeutic role of exercise-induced EVs in cardiovascular diseases. Emerging animal studies have confirmed the therapeutic effects of exercise-induced EVs [33, 81]. Liu et al. [33] isolated the circulating EVs from the plasma of rats subjected to 4 weeks of moderate aerobic exercise. The EVs were then added to human umbilical vein endothelial cells in vitro and cutaneous wounds in diabetic rats, respectively. The results demonstrated that EVs promoted angiogenesis and repair of skin defects, indicating that exercise-induced circulating EVs could be utilized as a therapy to active angiogenesis and diabetic wound healing. Alehossein et al. [81] found that treatment of sedentary mice with exosomes isolated from the plasma and the muscle of high intensity interval trained mice ameliorated glucose tolerance, insulin sensitivity, and reduced plasma levels of triglycerides. At present, the evaluation of therapeutic effects of exercise-induced EVs on cardiovascular protection has been focused on animal studies. Further human clinical trials are needed to confirm the effects of exercise-induced EVs for targeted therapy. Maybe, collecting EVs from healthy people or long-term exercisers and injecting into cardiovascular patients could be trialled, which may become a new approach for treating cardiovascular diseases. In addition, Liu et al. [101] uncovered that long-term caloric restriction alleviated aging-related-fibrosis of kidney through downregulation of miR-21 in EVs. Fasting regulated energy metabolism by promoting the secretion of EVs from adipose tissue [102]. Therefore, engineered EVs containing specific miRNAs or proteins could be constructed to mimic the effects of exercise or the other useful interventions, which may make cell-free targeted therapy for cardiovascular and other diseases possible.
Table 2.
The main research on the promotion of cardiovascular health by exercise-induced EVs.
| Research subjects | Exercise protocols | EVs cargo changes | Functional changes | Literature sources | ||
| Intensity | Duration and frequency | Forms | ||||
| Healthy adults (n = 3) | 50% VO2max | One time, 45 min | Treadmill running | SOD3 | Promoting angiogenesis in endothelial cells | [25] |
| Healthy men (n = 21) | 70% HRmax | One time, 30 min | Treadmill running | MAP2K1 | Protecting cardiomyocytes from oxidative stress | [91] |
| Healthy men (n = 10) | 60 s of exercise, with a 75 s rest in between, repeated for a total of 10 sets | One time | Cycling | miR-126-3p | Protecting endothelial cells from hypoxic damage | [11] |
| Healthy men (n = 40) | 70% VO2max | 20 min | Cycling | Nampt | Suppressing myocardial cell apoptosis and promoting their survival | [71] |
| Young man (n = 17) | 140% VO2max for 20 s and rest for 10 s in between, repeated for a total of 8 sets | 4 min | Cycling | CAT | Providing protective effects on the heart | [78] |
| 8–10 week-old C57BL/6J mice | 5 m/min | 60 min/d, 5 d/w, 4 w | Treadmill running | miR-126 | Inducing the recruitment of EPCs to the site of injury to maintain endothelial integrity | [81] |
| db/db mice | 7 m/min | 300 m/d, 8 w | Treadmill running | miR-445, miR-29b | Preventing myocardial fibrosis and uncoupling | [60] |
| Adult male | 6.59 m/min | 40 min/d, 6 d/w, 4 w | Wheel training | miR-338 | Protecting brain microvascular endothelial cells | [82] |
| Sprague-Dawley (SD) rats performed transient middle cerebral artery occlusion surgery | ||||||
| 8 week-old C57BL/6J mice | 18 m/min | 60 min/d, 9d | Treadmill running | miR-122-5p | Enhancing endothelial cell fatty acid utilization to promote angiogenesis | [46] |
| 7–8 week-old hypertensive transgenic mice | 10 m/min | 60 min/d, 5 d/w,8 w | Treadmill running | miR-27a | Suppressing ROS production in N2a cells to prevent damage caused by oxidative stress | [83] |
| 8–10 week-old C57BL/6J mice | 10 m/min | 5 d/w, 4 w | Treadmill running | miR-126 | Decreasing cell apoptosis | [28] |
| 2-month-old and 22-month-old Wistar rats | 60% VO2max | 20 min/time, 3 times/w, 12 w | Aerobic, acrobatic, resistance and combined | BDNF | Promoting the recovery of neurological function after stroke | [70] |
| 8–10 week-old C57BL/6J mice | - | 30 min/d, 4 w | Swimming | - | Alleviating atherosclerosis in ApoE-deficient mice | [35] |
| 6–8 week-old C57BL/6J mice | - | 90 min/d, 2 times/d, 4 w | Swimming | miR-125-5p miR-128-3p miR-30d-5p | Protecting the heart from myocardial ischemia/reperfusion injury | [8] |
| 6 week-old SD rats | - | 90 min/d, 7 times/w, 4 w | Swimming | miR342-5p | Protecting the heart against myocardial ischemia/reperfusion | [26] |
Notes: SOD3, extracellular superoxide dismutase; MAP2K1, mitogen-activated protein kinase kinase 1; Nampt, nicotinamide phosphoribosyltransferase; CAT, catalase; BDNF, brain-derived neurotrophic factor; EPCs, endothelial progenitor cells; ROS, reactive oxygen species; N2a cell, mouse neuroblastoma N2a cells; ApoE, apolipoprotein E; EVs, extracellular vesicles; VO2max, maximal oxygen uptake; HRmax, maximal heart rate; d, day; w, week; m, minute. indicates increase, indicates decrease.
7. Discussions and Conclusions
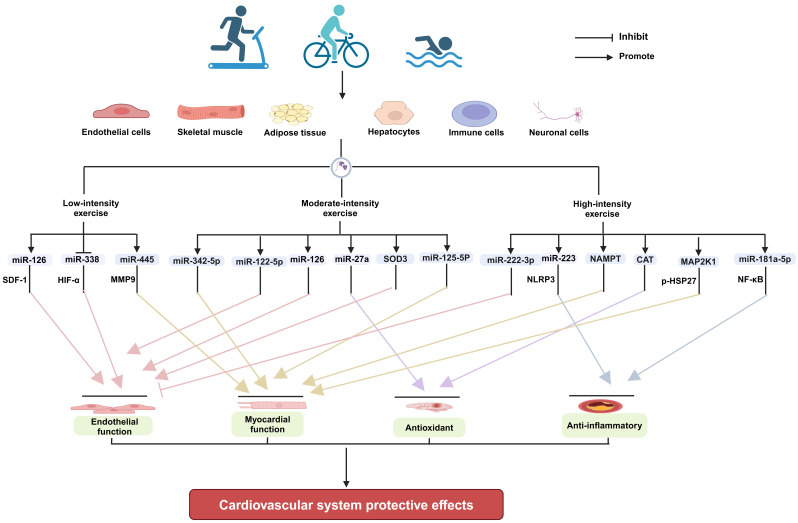
Exercise improving cardiovascular health is a systemic and integrative effect. Exercise can produce direct effects locally in tissues and organs, such as enhancing muscle strength, increasing stroke volume, promoting angiogenesis, and raising insulin sensitivity [103, 104]. Meanwhile, exercise can also elicit the secretion of cardioprotective exerkines, such as miRNAs from multiple tissues and organs of body, mediating tissue-organ interactions by endocrine and paracrine means [26, 105, 106]. The direct and indirect effects collectively contribute to cardiovascular health. EVs are important transportation vehicles for miRNAs and other types of exerkines. Exercise of different intensities can modulate the release of EVs with biological functions from specific tissue cells, such as endothelial cells, skeletal muscle, and adipose tissue, and exert a protective effect on the cardiovascular system by altering their contents (Fig. 1).
Fig. 1.
The mechanisms underlying the cardioprotective effects of EVs produced in response to exercise of varying intensities (Figure created with BioRender.com). EVs, extracellular vesicles; SOD3, extracellular superoxide dismutase; CAT, catalase; MAP2K1, mitogen-activated protein kinase kinase 1; SDF-1, stromal cell-derived factor-1; HIF-, hypoxia-inducible factor alpha; MMP9, matrix metallopeptidase 9; NLRP3, NOD-like receptor family pyrin domain-containing 3; HSP27, heat shock protein 27; NF-B, nuclear factor B; Nampt, nicotinamide phosphoribosyltransferase.
Current research generally suggests that all intensities of exercise are capable of increasing the secretion of EVs in serum/plasma in different populations, except for high intensity exercise performed by populations with cardiovascular disease or cardiovascular risk [32, 68, 71]. Moreover, in addition to high intensity continuous exercise, low, moderate and high intensity interval exercise can improve cardiovascular function through exercise-induced EVs [25, 60, 91, 107]. However, there is limited research on the regulatory law of EV secretion in response to low, moderate, and high intensities exercise, and comprehensive studies are needed. Meanwhile, at present, most studies are looking at plasma/serum samples, where it is very difficult to parse out differences between EVs and the proteins in the plasma/serum. It is well known that there are exercise-related proteins changes in blood [108]. These plasma proteins form protein corona of EVs, which may or may not be removed from the plasma depending on current EVs isolation methods, such as size-exclusion chromatography (SEC) and the ultracentrifuge method [109]. Therefore, the role of protein corona is ignored. It is also difficult to determine whether inner cargos of EVs (proteins, nucleic acids, lipids, etc.) or protein corona are playing a major role in improving cardiovascular diseases. In addition, for the inner cargo of EVs, each study identifies their changes, but these are generally poorly reproduced between studies, especially the studies with same exercise intensity. We may understand that exercise-secreted EVs exerting a cardioprotective function are controlled by multiple signaling pathways. All these proteins or nucleic acids play effective roles. However, their roles maybe not equal. Which protein or nucleic acid plays a key role remains to be determined. This is particularly important for precise treatment in cardiovascular diseases by simulating the effects of exercise in clinic. Therefore, there is still a long way to go through using exercise-secreted EVs or constructing engineered EVs mimicking the effects of exercise for clinical applications in cardiovascular or other diseases.
Acknowledgment
Not applicable.
Abbreviations
EVs, extracellular vesicles; ESEs, early-sorting endosomes; LSEs, late-sorting endosomes; MVBs, multivesicular bodies; Akt, protein kinase B; Jnk2, c-Jun N-terminal kinase 2; EPCs, endothelial progenitor cells; HSP60, heat shock protein 60; FNDC5, fibronectin type III domain-containing protein 5; MAPK, mitogen-activated protein kinase; MI/R, myocardial ischemia-reperfusion; LAMP1, lysosomal associated membrane protein 1; AGPAT1, 1-acyl-sn-glycerol-3-phosphate acyltransferase; VO2max, maximal oxygen uptake; HRmax, maximal heart rate; HRR, heart rate reserve; 1 RM, percentage of one repetition maximum; MMP9, matrix metallopeptidase 9; ELVs, exosome-like vesicles; HIF-, hypoxia-inducible factor alpha; BDNF, brain-derived neurotrophic factor; GluR2, glutamate receptor 2; NR2B, N-methyl-D-aspartate receptor 2B; NLRP3, NOD-like receptor family pyrin domain-containing 3; AS, atherosclerosis; SDF-1, stromal cell-derived factor-1; CCS, chronic coronary syndrome; NF-B, nuclear factor B; Nampt, nicotinamide phosphoribosyltransferase; NAD+, nicotinamide adenine dinucleotide; Nrf2, nuclear factor erythroid 2-related factor 2; HSP27, heat shock protein 27; miRNAs, microRNAs; CAT, catalase; VEGF, vascular endothelial growth factor; JAK, Janus tyrosine kinase; STAT, signal transducer and activator of transcription; AD, Alzheimer’s disease; CASP2, caspase 2; ApoE-/-, apolipoprotein E knock out; SOD3, extracellular superoxide dismutase; SD rats, Sprague-Dawley rats; ROS, reactive oxygen species; N2a cell, mouse neuroblastoma N2a cells; mPTP, mitochondrial permeability transition pores; MAP2K1, mitogen-activated protein kinase kinase 1; SEC, size-exclusion chromatograph; TSG101, tumor susceptibility gene; ATP, adenosine triphosphate.
Funding Statement
This research was supported by the Natural Science Foundation of Shandong Province (Grant No. ZR2020QC092), the National Natural Science Foundation of China (Grant No. 32000927) and the Graduate Student Research Grant from Shandong Second Medical University (Grant No. 2023YJSCX020).
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Min Cheng, Email: mincheng@sdsmu.edu.cn.
Yan-Xia Wang, Email: wangyanxia6666@sdsmu.edu.cn.
Author Contributions
All authors contributed significantly to searching the literature and writing original manuscript. YXW and MC had the idea for the paper, reviewed and edited it critically for important intellectual content. PS, YXW, and YYS performed the literature search. PS, YQ, YYJ and XMG substantially contributed to the conception of the paper, wrote the original draft and designed the figures. YXW, MC and XMG edited and reviewed the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Not applicable.
Funding
This research was supported by the Natural Science Foundation of Shandong Province (Grant No. ZR2020QC092), the National Natural Science Foundation of China (Grant No. 32000927) and the Graduate Student Research Grant from Shandong Second Medical University (Grant No. 2023YJSCX020).
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Tang J, Wei Y, Pi C, Zheng W, Zuo Y, Shi P, et al. The therapeutic value of bifidobacteria in cardiovascular disease. NPJ Biofilms and Microbiomes . 2023;9:82. doi: 10.1038/s41522-023-00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Femminò S, Penna C, Margarita S, Comità S, Brizzi MF, Pagliaro P. Extracellular vesicles and cardiovascular system: Biomarkers and Cardioprotective Effectors. Vascular Pharmacology . 2020;135:106790. doi: 10.1016/j.vph.2020.106790. [DOI] [PubMed] [Google Scholar]
- [3].Frühbeis C, Helmig S, Tug S, Simon P, Krämer-Albers EM. Physical exercise induces rapid release of small extracellular vesicles into the circulation. Journal of Extracellular Vesicles . 2015;4:28239. doi: 10.3402/jev.v4.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guescini M, Canonico B, Lucertini F, Maggio S, Annibalini G, Barbieri E, et al. Muscle Releases Alpha-Sarcoglycan Positive Extracellular Vesicles Carrying miRNAs in the Bloodstream. PloS One . 2015;10:e0125094. doi: 10.1371/journal.pone.0125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nature Reviews. Molecular Cell Biology . 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- [6].Lai Z, Liang J, Zhang J, Mao Y, Zheng X, Shen X, et al. Exosomes as a delivery tool of exercise-induced beneficial factors for the prevention and treatment of cardiovascular disease: a systematic review and meta-analysis. Frontiers in Physiology . 2023;14:1190095. doi: 10.3389/fphys.2023.1190095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nie Y, Sato Y, Garner RT, Kargl C, Wang C, Kuang S, et al. Skeletal muscle-derived exosomes regulate endothelial cell functions via reactive oxygen species-activated nuclear factor-κB signalling. Experimental Physiology . 2019;104:1262–1273. doi: 10.1113/EP087396. [DOI] [PubMed] [Google Scholar]
- [8].Zhao H, Chen X, Hu G, Li C, Guo L, Zhang L, et al. Small Extracellular Vesicles From Brown Adipose Tissue Mediate Exercise Cardioprotection. Circulation Research . 2022;130:1490–1506. doi: 10.1161/CIRCRESAHA.121.320458. [DOI] [PubMed] [Google Scholar]
- [9].Siqueira IR, Palazzo RP, Cechinel LR. Circulating extracellular vesicles delivering beneficial cargo as key players in exercise effects. Free Radical Biology & Medicine . 2021;172:273–285. doi: 10.1016/j.freeradbiomed.2021.06.007. [DOI] [PubMed] [Google Scholar]
- [10].Li J, Wang J, Wang Z. Circ_0006768 upregulation attenuates oxygen-glucose deprivation/reoxygenation-induced human brain microvascular endothelial cell injuries by upregulating VEZF1 via miR-222-3p inhibition. Metabolic Brain Disease . 2021;36:2521–2534. doi: 10.1007/s11011-021-00775-8. [DOI] [PubMed] [Google Scholar]
- [11].D’Souza RF, Woodhead JST, Zeng N, Blenkiron C, Merry TL, Cameron-Smith D, et al. Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. American Journal of Physiology. Endocrinology and Metabolism . 2018;315:E723–E733. doi: 10.1152/ajpendo.00138.2018. [DOI] [PubMed] [Google Scholar]
- [12].Badacz R, Kleczyński P, Legutko J, Żmudka K, Gacoń J, Przewłocki T, et al. Expression of miR-1-3p, miR-16-5p and miR-122-5p as Possible Risk Factors of Secondary Cardiovascular Events. Biomedicines . 2021;9:1055. doi: 10.3390/biomedicines9081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sahoo S, Adamiak M, Mathiyalagan P, Kenneweg F, Kafert-Kasting S, Thum T. Therapeutic and Diagnostic Translation of Extracellular Vesicles in Cardiovascular Diseases: Roadmap to the Clinic. Circulation . 2021;143:1426–1449. doi: 10.1161/CIRCULATIONAHA.120.049254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tetta C, Bruno S, Fonsato V, Deregibus MC, Camussi G. The role of microvesicles in tissue repair. Organogenesis . 2011;7:105–115. doi: 10.4161/org.7.2.15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Y, Chen LM, Liu ML. Microvesicles and diabetic complications–novel mediators, potential biomarkers and therapeutic targets. Acta Pharmacologica Sinica . 2014;35:433–443. doi: 10.1038/aps.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wilhelm EN, Mourot L, Rakobowchuk M. Exercise-Derived Microvesicles: A Review of the Literature. Sports Medicine (Auckland, N.Z.) . 2039;48:2025–2039. doi: 10.1007/s40279-018-0943-z. [DOI] [PubMed] [Google Scholar]
- [17].Zhang Q, Higginbotham JN, Jeppesen DK, Yang YP, Li W, McKinley ET, et al. Transfer of Functional Cargo in Exomeres. Cell Reports . 2019;27:940–954.e6. doi: 10.1016/j.celrep.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nature Cell Biology . 2018;20:332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang K, Xiao Q, Niu M, Pan X, Zhu X. Exosomes in atherosclerosis: Convergence on macrophages. International Journal of Biological Sciences . 2022;18:3266–3281. doi: 10.7150/ijbs.71862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Krylova SV, Feng D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. International Journal of Molecular Sciences . 2023;24:1337. doi: 10.3390/ijms24021337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yue B, Yang H, Wang J, Ru W, Wu J, Huang Y, et al. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Proliferation . 2020;53:e12857. doi: 10.1111/cpr.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (New York, N.Y.) . 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jeppesen DK, Zhang Q, Franklin JL, Coffey RJ. Extracellular vesicles and nanoparticles: emerging complexities. Trends in Cell Biology . 2023;33:667–681. doi: 10.1016/j.tcb.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chung J, Kim KH, Yu N, An SH, Lee S, Kwon K. Fluid Shear Stress Regulates the Landscape of microRNAs in Endothelial Cell-Derived Small Extracellular Vesicles and Modulates the Function of Endothelial Cells. International Journal of Molecular Sciences . 2022;23:1314. doi: 10.3390/ijms23031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abdelsaid K, Sudhahar V, Harris RA, Das A, Youn SW, Liu Y, et al. Exercise improves angiogenic function of circulating exosomes in type 2 diabetes: Role of exosomal SOD3. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology . 2022;36:e22177. doi: 10.1096/fj.202101323R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hou Z, Qin X, Hu Y, Zhang X, Li G, Wu J, et al. Longterm Exercise-Derived Exosomal miR-342-5p: A Novel Exerkine for Cardioprotection. Circulation Research . 2019;124:1386–1400. doi: 10.1161/CIRCRESAHA.118.314635. [DOI] [PubMed] [Google Scholar]
- [27].Ma C, Wang J, Liu H, Chen Y, Ma X, Chen S, et al. Moderate Exercise Enhances Endothelial Progenitor Cell Exosomes Release and Function. Medicine and Science in Sports and Exercise . 2018;50:2024–2032. doi: 10.1249/MSS.0000000000001672. [DOI] [PubMed] [Google Scholar]
- [28].Wang J, Liu H, Chen S, Zhang W, Chen Y, Yang Y. Moderate exercise has beneficial effects on mouse ischemic stroke by enhancing the functions of circulating endothelial progenitor cell-derived exosomes. Experimental Neurology . 2020;330:113325. doi: 10.1016/j.expneurol.2020.113325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature Reviews. Endocrinology . 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- [30].Li T, Han X, Chen S, Wang B, Teng Y, Cheng W, et al. Effects of Exercise on Extracellular Vesicles in Patients with Metabolic Dysfunction: a Systematic Review. Journal of Cardiovascular Translational Research . 2023;16:97–111. doi: 10.1007/s12265-022-10282-5. [DOI] [PubMed] [Google Scholar]
- [31].Karvinen S, Korhonen TM, Sievänen T, Karppinen JE, Juppi HK, Jakoaho V, et al. Extracellular vesicles and high-density lipoproteins: Exercise and oestrogen-responsive small RNA carriers. Journal of Extracellular Vesicles . 2023;12:e12308. doi: 10.1002/jev2.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Just J, Yan Y, Farup J, Sieljacks P, Sloth M, Venø M, et al. Blood flow-restricted resistance exercise alters the surface profile, miRNA cargo and functional impact of circulating extracellular vesicles. Scientific Reports . 2020;10:5835. doi: 10.1038/s41598-020-62456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu HY, Yu LF, Zhou TG, Wang YD, Sun DH, Chen HR, et al. Lipopolysaccharide-stimulated bone marrow mesenchymal stem cells-derived exosomes inhibit H2O2-induced cardiomyocyte inflammation and oxidative stress via regulating miR-181a-5p/ATF2 axis. European Review for Medical and Pharmacological Sciences . 2020;24:10069–10077. doi: 10.26355/eurrev_202010_23224. [DOI] [PubMed] [Google Scholar]
- [34].Barone R, Macaluso F, Sangiorgi C, Campanella C, Marino Gammazza A, Moresi V, et al. Skeletal muscle Heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 α1 expression. Scientific Reports . 2016;6:19781. doi: 10.1038/srep19781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang Y, Liu Y, Zhang S, Li N, Xing C, Wang C, et al. Exercise Improves Metabolism and Alleviates Atherosclerosis via Muscle-Derived Extracellular Vesicles. Aging and Disease . 2023;14:952–965. doi: 10.14336/AD.2022.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chi C, Fu H, Li YH, Zhang GY, Zeng FY, Ji QX, et al. Exerkine fibronectin type-III domain-containing protein 5/irisin-enriched extracellular vesicles delay vascular ageing by increasing SIRT6 stability. European Heart Journal . 2022;43:4579–4595. doi: 10.1093/eurheartj/ehac431. [DOI] [PubMed] [Google Scholar]
- [37].Shi H, Hao X, Sun Y, Zhao Y, Wang Y, Cao X, et al. Exercise-inducible circulating extracellular vesicle irisin promotes browning and the thermogenic program in white adipose tissue. Acta Physiologica (Oxford, England) . 2024;240:e14103. doi: 10.1111/apha.14103. [DOI] [PubMed] [Google Scholar]
- [38].Di W, Amdanee N, Zhang W, Zhou Y. Long-term exercise-secreted extracellular vesicles promote browning of white adipocytes by suppressing miR-191a-5p. Life Sciences . 2020;263:118464. doi: 10.1016/j.lfs.2020.118464. [DOI] [PubMed] [Google Scholar]
- [39].Kermanizadeh A, Gaiser BK, Johnston H, Brown DM, Stone V. Toxicological effect of engineered nanomaterials on the liver. British Journal of Pharmacology . 2014;171:3980–3987. doi: 10.1111/bph.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Li W, Yu L. Role and therapeutic perspectives of extracellular vesicles derived from liver and adipose tissue in metabolic dysfunction-associated steatotic liver disease. Artificial Cells, Nanomedicine, and Biotechnology . 2024;52:355–369. doi: 10.1080/21691401.2024.2360008. [DOI] [PubMed] [Google Scholar]
- [41].Zhao J, Hu L, Gui W, Xiao L, Wang W, Xia J, et al. Hepatocyte TGF-β Signaling Inhibiting WAT Browning to Promote NAFLD and Obesity Is Associated With Let-7b-5p. Hepatology Communications . 2022;6:1301–1321. doi: 10.1002/hep4.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jung JW, Kim JE, Kim E, Lee H, Lee H, Shin EA, et al. Liver-originated small extracellular vesicles with TM4SF5 target brown adipose tissue for homeostatic glucose clearance. Journal of Extracellular Vesicles . 2022;11:e12262. doi: 10.1002/jev2.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu X, Shao Y, Han L, Zhang R, Chen J. Emerging Evidence Linking the Liver to the Cardiovascular System: Liver-derived Secretory Factors. Journal of Clinical and Translational Hepatology . 2023;11:1246–1255. doi: 10.14218/JCTH.2022.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zuo R, Ye LF, Huang Y, Song ZQ, Wang L, Zhi H, et al. Hepatic small extracellular vesicles promote microvascular endothelial hyperpermeability during NAFLD via novel-miRNA-7. Journal of Nanobiotechnology . 2021;19:396. doi: 10.1186/s12951-021-01137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yilmaz Y, Kurt R, Yonal O, Polat N, Celikel CA, Gurdal A, et al. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Atherosclerosis . 2010;211:182–186. doi: 10.1016/j.atherosclerosis.2010.01.049. [DOI] [PubMed] [Google Scholar]
- [46].Lou J, Wu J, Feng M, Dang X, Wu G, Yang H, et al. Exercise promotes angiogenesis by enhancing endothelial cell fatty acid utilization via liver-derived extracellular vesicle miR-122-5p. Journal of Sport and Health Science . 2022;11:495–508. doi: 10.1016/j.jshs.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Robbins JM, Gerszten RE. Exercise, exerkines, and cardiometabolic health: from individual players to a team sport. The Journal of clinical investigation . 2023;133:e168121. doi: 10.1172/JCI168121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ohayon L, Zhang X, Dutta P. The role of extracellular vesicles in regulating local and systemic inflammation in cardiovascular disease. Pharmacological Research . 2021;170:105692. doi: 10.1016/j.phrs.2021.105692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xiong YY, Gong ZT, Tang RJ, Yang YJ. The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction. Theranostics . 2021;11:1046–1058. doi: 10.7150/thno.53326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Brahmer A, Neuberger E, Esch-Heisser L, Haller N, Jorgensen MM, Baek R, et al. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. Journal of Extracellular Vesicles . 2019;8:1615820. doi: 10.1080/20013078.2019.1615820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Highton PJ, White AEM, Nixon DGD, Wilkinson TJ, Neale J, Martin N, et al. Influence of acute moderate- to high-intensity aerobic exercise on markers of immune function and microparticles in renal transplant recipients. American Journal of Physiology. Renal Physiology . 2020;318:F76–F85. doi: 10.1152/ajprenal.00332.2019. [DOI] [PubMed] [Google Scholar]
- [52].Wang H, Zheng Q, Lu Z, Wang L, Ding L, Xia L, et al. Role of the nervous system in cancers: a review. Cell Death Discovery . 2021;7:76. doi: 10.1038/s41420-021-00450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dresselhaus EC, Harris KP, Blanchette CR, Koles K, Del Signore SJ, Pescosolido MF, et al. ESCRT disruption provides evidence against trans-synaptic signaling via extracellular vesicles. The Journal of Cell Biology . 2024;223:e202405025. doi: 10.1083/jcb.202405025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mason AJ, Deppmann C, Winckler B. Emerging Roles of Neuronal Extracellular Vesicles at the Synapse. The Neuroscientist: a Review Journal Bringing Neurobiology, Neurology and Psychiatry . 2024;30:199–213. doi: 10.1177/10738584231160521. [DOI] [PubMed] [Google Scholar]
- [55].Zumkehr J, Rodriguez-Ortiz CJ, Medeiros R, Kitazawa M. Inflammatory Cytokine, IL-1β, Regulates Glial Glutamate Transporter via microRNA-181a in vitro. Journal of Alzheimer’s Disease: JAD . 2018;63:965–975. doi: 10.3233/JAD-170828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Luo H, Ye G, Liu Y, Huang D, Luo Q, Chen W, et al. miR-150-3p enhances neuroprotective effects of neural stem cell exosomes after hypoxic-ischemic brain injury by targeting CASP2. Neuroscience Letters . 2022;779:136635. doi: 10.1016/j.neulet.2022.136635. [DOI] [PubMed] [Google Scholar]
- [57].Wang J, Cao B, Sun R, Chen Y, Feng J. Exosome-transported Long Non-coding Ribonucleic Acid H19 Induces Blood-brain Barrier Disruption in Cerebral Ischemic Stroke Via the H19/micro Ribonucleic Acid-18a/Vascular Endothelial Growth factor Axis. Neuroscience . 2022;500:41–51. doi: 10.1016/j.neuroscience.2022.07.028. [DOI] [PubMed] [Google Scholar]
- [58].Katsur M, He Z, Vinokur V, Corteling R, Yellon DM, Davidson SM. Exosomes from neuronal stem cells may protect the heart from ischaemia/reperfusion injury via JAK1/2 and gp130. Journal of Cellular and Molecular Medicine . 2021;25:4455–4465. doi: 10.1111/jcmm.16515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Delgado-Peraza F, Nogueras-Ortiz C, Simonsen AH, Knight DD, Yao PJ, Goetzl EJ, et al. Neuron-derived extracellular vesicles in blood reveal effects of exercise in Alzheimer’s disease. Alzheimer’s Research & Therapy . 2023;15:156. doi: 10.1186/s13195-023-01303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chaturvedi P, Kalani A, Medina I, Familtseva A, Tyagi SC. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. Journal of Cellular and Molecular Medicine . 2015;19:2153–2161. doi: 10.1111/jcmm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fakouri A, Razavi ZS, Mohammed AT, Hussein AHA, Afkhami H, Hooshiar MH. Applications of mesenchymal stem cell-exosome components in wound infection healing: new insights. Burns & Trauma . 2024;12:tkae021. doi: 10.1093/burnst/tkae021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li QQ, Qin KR, Zhang W, Guan XM, Cheng M, Wang YX. Advancements in the Regulation of Different-Intensity Exercise Interventions on Arterial Endothelial Function. Reviews in Cardiovascular Medicine . 2023;24:306. doi: 10.31083/j.rcm2411306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].American College of Sports Medicine Position Stand American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Medicine and Science in Sports and Exercise . 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- [64].Laurent CM, Vervaecke LS, Kutz MR, Green JM. Sex-specific responses to self-paced, high-intensity interval training with variable recovery periods. Journal of Strength and Conditioning Research . 2014;28:920–927. doi: 10.1519/JSC.0b013e3182a1f574. [DOI] [PubMed] [Google Scholar]
- [65].Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology . 1979;47:1278–1283. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- [66].Oliveira GP, Jr, Porto WF, Palu CC, Pereira LM, Petriz B, Almeida JA, et al. Effects of Acute Aerobic Exercise on Rats Serum Extracellular Vesicles Diameter, Concentration and Small RNAs Content. Frontiers in Physiology . 2018;9:532. doi: 10.3389/fphys.2018.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Xhuti D, Nilsson MI, Manta K, Tarnopolsky MA, Nederveen JP. Circulating exosome-like vesicle and skeletal muscle microRNAs are altered with age and resistance training. The Journal of Physiology . 2023;601:5051–5073. doi: 10.1113/JP282663. [DOI] [PubMed] [Google Scholar]
- [68].Warnier G, De Groote E, Britto FA, Delcorte O, Nederveen JP, Nilsson MI, et al. Effects of an acute exercise bout in hypoxia on extracellular vesicle release in healthy and prediabetic subjects. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology . 2022;322:R112–R122. doi: 10.1152/ajpregu.00220.2021. [DOI] [PubMed] [Google Scholar]
- [69].Mohammad S, Hutchinson KA, da Silva DF, Bhattacharjee J, McInnis K, Burger D, et al. Circulating small extracellular vesicles increase after an acute bout of moderate-intensity exercise in pregnant compared to non-pregnant women. Scientific Reports . 2021;11:12615. doi: 10.1038/s41598-021-92180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Barcellos N, Cechinel LR, de Meireles LCF, Lovatel GA, Bruch GE, Carregal VM, et al. Effects of exercise modalities on BDNF and IL-1β content in circulating total extracellular vesicles and particles obtained from aged rats. Experimental Gerontology . 2020;142:111124. doi: 10.1016/j.exger.2020.111124. [DOI] [PubMed] [Google Scholar]
- [71].Chong MC, Silva A, James PF, Wu SSX, Howitt J. Exercise increases the release of NAMPT in extracellular vesicles and alters NAD+ activity in recipient cells. Aging Cell . 2022;21:e13647. doi: 10.1111/acel.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chong MC, Shah AD, Schittenhelm RB, Silva A, James PF, Wu SSX, et al. Acute exercise-induced release of innate immune proteins via small extracellular vesicles changes with aerobic fitness and age. Acta Physiologica (Oxford, England) . 2024;240:e14095. doi: 10.1111/apha.14095. [DOI] [PubMed] [Google Scholar]
- [73].Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metabolism . 2018;27:237–251.e4. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- [74].Apostolopoulou M, Mastrototaro L, Hartwig S, Pesta D, Straßburger K, de Filippo E, et al. Metabolic responsiveness to training depends on insulin sensitivity and protein content of exosomes in insulin-resistant males. Science Advances . 2021;7:eabi9551. doi: 10.1126/sciadv.abi9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dimassi S, Karkeni E, Laurant P, Tabka Z, Landrier JF, Riva C. Microparticle miRNAs as Biomarkers of Vascular Function and Inflammation Response to Aerobic Exercise in Obesity? Obesity (Silver Spring, Md.) . 2018;26:1584–1593. doi: 10.1002/oby.22298. [DOI] [PubMed] [Google Scholar]
- [76].Van Craenenbroeck EM, Frederix G, Pattyn N, Beckers P, Van Craenenbroeck AH, Gevaert A, et al. Effects of aerobic interval training and continuous training on cellular markers of endothelial integrity in coronary artery disease: a SAINTEX-CAD substudy. American Journal of Physiology. Heart and Circulatory Physiology . 2015;309:H1876–H1882. doi: 10.1152/ajpheart.00341.2015. [DOI] [PubMed] [Google Scholar]
- [77].Zhang R, Liang X, Tang S, Song L, Zhang J, Du Y. Short-Term High-Intensity Treadmill Exercise Promotes Ceramide-Dependent Extracellular Vesicle Secretion in the Central Nervous System of Mice. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research . 2021;27:e929609. doi: 10.12659/MSM.929609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kobayashi Y, Eguchi A, Tamai Y, Fukuda S, Tempaku M, Izuoka K, et al. Protein Composition of Circulating Extracellular Vesicles Immediately Changed by Particular Short Time of High-Intensity Interval Training Exercise. Frontiers in Physiology . 2021;12:693007. doi: 10.3389/fphys.2021.693007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Pu L, Wang L, Zhang R, Zhao T, Jiang Y, Han L. Projected Global Trends in Ischemic Stroke Incidence, Deaths and Disability-Adjusted Life Years From 2020 to 2030. Stroke . 2023;54:1330–1339. doi: 10.1161/STROKEAHA.122.040073. [DOI] [PubMed] [Google Scholar]
- [80].Harraz MM, Eacker SM, Wang X, Dawson TM, Dawson VL. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America . 2012;109:18962–18967. doi: 10.1073/pnas.1121288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Alehossein P, Taheri M, Tayefeh Ghahremani P, Dakhlallah D, Brown CM, Ishrat T, et al. Transplantation of Exercise-Induced Extracellular Vesicles as a Promising Therapeutic Approach in Ischemic Stroke. Translational Stroke Research . 2023;14:211–237. doi: 10.1007/s12975-022-01025-4. [DOI] [PubMed] [Google Scholar]
- [82].Huang M, Xiao C, Zhang L, Li L, Luo J, Chen L, et al. Bioinformatic Analysis of Exosomal MicroRNAs of Cerebrospinal Fluid in Ischemic Stroke Rats After Physical Exercise. Neurochemical Research . 2021;46:1540–1553. doi: 10.1007/s11064-021-03294-1. [DOI] [PubMed] [Google Scholar]
- [83].Chen S, Sigdel S, Sawant H, Bihl J, Wang J. Exercise-Intervened Endothelial Progenitor Cell Exosomes Protect N2a Cells by Improving Mitochondrial Function. International Journal of Molecular Sciences . 2024;25:1148. doi: 10.3390/ijms25021148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sha R, Zhang B, Han X, Peng J, Zheng C, Zhang F, et al. Electroacupuncture Alleviates Ischemic Brain Injury by Inhibiting the miR-223/NLRP3 Pathway. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research . 2019;25:4723–4733. doi: 10.12659/MSM.917213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Doncheva AI, Romero S, Ramirez-Garrastacho M, Lee S, Kolnes KJ, Tangen DS, et al. Extracellular vesicles and microRNAs are altered in response to exercise, insulin sensitivity and overweight. Acta Physiologica (Oxford, England) . 2022;236:e13862. doi: 10.1111/apha.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation . 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- [87].Medina-Leyte DJ, Zepeda-García O, Domínguez-Pérez M, González-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. International Journal of Molecular Sciences . 2021;22:3850. doi: 10.3390/ijms22083850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kränkel N, Strässler E, Uhlemann M, Müller M, Briand-Schumacher S, Klingenberg R, et al. Extracellular vesicle species differentially affect endothelial cell functions and differentially respond to exercise training in patients with chronic coronary syndromes. European Journal of Preventive Cardiology . 2021;28:1467–1474. doi: 10.1177/2047487320919894. [DOI] [PubMed] [Google Scholar]
- [89].Su Y, Yuan J, Zhang F, Lei Q, Zhang T, Li K, et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death & Disease . 2019;10:365. doi: 10.1038/s41419-019-1599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circulation Research . 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lisi V, Senesi G, Bertola N, Pecoraro M, Bolis S, Gualerzi A, et al. Plasma-derived extracellular vesicles released after endurance exercise exert cardioprotective activity through the activation of antioxidant pathways. Redox Biology . 2023;63:102737. doi: 10.1016/j.redox.2023.102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Guo Y, Zhang Q, Yang D, Chen P, Xiao W. HIIT Promotes M2 Macrophage Polarization and Sympathetic Nerve Density to Induce Adipose Tissue Browning in T2DM Mice. Biomolecules . 2024;14:246. doi: 10.3390/biom14030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Guo Y, Zhang Q, Zheng L, Shou J, Zhuang S, Xiao W, et al. Depot-specific adaption of adipose tissue for different exercise approaches in high-fat diet/streptozocin-induced diabetic mice. Frontiers in physiology . 2023;14:1189528. doi: 10.3389/fphys.2023.1189528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Khalafi M, Mohebbi H, Symonds ME, Karimi P, Akbari A, Tabari E, et al. The Impact of Moderate-Intensity Continuous or High-Intensity Interval Training on Adipogenesis and Browning of Subcutaneous Adipose Tissue in Obese Male Rats. Nutrients . 2020;12:925. doi: 10.3390/nu12040925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Peng H, Zhang J, Zhang Z, Turdi S, Han X, Liu Q, et al. Cardiac-specific overexpression of catalase attenuates lipopolysaccharide-induced cardiac anomalies through reconciliation of autophagy and ferroptosis. Life Sciences . 2023;328:121821. doi: 10.1016/j.lfs.2023.121821. [DOI] [PubMed] [Google Scholar]
- [96].Sayed ASM, Xia K, Salma U, Yang T, Peng J. Diagnosis, prognosis and therapeutic role of circulating miRNAs in cardiovascular diseases. Heart, Lung & Circulation . 2014;23:503–510. doi: 10.1016/j.hlc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- [97].Gan CS, Wang CW, Tan KS. Circulatory microRNA-145 expression is increased in cerebral ischemia. Genetics and Molecular Research: GMR . 2012;11:147–152. doi: 10.4238/2012.January.27.1. [DOI] [PubMed] [Google Scholar]
- [98].Ji X, Takahashi R, Hiura Y, Hirokawa G, Fukushima Y, Iwai N. Plasma miR-208 as a biomarker of myocardial injury. Clinical Chemistry . 2009;55:1944–1949. doi: 10.1373/clinchem.2009.125310. [DOI] [PubMed] [Google Scholar]
- [99].Gidlöf O, Andersson P, van der Pals J, Götberg M, Erlinge D. Cardiospecific microRNA plasma levels correlate with troponin and cardiac function in patients with ST elevation myocardial infarction, are selectively dependent on renal elimination, and can be detected in urine samples. Cardiology . 2011;118:217–226. doi: 10.1159/000328869. [DOI] [PubMed] [Google Scholar]
- [100].Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. European Heart Journal . 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- [101].Liu JR, Cai GY, Ning YC, Wang JC, Lv Y, Guo YN, et al. Caloric restriction alleviates aging-related fibrosis of kidney through downregulation of miR-21 in extracellular vesicles. Aging . 2020;12:18052–18072. doi: 10.18632/aging.103591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhou Y, Tan C. miRNAs in Adipocyte-Derived Extracellular Vesicles: Multiple Roles in Development of Obesity-Associated Disease. Frontiers in Molecular Biosciences . 2020;7:171. doi: 10.3389/fmolb.2020.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee DC, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circulation Research . 2015;117:207–219. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Thijssen DHJ, Cable NT, Green DJ. Impact of exercise training on arterial wall thickness in humans. Clinical Science (London, England: 1979) . 2012;122:311–322. doi: 10.1042/CS20110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhou X, Xu M, Bryant JL, Ma J, Xu X. Exercise-induced myokine FNDC5/irisin functions in cardiovascular protection and intracerebral retrieval of synaptic plasticity. Cell & Bioscience . 2019;9:32. doi: 10.1186/s13578-019-0294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Wu G, Zhang X, Gao F. The epigenetic landscape of exercise in cardiac health and disease. Journal of Sport and Health Science . 2021;10:648–659. doi: 10.1016/j.jshs.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Shill DD, Lansford KA, Hempel HK, Call JA, Murrow JR, Jenkins NT. Effect of exercise intensity on circulating microparticles in men and women. Experimental Physiology . 2018;103:693–700. doi: 10.1113/EP086644. [DOI] [PubMed] [Google Scholar]
- [108].Horowitz AM, Fan X, Bieri G, Smith LK, Sanchez-Diaz CI, Schroer AB, et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science (York, N.Y.) . 2020;369:167–173. doi: 10.1126/science.aaw2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Simonsen JB. What Are We Looking At? Extracellular Vesicles, Lipoproteins, or Both? Circulation Research . 2017;121:920–922. doi: 10.1161/CIRCRESAHA.117.311767. [DOI] [PubMed] [Google Scholar]