Summary
Cigarette smoking addiction is a leading cause of morbidity and mortality and presents a challenging interventional target. Interventions for stopping smoking offer trade-offs in ability to displace or blunt the effects of cigarettes, which include positive and negative reinforcement, psychological reward, aversiveness, and sensory enjoyment, and which are mediated through nicotine and non-nicotine elements of smoking. Established therapies, which include nicotine replacement therapies (NRTs), varenicline, and bupropion are being supplemented with a growing evidence base for cytisine and nicotine substitution products, with more rapid acting NRTs on the horizon, all of which are expanding individual choice. An understanding of determinants of efficacy can inform a personalized and adaptive approach to smoking cessation, which presents an opportunity to further improve outcomes. This includes tailoring cessation treatment plans based on initial individual response, preference, and tolerability to first line interventions and considering second-line options (including evidence-based combination therapies) when needed.
Video Abstract
Subject areas: Health sciences, Medicine, Social sciences
Graphical abstract
Health sciences; Medicine; Social sciences
Introduction: Cigarette smoking remains a persistent and urgent public health challenge
Forty years after smoking cessation products were first approved by the FDA, cigarette addiction remains a persistent cause of preventable morbidity and mortality in the US and globally. A recent analysis of four national databases representing 23 million person-years highlighted that abstention from smoking for as little as 3 years had a dramatic benefit across all ages, reducing excess mortality risk by over 50% in those who were 50–59 years old, with even greater benefit for younger cohorts.1 And yet, the efficacy of cessation products is minimal in absolute terms, with an average of 30 quit attempts required per successful cessation outcome.2 This article explores mechanisms of efficacy and limitations of established cessation therapies, identifies emerging products expanding the range of personalized options, and describes an opportunity for further improving outcomes through evidence-based adaptive approaches.
Cigarette addiction represents a complex and challenging interventional target
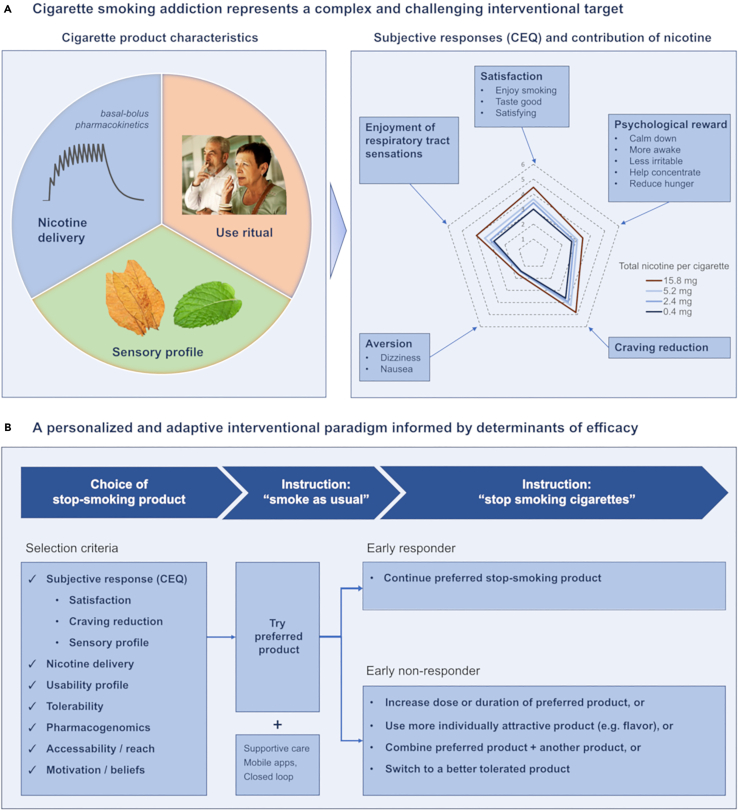
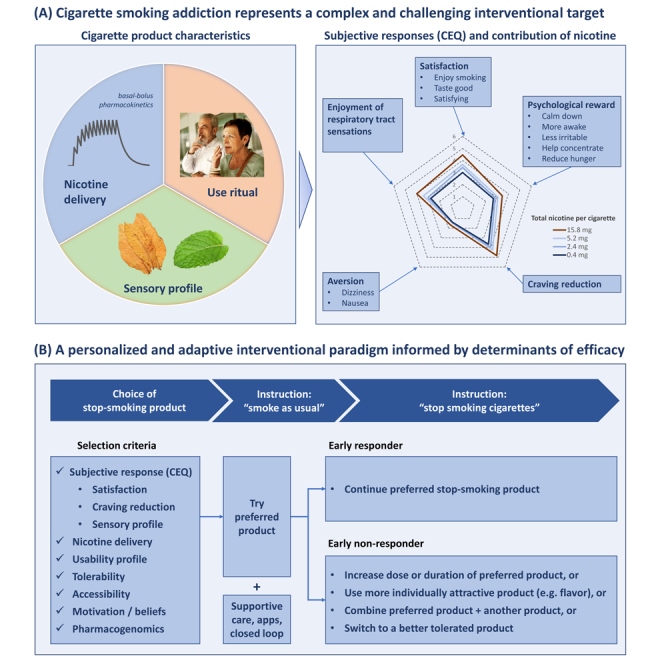
To understand mechanisms of action of products for stopping smoking, one first must understand the pharmacological, physiological, and behavioral mechanisms which contribute to smoking addiction. As illustrated in Figure 1A, cigarette addiction represents a highly complex interventional target. Cigarettes emit both nicotine and sensory flavorants, and are consumed via ritualistic behaviors, reinforced over years or decades, that include hand-to-mouth repetitive actions, and integration into pro-social behaviors (e.g., sharing of a cigarette) and individual relaxation habits (e.g., smoking breaks). These pharmacologic and behavioral stimuli result in positive and negative reinforcement and psychological reward which contribute to continued smoking and relapse.
Figure 1.
Graphical abstract
(A) Cigarette smoking addiction represents a complex and challenging interventional target. Cigarettes provide bolus nicotine delivery which accumulates over the course of a day causing a basal-bolus pharmacokinetic pattern, and flavorants from tobacco, menthol, additives, and the combustion process. Consumption is also integrated with habituated use rituals. Cigarettes elicit subjective responses which can be quantified by the Cigarette Evaluation Questionnaire (CEQ). The CEQ consists of 12 questions which segment into five latent categories: satisfaction, enjoyment of respiratory tract sensations, psychological reward, craving reduction, and aversion. Responses are reported on a rating scale of (0–6). The spider chart shown represents mean responses for each category after smoking a combusted cigarette.3 Responses to acute use of combusted cigarettes with nicotine content ranging from very low (0.4 mg, darkest blue) to typical (15.8 mg, red) show a dose-response impact on the CEQ.4
(B) Determinants of cessation efficacy can inform a personalized and adaptive interventional paradigm. A stop-smoking product aligned with personal choice and other predictive selection criteria can be tried during an initial pre-quit period. If initial response is below a minimal threshold, adjustments can be made, including change of dosing or flavor, combination with an adjunctive product, or switching to a better tolerated product. Provision of supportive care or digital closed loop technologies can further improve outcomes as well as accuracy of measuring initial response (i.e., reduction in exhaled CO in the pre-quit period).
Nicotine delivery by cigarettes elicits positive and negative reinforcement, psychological reward, and aversiveness through centrally mediated mechanisms
Nicotine, the primary active ingredient of cigarettes, is an agonist of excitatory neuronal nicotinic acetylcholine receptors (nAChRs). Neuronal nAChRs consist of pentameric combinations of alpha and sometimes also beta subunits which can vary in nicotine affinity, localization, and impact. The reinforcing effects of nAChRs have classically been viewed as resulting from induction of dopamine release via mesolimbic pathways.5 More recently, increased focus has been given to aversive influences that are involved in nicotine withdrawal symptoms and which also limit excessive nicotine self-administration, via involvement of the medial and lateral habenula and the interpeduncular nucleus.6 Nicotine also modulates attention and higher-order cognitive processes through a thalamo-cortical feedback circuit.7 The typical daily smoking pattern results in basal systemic nicotine pharmacokinetics that persist throughout the day, with superimposed bolus peaks associated with each cigarette smoked (i.e., describing a “basal-bolus” pattern, see Figure 1A, left panel).8 Acute use of cigarettes elicits subjective responses associated with psychological reward, satisfaction, craving reduction (i.e., reversal of withdrawal symptoms), and aversiveness, as measured through the Cigarette Evaluation Questionnaire (CEQ). The magnitude of these responses exhibit a dose-response relationship to cigarette nicotine content and associated bolus nicotine delivery (Figure 1A, right panel).
Cigarettes also provide nicotine and non-nicotine mediated sensory stimuli
Cigarettes induce sensory inputs which are both positive (“enjoyment of respiratory tract sensations” as measured by the CEQ), and negative in valence (“aversiveness”, which may be both centrally and peripherally mediated). Nicotine can directly activate trigeminal sensory neurons. Cigarette combustion products and flavorants present in tobacco or menthol-flavored cigarettes can also activate transient receptor potential (TRP) and other sensory receptors in the oropharynx and olfactory system as well as the rest of the respiratory tract.9 Even cigarettes with minimal nicotine can elicit a significant portion of each of the CEQ responses in people who smoke (Figure 1A, right panel). Likewise, while IV nicotine infusions can reduce concomitant ad libitum cigarette smoking, smoking of usual cigarettes is further reduced when these infusions are supplemented with denicotinized cigarettes.10
In summary, both the nicotine and non-nicotine elements of smoking are each necessary but not sufficient to individually comprise the cigarette use sensorial experience of enjoyment of respiratory tract sensations and aversiveness, and subjective responses, which include positive and negative reinforcement, and psychological reward.
Cessation products offer trade-offs in approaches to displace or blunt the actions of cigarettes
Three established classes of stop-smoking products utilize different mechanisms to displace or blunt the actions of cigarettes. Nicotine delivery products are designed to replace, to varying degrees, the bolus and/or basal nicotine delivery and sensory stimuli provided by cigarettes, and can also blunt subjective responses to cigarette use. Nicotinic partial agonists blunt the bolus effects of nicotine while providing an alternative source of basal nicotinic stimulation. Anti-depressants may modulate aspects of downstream behavioral responses of cigarettes, such as psychological reward, and also have a modulatory impact on nicotinic signaling. These products may be used either as monotherapies, or in some cases, in combination, to provide a personalized approach tuned to meet the preferences of the individual who smokes.
Nicotine delivery products for stopping smoking encompass evolving categories, spanning a range of nicotine delivery and sensory profiles
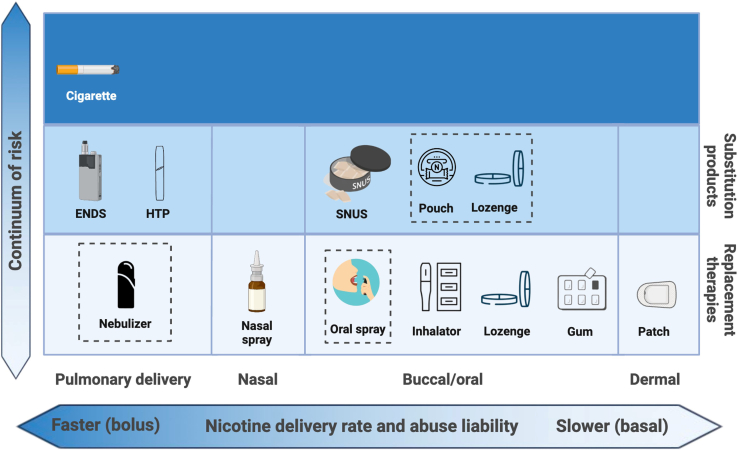
The products most commonly used to stop smoking in the US are nicotine delivery products, which include medicinal nicotine replacement therapies (NRTs) and consumer nicotine substitution products.11 These products are intended to replace the nicotine delivered by cigarettes, and to varying degrees, their sensorial experience, and can also blunt subjective responses to cigarettes. Table 1 and Figure 2 provide an overview of these products (for brevity, this review will primarily reflect US regulatory status).
Table 1.
Pharmacologic approaches for stopping smoking
| Product category | Mechanism | Route of administration | Rate of nicotine delivery | Flavor profile | Stop smoking efficacy | Regulatory status (US) | Reference |
|---|---|---|---|---|---|---|---|
| Medicinal nicotine replacement therapies: FDA approved products | |||||||
| Transdermal patch | Nicotinic agonist | Dermal | Slowest (basal) | N/A | 1.37 OR, 105 RCTs, n = 37,319 | FDA approved: cessation. OTC | Lindson et al.12 |
| Gum | Nicotinic agonist | Oral | > patch, < cigarette |
Mint, fruits, spices | 1.49 OR, 56 RCTs, n = 22,581 | FDA approved: cessation, OTC | Hartmann-Boyce et al.13 |
| Tablet, lozenge | Nicotinic agonist | Oral | > patch, < cigarette |
Mint, fruits, spices | 1.52 OR, 8 RCTs, n = 4,439 | FDA approved: cessation, OTC | Hartmann-Boyce et al.13 |
| Inhalator | Nicotinic agonist | Buccal/oral | > gum, < cigarette |
N/A | 1.90 OR, 4 RCTs, n = 976 | FDA approved: cessation. Rx | Hartmann-Boyce et al.13 |
| Nasal spray | Nicotinic agonist | Nasal | > inhalator, < cigarette |
N/A | 2.02 OR, 4 RCTs, n = 887 | FDA approved: cessation, Rx | Hartmann-Boyce et al.13 |
| NRT combination | Nicotinic agonist | Mixed | < cigarette | N/A | 1.93 OR, 86 study arms | N/A (Typically patch + oral NRT) | Lindson et al.12 |
| Medicinal nicotine replacement therapies: products under development | |||||||
| HFA inhaler | Nicotinic agonist | Buccal/oral | Bioequivalent to nicotine inhaler | Unflavored | Limited data | Not developed in US Approved in UK for cessation; discontinued |
– |
| Oral mist | Nicotinic agonist | Buccal/oral | Intermediate | TBD (mint, fruit ex-US) | Limited data | US: Positive advisory panel (OTC) Ex-US: approved in many countries |
– |
| Medicinal ENDS | Nicotinic agonist | Pulmonary | Cigarette-like | TBD | See nicotine substitution products, below | Pre-clinical development in US Clinical development, UK |
– |
| Nebulizer | Nicotinic agonist | Pulmonary | Potentially cigarette-like | Unflavored | Limited data | Clinical development, UK Entering clinical development, US |
– |
| Rapid-acting sublingual tablet | Nicotinic agonist | Buccal/sublingual | Faster than NRT lozenge; < cigarette | TBD | Limited data | Under clinical development | – |
| Medicinal therapies, mechanisms beyond nicotine replacement: FDA approved products | |||||||
| Varenicline | Nicotinic partial agonist | Oral | N/A | N/A | 2.33 OR, 67 RCTs, n = 16,430 | FDA approved: cessation, Rx | Lindson et al.12 |
| Bupropion | Nicotinic antagonist; DA and NA reuptake inhibitor | Oral | N/A | N/A | 1.43 OR, 71 RCTs, n = 14,759 | FDA approved: cessation, Rx | Lindson et al.12 |
| Medicinal therapies, mechanisms beyond nicotine replacement: products under development | |||||||
| Cytisine | Nicotinic partial agonist | Oral | N/A | N/A | 2.21 OR, 7 RCTs, n = 3,848 | Eastern EU: history of use | Lindson et al.12 |
| Cytisinicline | Nicotinic partial agonist | Oral | N/A | N/A | Limited data | US: late stage clinical/FDA review | – |
| Nicotine substitution products: FDA authorized products and categories under FDA review | |||||||
| ENDS | Nicotinic agonist | Pulmonary | Cigarette-like | Tobacco, menthol | 2.37 OR, 16 RCTs, n = 3,828 | FDA authorized: APPH MRTP applications in clinical phase |
Lindson et al.12 |
| HTP | Nicotinic agonist | Pulmonary | Cigarette-like | Tobacco, menthol | Limited data | FDA authorized: APPH, MRTP (Reduced toxicant exposure vs. cigarettes) | Tattan-Birch et al.14 |
| Snus | Nicotinic agonist | Buccal | Intermediate | Tobacco, mint, winter-green | Limited data | FDA authorized: APPH, MRTP (Reduced risk of mouth cancer, heart disease, lung cancer, stroke, emphysema, and chronic bronchitis vs. cigarettes) | Lindson-Hawley et al.15 |
| Snuff | Nicotinic agonist | Buccal | Intermediate | Tobacco-flavored | Limited data | FDA authorized: MRTP (Reduced risk of lung cancer vs. cigarettes) | – |
| Nicotine pouches | Nicotinic agonist | Buccal | Intermediate | TBD | Limited data | Under FDA review | – |
| VLNC cigarettes | Nicotinic agonist | Pulmonary | Lower nicotine emissions vs. full strength cigarettes | Tobacco, menthol | Limited data | FDA authorized: APPH, MRTP (Reduced nicotine exposure vs. other cigarettes; helps you smoke less) | – |
Abbreviations: APPH (appropriate for the protection of the public health; FDA marketing authorization regulatory standard); ENDS (electronic nicotine delivery system; e-cigarette); HFA (hydrofluoroalkane inhaler); HTP (heated tobacco product); OTC (over-the-counter medicine; no prescription required); Rx (prescribed medicine); RCT (randomized controlled trial); OR (odds ratio vs. placebo); MHRA (Medicines and Healthcare Products Regulatory Agency, UK); MRTP (modified risk tobacco product; FDA review has indicated reduced toxicity, risk or harm vs. use of other tobacco products such as cigarettes); VLNC (very low nicotine content cigarette). “Limited data” indicates meta-analysis not available.
Figure 2.
Nicotine delivery products for stopping smoking
Cigarettes and nicotine delivery products span a continuum of risk (X axis). They also vary in route of administration and nicotine delivery rates (Y axis), which impact abuse liability and efficacy in stopping smoking. Nicotine replacement therapies meet medicinal regulatory standards of safety and are lowest on the harm continuum of nicotine products. NRTs approved in the US include patches, gum, lozenges, inhalators, and nasal inhaler. Pipeline products (dotted lines) include oral spray, nebulizer, and medicinal ENDS (in England). Nicotine substitution products are intermediate in the continuum of risk. Products which the FDA-CTP (Center for Tobacco Products) has scientifically reviewed and designated as meeting the APPH (appropriate for the protection of the public health) marketing authorization regulatory standard include ENDS, HTP, and SNUS products. Nicotine pouches and lozenges are under FDA review. Not shown: very low nicotine content cigarettes (VLNCs), which provide reduced nicotine exposure vs. conventional cigarettes, although with similarly toxic smoke. Created in BioRender. Mangione, M. (2024) BioRender.com/q50z218.
Medicinal nicotine replacement therapies offer trade-offs in nicotine delivery and attractiveness
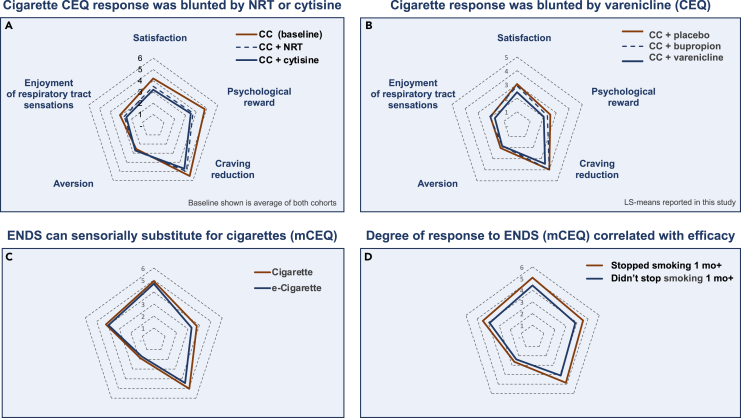
NRTs include medicinal nicotine patches, gums, lozenges, inhalers, and nasal sprays. Each form presents distinct advantages and considerations to suit personal preferences. The products with the most cigarette-like nicotine delivery have the highest efficacy in transferring dependence and stopping smoking, which also corresponds to an increased risk of abuse. Intra-nasal nicotine provides the fastest-acting nicotine delivery of NRT products currently available, and is associated with an OR (odds ratio for cessation vs. placebo) of 2.0 (see Table 1). Intra-nasal nicotine may be somewhat limited in its attractiveness to people who smoke because it cannot mimic the sensory and hand-to-mouth behavioral components of the smoking ritual.16 Patches provide a compliance benefit of all-day nicotine release from a single application. However, they provide neither the bolus nicotine delivery nor the sensory stimuli and behavioral rituals associated with smoking, and may cause sleep disruption and skin irritation.17 Oral NRTs provide an intermediate flux of nicotine and also provide oral tactile stimuli and flavorings, with mint flavors being most popular in the US.18 Nicotine patches and oral NRTs demonstrated OR of 1.37 and ∼1.5, respectively, in recent meta-analyses (see Table 1). In addition to providing a replacement source of nicotine, as shown in Figure 3A, use of NRTs somewhat blunts the acute subjective impacts of cigarette use, thus reducing the attractiveness of cigarettes and making it easier to replace cigarette use with NRTs.
Figure 3.
Subjective impact of stop-smoking products
(A) Responses to acute use of cigarettes, as measured by CEQ, comparing baseline response vs. response to cigarettes after 1 week of usage of NRT or cytisine (shown in red, dotted line, or blue, respectively).19
(B) CEQ responses to combusted cigarettes (CC) in conjunction with placebo, bupropion, or varenicline (shown in red, dotted line, or blue, respectively).20
(C) ENDS (blue) can sensorially substitute for CC (red).3
(D) degree of mCEQ response to ENDS correlates with efficacy in stopping smoking for at least one month.21 Note: (A, B, and C) were reported from participants who as yet had not stopped smoking at the time of the questionnaire, and therefore may not fully highlight responses representative of complete cessation or complete switching and maximal displacement of dependency from CC to ENDS. Responses to ENDS products were measured with a modified CEQ survey (mCEQ). The CEQ consists of 12 questions which segment into five latent categories: satisfaction, enjoyment of respiratory tract sensations, psychological reward, craving reduction, and aversion. Responses are reported on a rating scale of (0–6). The spider charts shown represent mean responses for each category after smoking a combusted cigarette (CEQ) or using an e-cigarette (mCEQ).
NRTs under development include faster-acting products which offer the potential for increased efficacy
Several categories of buccally absorbed NRTs are available ex-US or are in development. Buccally absorbed products are somewhat faster acting than products absorbed gastrically, and thus should be moderately more efficacious, along with having fewer gastric side effects. An over-the-counter nicotine oral spray is on the market ex-US and has received a favorable FDA advisory panel opinion. Biochemically verified continuous smoking abstinence from week 2 to week 6 was doubled vs. placebo (5.0% vs. 2.5%) in a pivotal randomized controlled trial (RCT).22 Nicotine strips and other novel oral formulations are also being introduced outside the US. A rapidly acting sublingual tablet under clinical development in the US has demonstrated rapid relief from withdrawal symptoms in an initial study.23
NRTs with rapid pulmonary delivery offer the potential for superior efficacy vs. most other NRTs, although this will need to be validated in clinical trials. A previous generation of pulmonary NRTs and medicinal ENDS (electronic nicotine delivery system, or e-cigarette) products (Voke/e-Voke, AERx, Staccato, Hava Health) have discontinued development or are not available.24 Products currently in development include an NRT nebulizer (Qnovia) in England and the US,83 and medicinal ENDS (electronic nicotine delivery products, or e-cigarettes; NJOY and others) in England.84
Nicotine substitution products have an emerging role in stopping smoking
The FDA is increasingly recognizing addiction to cigarette use as a chronic relapsing condition requiring extended dosing of products for stopping smoking. In 2013, labeling for NRT duration of use was extended, and more recently, FDA guidance has proposed expanded indications for maintenance use of cessation products after the quit attempt to prevent relapse.25,26 Similarly, the FDA Center for Tobacco Products (FDA-CTP) has been authorizing nicotine substitution products, with diverse formulations, delivery methods, and pharmacokinetic and sensory profiles, as harm reduction options for people who can’t or won’t otherwise quit smoking.
In the U.S., ENDS represent the most prevalent nicotine substitution products, and were used every day or some days by 11–17 million U.S. adults in 2021.27 In a survey of adults who vape in the US, UK, and Canada, 73% reported wanting to quit smoking as a reason for use.28 Evidence for the cessation efficacy of ENDS has reached a “tipping point” for many in the scientific community.29,30 A recent meta-analysis of 7 RCTs (n = 2,544) indicated that ENDS representative of the past decade have been ∼59% more efficacious than NRT monotherapies for smoking cessation.31 Early products (2008–2014) were inefficient in pulmonary nicotine delivery, utilizing unprotonated (“freebase”) nicotine formulations, but proved to be attractive alternatives to cigarettes for adults who smoke because of a diversity of flavor availability, which has been associated with higher quit rates.32,33 The introduction of protonated (“nicotine salt”) formulations in 2013–2015 (VUSE Solo, JUUL, NJOY Daily) and subsequent changes from wick-and-coil heaters to larger surface area and ceramic heater technologies c. 2018 (NJOY ACE, VUSE Alto), has led to nicotine delivery rates more similar to cigarettes, with a commensurate increase in efficacy.34,35 Figure 3C illustrates that e-cigarettes can provide a substitute for the sensory and behavioral effects of cigarettes. In a recent RCT in adults who smoke, reported side effects included coughing, headaches, and increased phlegm (although these were also reported in the control group).36 A comprehensive discussion of the physiological impact of switching from cigarettes to ENDS would require a review of its own, but improvements in relevant blood and urine biomarker levels and in respiratory- and cardiac-related endpoints have been reported in some randomized studies.37,38
Heated tobacco products (HTP) consist of a device that transfers controlled heat to tobacco sticks, plugs, or capsules that generate nicotine-containing aerosols. In the US, HTP have faced limited distribution due to patent disputes, but new products are pending. In Japan, HTP, launched in 2014, now represent ∼1/3 of the tobacco stick market. Reported HTP population-level displacement of cigarettes has varied across different survey methodologies and countries.14,39 An RCT of HTPs coupled with counseling reported 39% biochemically verified 3-month abstinence from cigarette smoking.40
Oral nicotine products, such as snus, pouches, and lozenges, are designed to deliver nicotine through oral mucosal absorption without the need for combustion. Oral products represent the fastest-growing consumer tobacco segment in the US and can resemble oral NRTs in composition and flavoring.41 In an initial open-label actual-use study, self-reported 7-day stop-smoking rates of 27% were observed after 1 week of product trial followed by 6 weeks of use of preferred flavor(s). Choosing multiple flavors was associated with greater reductions in smoking; further studies are warranted.42
Nicotinic partial agonists and anti-depressants offer additional options
Evidence for cytisine as an alternative to varenicline has recently been bolstered
Varenicline has historically been the most effective cessation product in RCTs, with an OR (odds ratio vs. placebo) of 2.33 reported in a recent meta-analysis.12 Varenicline’s activity includes partial agonism of β2∗ and full agonism of α7 nAChR subtypes.43 The partial agonist actions of varenicline have been observed to elevate tonic levels of dopamine.44 Varenicline concomitantly selectively suppresses cigarette-induced dopamine release.45 Varenicline has been shown to dampen smoking satisfaction, as well as craving and negative affect46 (see also Figure 3C). Dosing is once or twice per day; side effects include sleep disturbances and nausea.47,48
Cytisine (historically used in eastern Europe) is similarly a partial agonist of β2∗ nAChRs.49 Recent meta-analyses have revised the OR of cytisine upward to ∼2.2, approaching the efficacy of varenicline.12,50 Cytisine also has a blunting effect on cigarette behavioral and responses which is similar to varenicline (Figure 3A). Cytisine offers a meaningfully lower rate of nausea incidence vs. varenicline, consistent with its lower affinity for 5-HT3 receptors.47,48 A drawback is its rapid metabolism, typically requiring dosing of 6 times per day. A more convenient 3 times per day dosing regimen (cytisinicline) is in late stage development.51
Antidepressants remain less effective than varenicline
Bupropion was initially developed and approved as an antidepressant and later discovered to be effective for smoking cessation. Inhibition of the neuronal reuptake of dopamine and norepinephrine, and antagonism of nAChRs are thought to contribute to its efficacy.5 A RCT reported that bupropion was somewhat less efficacious than varenicline in blunting the acute impact of cigarettes (see Figure 3C). More recently, negative allosteric modulation of 5-HT3A receptors by bupropion has been described, which may contribute to its lower incidence of nausea vs. varenicline.52 Bupropion can induce seizures, and similarly to SSRIs, may cause neuropsychiatric adverse reactions.5 A meta-analysis reported cessation OR of 1.43 vs. placebo.12 Nortriptyline, another antidepressant, demonstrated OR of 1.35.12
The efficacy of stop smoking products is low in absolute terms
While stop-smoking products have validated efficacy in RCTs (see Table 1), absolute efficacy rates are low. Varenicline, for instance, only increases odds of cessation in RCTs by 8 in 100.12 The other leading cessation medications are even less efficacious; oral NRTs, nicotine patches, and bupropion increase rates of cessation by 2–3 per 100 attempts in RCTs.12
Furthermore, the net real-world efficacy of stop-smoking products is more limited than what is observed in randomized controlled trials. In the US, an analysis of the PATH study dataset showed a 10% twelve-month smoking quit rate for people who used pharmacotherapy (including NRTs), a rate comparable to those who did not use pharmacotherapy.53 Likewise, results from the Smoking Toolkit study in the UK indicated that, at the population level, NRT use heightened motivation to quit but did not necessarily lead to a reduction in cigarette intake.54 On average, it takes 30 real-world quit attempts to successfully stop smoking; this relapse pattern causes reductions in perceived self-efficacy and motivation which further inhibit quitting.2,55
Determinants of efficacy offer insight into opportunities for improving individualized outcomes
In spite of these low net rates of efficacy, it is also true that millions of people have stopped smoking with these products. As discussed previously, individual determinants of efficacy include substitutability for the sensory and nicotine delivery profile of cigarettes and tolerability. Additional critical determinants include sufficiency of dosing, and supportive care and motivational factors. These insights can inform approaches for improving outcomes at the individual and population level.
Sufficiency of dosing is often unmet for stop-smoking products
A central yet often overlooked insight is that efficacy is highly dependent on sufficient dosing and adherence, and these sufficiency criteria are often not met, both in RCTs and in real-world use. The UK Smoking Toolkit analysis, for example, noted that daily use of nicotine patch or use at least 4 times per day of oral NRTs was associated with smoking reduction, while less frequent use was not. Increasing duration of use of NRT up to 12 weeks was also positively associated with smoking reduction.54 A meta-analysis found that adherence to NRT use was only 61% in RCTs and 26% in real-world studies; adherence was associated with an OR of 2.17 vs. non-adherence.56 Likewise, for ENDS products, daily use, but not non-daily use, was associated with increased odds of stopping smoking in a meta-analysis and in a four-country survey of real-world use patterns.57,58 Similarly, in RCTs, cytisine was found to be more effective when dosed for an extended course of up to 84 days (similar to varenicline) vs. shorter durations of 25 days (as labeled in Europe).51
In addition to adherence to dosing over time, sufficient dosing per use is also critical, particularly for nicotine delivery products. A recent observational study reported that successful quitting was likeliest among those receiving an initial NRT dose of at least 2–3 mg per day per daily cigarette smoked.59 Several studies have similarly observed a dose-response relationship between nicotine delivery and rates of stopping smoking with ENDS. An RCT of 36 vs. 8 vs. 0 mg/mL of freebase nicotine reported biochemically-confirmed stop-smoking rates of 10.8%, 4.6%, and 0.8%, respectively, at week 24, in a population with no initial intent to stop smoking.60 In a comparison of real-world use patterns of the same pod-based device in adults who purchased a starter pack in North America (59 mg/mL nicotine salt) vs. UK (18 mg/mL nicotine salt), self-reported stop-smoking rates of 38% vs. 28%, respectively, were observed after 24 weeks of use.35
Supportive care and motivational factors are also important for efficacy
Like any significant modification of a habituated behavior, whether involving consumption of food, exercise, or other chronic habits, supportive care is a validated and efficacious component. In smoking cessation, provision of supportive care has been associated with an increased OR of 1.57.61 For instance, a meta-analysis indicated that when varenicline is combined with supportive care, cessation rates of ∼23% are achieved on average.62
Resources within the healthcare system for supportive care are limited, and thus mobile apps have been developed to expand access and automate supportive care. A recent systematic review of studies published in 2017–2022 found evidence for short-term improvement in cessation rates in adult smokers with intent to quit who used mobile app, web or texting based programs. Longer term benefits overall were limited in this generation of products, but results were improved in programs which incorporated personalized features.63 This highlights the opportunity for mobile platforms to integrate digital diagnostics such as handheld monitors of exhaled carbon monoxide for closed-loop personalized feedback and reinforcement of progress in stopping smoking. A recent RCT evaluated cessation efficacy of NRT in conjunction with either an intensive digital platform combining remote CO monitoring, SMS text-messaging based counseling with a tobacco cessation coach, a web-based community forum, and an app vs. app only. 1-year, CO-validated continuous abstinence was 19.1% in the intensive support group, vs. 8.5% in the app-only group. Free NRTs were offered every 2 weeks for up to 12 weeks, and cumulative dose of NRT utilized was higher in the intensive support group (3.3 orders vs. 1.8), suggesting an adherence benefit.64
Personal beliefs can also impact motivation and adherence for nicotine products. Qualitative studies have indicated that many adults who smoke have concerns about the safety and side effect profiles of NRT and ENDS, as well as concerns about the potential continuation of nicotine addiction.65,66 Data from the PATH study indicated that a belief that electronic cigarettes were less harmful was associated with a doubling of the odds of successfully switching from cigarettes to ENDS and with a further halving of the odds of subsequent relapse back to smoking for successful switchers.67 Education of patients who smoke about the relative benefits and risks of cessation and harm reduction approaches may have a significant public health impact.68 One challenge is that a substantial majority of US physicians believe that nicotine is a directly causative agent of tobacco-related diseases such as cancer and COPD.69
Evidence-based adaptive approaches may further increase cessation efficacy
Adaptive approaches can include use of evidence-based combinations and multiple lines of therapy
Adaptive treatment approaches have an established history in medicine and hold promise for further enhancing smoking cessation adherence and outcomes. These approaches, as illustrated in Figure 1B, include tuning choice of therapies based on individual product preferences and early predictors of efficacy. They may also entail a step-up of dosing, a switch to another better tolerated or more attractive product with higher likelihood of adherence, or supplementation with another product, following an incomplete response or non-response to an initial product, and may be combined with supportive care including mobile CO monitoring technologies.
Recent RCTs incorporating adaptive protocols provide insights for future research
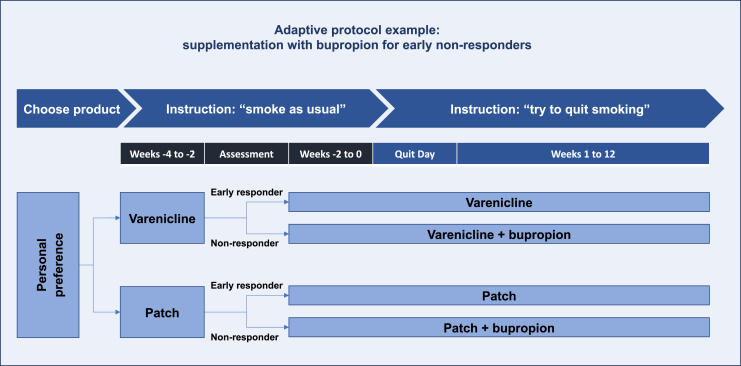
A recent RCT compared adaptive vs. standard approaches to first-line use of varenicline or nicotine patch. The adaptive study arms provided the addition of bupropion in the pre-quit period for “early non-responders” to initial monotherapy (i.e., less than 50% smoking reduction during the first 2 weeks; see Figure 4). Biochemically verified 30-day continuous abstinence rates at 12 weeks were higher in the adaptive arms (28% vs. 8% for varenicline adaptive protocol vs. non-adaptive protocol; 16% vs. 10% for NRT adaptive protocol vs. non-adaptive protocol).70
Figure 4.
Adaptive protocol example: supplementation with bupropion for early non-responders to NRT or varenicline
This figure illustrates an adaptive protocol which improved cessation outcomes in an RCT comparing randomization to an adaptive vs. non-adaptive protocol. In the adaptive protocol arm, participants chose between nicotine patch or varenicline, early response was monitored, and for early non-responders (less than 50% reduction in smoking), bupropion was added to the initially preferred product.70 Study participants who were randomized to a non-adaptive protocol continued on their initially preferred monotherapy regardless of early responder status (not shown).
In a prior RCT, supplementing nicotine patch with bupropion for early non-responders to patch (after 1 week of use in the pre-quit period) greatly increased 6 months point abstinence rates (17.2% vs. 6.6% for those randomized to combination vs. patch only). However, provision of adjunctive bupropion later in treatment for early patch responders who relapsed was not beneficial (10.0% for relapsers who were randomized to combination vs. vs. 13.3% for those who remained on patch only).71 This highlights that pre-quit trial use of product can be highly informative for whether treatment should be adjusted or not in order to improve outcomes.
Interventional adjustments for early non-responders should be supported by empiric evidence, as not all adjustments are beneficial. An RCT observed that escalation of dose for early non-responders to varenicline did not increase smoking cessation rates, possibly in part because nausea incidence further increased.72 Alternate adaptive protocols might include addition of ENDS or fast-acting NRT to varenicline, step-up in varenicline dose only for non-responders who do not experience nausea, or switching to cytisine for non-responders who do experience nausea.
Beyond actual reduction in smoking over 1–2 weeks, acutely measured metrics such as individual CEQ response upon product trial may provide an early prediction of efficacy. Figure 3D shows that larger magnitude early CEQ responses upon trial of e-cigarettes were correlated with subsequent smoking abstinence of at least 1 month or more. Likewise, initial positive reactions were predictive of ultimate successful treatment outcome for a nicotine nasal spray.16
Evidence-based combinations may have utility in adaptive protocols
Adaptive protocols may identify individuals for whom the efficacy benefits of combinations outweigh the potential for increased compliance burden or side effects. Beyond the bupropion combinations described previously, combinations which have been observed to be incrementally beneficial over monotherapy and which might be incorporated into adaptive protocols include the pairing of oral NRT, ENDS, or varenicline with nicotine patches, as well as varenicline combined with ENDS. Other combinations incorporating emerging or pipeline products, including cytisine, pulmonary NRTs, and other nicotine substitution products may also hold relevance.
The combination of patch + oral NRT is more efficacious vs. either monotherapy alone
Combinations of NRTs (typically oral NRT + patch) can more closely approximate the nicotine dosage levels and basal-bolus pharmacokinetic pattern associated with cigarettes and are well established as being more efficacious than monotherapies, with an OR of 1.93 vs. placebo (see Table 1).
Adding ENDS to patch increased efficacy in an RCT
In a recent RCT exploring 2x2 combinations of active or placebo nicotine patches and ENDS, enhanced smoking reduction was observed with this combined approach.73 Sample sizes were small, however, and additional studies are warranted to evaluate the combination of pulmonary nicotine delivery with nicotine patch for stopping smoking. This type of pairing is aligned with the US regulatory context, in the most typical instance where NRT use is likely to result in dual-use of NRT and cigarettes, where persisting cigarette usage is substituted with a harm reduction alternative.
Augmenting nicotine patch with varenicline increased efficacy but adding patch to varenicline has yielded mixed results
Combining varenicline with NRT has yielded mixed results. In two recent 2 RCTs, varenicline showed a benefit vs. placebo when added to nicotine patch, while adding patch to varenicline did not provide benefit in a large study (n = 1251).74,75 However, an earlier 2015 meta-analysis of three trials did show a benefit when an active patch (vs. placebo patch) was added to varenicline.76 The basal nicotine delivery provided by patches desensitizes nAChRs, while also providing ongoing agonism, which is possibly too similar mechanistically to the partial agonism induced by varenicline to provide extensive benefit beyond that of varenicline alone, given that varenicline is more efficacious than nicotine patches as a monotherapy.
The combination of varenicline with ENDS demonstrated incremental benefit in 2 RCTs
An RCT combining ENDS (providing rapid nicotine delivery) with varenicline (providing basal partial agonism of nAChRs) indicated a trend toward higher efficacy for the combination vs. varenicline alone, with a 43% lower hazard of relapse for the group receiving the combination treatment.77 Likewise, in a population which dual-used cigarettes and ENDS, an RCT of adjunctive varenicline showed increased continuous smoking abstinence between weeks 4 and 24 (49% vs. 14% for placebo). ENDS e-liquid consumption increased in the varenicline arm, but not the placebo arm, suggesting a synergistic interaction of ENDS and varenicline.78
Cessation of the use of nicotine as a multi-step journey
Finally, as articulated recently by the FDA-CTP Center Director, nicotine substitution can be an important interim harm reduction step on a multi-step journey toward ultimate nicotine cessation for people who smoke.79 Additionally, the use of nicotine substitution products should ideally not divert those who are interested in and likely to succeed in nicotine cessation.29 There is real-world evidence that stepping down of ENDS e-liquid nicotine concentration to zero is possible.33 Both varenicline80 and cytisine81 have demonstrated efficacy in cessation of nicotine vaping. Furthermore, a texting-based digital intervention increased vaping cessation rates for adolescents, and the combination of digital and pharmaceutical approaches might prove synergistic in the future.82
Discussion: Conclusion and future perspectives
Cigarette addiction is a chronic, relapsing condition with complex drivers. Personalized and adaptive approaches present an opportunity to improve outcomes. This includes tailoring cessation treatment plans based on initial individual response, preference, and tolerability to first line interventions and considering second-line options (including evidence-based combination therapies) when needed.
Several research directions may hold promise for further exploration. Firstly, efficacy should be verified across multiple RCTs for emerging products such as HTPs and oral nicotine products and pipeline products such as faster-acting NRTs. Predictive factors, such as personal preferences, CEQ responses, genetic predispositions, and early responses to pre-quit trial of cessation products can be studied to help define individualized treatment approaches. Thirdly, sequences and combinations of products can be explored to optimize adaptive protocols and to define populations most likely to positively respond to nicotine substitution, while not interfering with and enhancing the rate of nicotine cessation in early responders to cessation approaches. Lastly, the role of stop-smoking adaptive interventions during different phases of cessation, including pre-quit attempt, during the primary quit attempt, and afterward to prevent relapse of smoking, each provide opportunities for future studies.
Limitations of the study
This review reflected a comprehensive assessment of the literature, but was not formally a systematic review, and thus was submitted as a perspective article. However, the core data table (Table 1) referenced efficacy odds ratios from the Cochrane Library of systematic reviews, which are executed by an independent non-profit organization with the following policy statement: “We do not accept commercial or conflicted funding. This is vital for us to generate authoritative and reliable information, working freely, unconstrained by commercial and financial interests.”85
Acknowledgments
The authors thank Marta Mangione for assistance with formatting figures. The authors also thank the reviewers for their suggestions and Jerry Madukwe for helpful comments on initial drafts. J.E.R. and G.C. were supported by salaries from the Rose Research Center, and C.M.B., R.B., and R.P. were supported by salaries from the University of Catania. The writing of this paper was not sponsored by any external entities.
Author contributions
All authors contributed to the conception and design of the analysis and to the identification and collection of cited data and references. G.C. was the lead writer, and all authors contributed narrative segments and/or edits.
Declaration of interests
J.E.R. and G.C. are employees of Rose Research Center (RRC). RRC is an independent contract research organization that performs studies pertaining to smoking cessation and tobacco harm reduction. Other research support: National Institute on Drug Abuse; Foundation for a Smoke-Free World, Inc. (“FSFW”), a US nonprofit 501(c)(3) private foundation; Nicotine BRST LLC; JUUL Labs; Altria; Embera Neurotherapeutics, Inc.; Otsuka Pharmaceutical; Swedish Match, Philip Morris International. Patent applications filed for bupropion/zonisamide and related drug combinations. Patent purchase agreement with Philip Morris International for nicotine inhalation system patents, final payment 2014. Patent payments from Novartis through University of California patent license for nicotine skin patch, final payment 2008. RRC is the developer of the eResearch integrated digital platform for decentralized clinical trials of stop smoking products. J.E.R. Consulting: Philip Morris International, JT International, SA. G.C. was previously a Principal Scientist at JUUL Labs, a developer of non-combusted tobacco products, provides limited ongoing scientific consulting, and was previously employed by its predecessor companies. He also was employed at Nektar Therapeutics, whose pipeline included an inhaled NRT. Stock holdings in Qnovia, a developer of an inhaled NRT, and JUUL Labs.
R.P. is full tenured professor of Internal Medicine at the University of Catania (Italy) and Medical Director of the Institute for Internal Medicine and Clinical Immunology at the same University. He has received grants from U-BIOPRED and AIR-PROM, Integral Rheumatology & Immunology Specialists Network (IRIS), Foundation for a Smoke Free World, Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, Merk Sharp & Dohme, Boehringer Ingelheim, Novartis, Arbi Group Srl., Duska Therapeutics, Forest Laboratories, Ministero dell Universita’ e della Ricerca (MUR) Bando PNRR 3277/2021 (CUP E63C22000900006) and 341/2022 (CUP E63C22002080006), funded by NextGenerationEU of the European Union (EU), and the ministerial grant PON REACT-EU 2021 GREEN- Bando 3411/2021 by Ministero dell Universita’ e (MUR)—PNRR EU Community. He is founder of the Center for Tobacco Prevention and Treatment (CPCT) at the University of Catania and of the Center of Excellence for the Acceleration of Harm Reduction at the same university. He receives consultancy fees from Pfizer, Boehringer Ingelheim, Duska Therapeutics, Forest Laboratories, CV Therapeutics, Sermo Inc., GRG Health, Clarivate Analytics, Guidepoint Expert Network, and GLG Group. He receives textbooks royalties from Elsevier. He is also involved in a patent application for ECLAT Srl. He is a pro bono scientific advisor for Lega Italiana Anti Fumo (LIAF) and the International Network of Nicotine Consumers Organizations (INNCO); and he is Chair of the European Technical Committee for Standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4). R.P. has submitted an international patent application for a tracker application designed for monitoring nicotine and tobacco products. This application was filed through ECLAT Srl., a spin-off company of the University of Catania, Italy. Additionally, the authors affiliated with the RRC declare the following: They have filed patent applications related to bupropion/zonisamide and associated drug combinations. They have entered into a patent purchase agreement with Philip Morris International regarding nicotine inhalation system patents, with the final payment completed in 2014. They have received patent payments from Novartis, facilitated through a patent license with the University of California, for a nicotine skin patch, with the final payment received in 2008.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111090.
A video abstract is available at https://doi.org/10.1016/j.isci.2024.111090#mmc1.
Appendix
APPH: Appropriate for the protection of the public health; FDA marketing authorization regulatory standard for consumer tobacco and nicotine products.
CDER: Center for Drug Evaluation and Research; the FDA center which regulates pharmaceutical smoking cessation products.
CTP: Center for Tobacco Products; the FDA center which regulates consumer tobacco and nicotine products.
ENDS: Electronic nicotine delivery system (e-cigarettes).
HTP: Heated tobacco product.
MHRA: UK Medicines and Healthcare Products Regulatory Agency
MRTP: Modified risk tobacco product; FDA review of an APPH product supports a labeling expansion communicating reduced toxicity, risk or harm vs. use of other tobacco products such as cigarettes.
OR: Odds ratio. This is the ratio of the outcome rate associated with a given intervention vs. the outcome rate seen under control conditions in the absence of the intervention.
RCT: Randomized controlled trial.
Supplemental information
References
- 1.Cho E.R., Brill I.K., Gram I.T., Brown P.E., Jha P. Smoking Cessation and Short- and Longer-Term Mortality. NEJM Evid. 2024;3 doi: 10.1056/EVIDoa2300272. [DOI] [PubMed] [Google Scholar]
- 2.Chaiton M., Diemert L., Cohen J.E., Bondy S.J., Selby P., Philipneri A., Schwartz R. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvanko A.M., St Helen G., Nardone N., Addo N., Benowitz N.L. Twenty-four-hour subjective and pharmacological effects of ad-libitum electronic and combustible cigarette use among dual users. Addiction. 2020;115:1149–1159. doi: 10.1111/add.14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins S.T., Bergeria C.L., Davis D.R., Streck J.M., Villanti A.C., Hughes J.R., Sigmon S.C., Tidey J.W., Heil S.H., Gaalema D.E., et al. Response to reduced nicotine content cigarettes among smokers differing in tobacco dependence severity. Prev. Med. 2018;117:15–23. doi: 10.1016/j.ypmed.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polosa R., Benowitz N.L. Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol. Sci. 2011;32:281–289. doi: 10.1016/j.tips.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wills L., Ables J.L., Braunscheidel K.M., Caligiuri S.P.B., Elayouby K.S., Fillinger C., Ishikawa M., Moen J.K., Kenny P.J. Neurobiological Mechanisms of Nicotine Reward and Aversion. Pharmacol. Rev. 2022;74:271–310. doi: 10.1124/pharmrev.121.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh V., Bangasser D.A. In: Behavioral Pharmacology of the Cholinergic System Current Topics in Behavioral Neurosciences. Shoaib M., Wallace T.L., editors. Springer International Publishing; 2020. Cholinergic Signaling Dynamics and Cognitive Control of Attention; pp. 71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benowitz N.L., St.Helen G., Liakoni E. Clinical Pharmacology of Electronic Nicotine Delivery Systems (ENDS): Implications for Benefits and Risks in the Promotion of the Combusted Tobacco Endgame. J. Clin. Pharmacol. 2021;61:S18. doi: 10.1002/jcph.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carstens E., Carstens M.I. Sensory Effects of Nicotine and Tobacco. Nicotine Tob. Res. 2022;24:306–315. doi: 10.1093/ntr/ntab086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westman E.C., Behm F.M., Rose J.E. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol. Biochem. Behav. 1996;53:309–315. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- 11.Plurphanswat N., Rodu B. Why can’t smokers quit? Longitudinal study of smokers in the US using the Population Assessment of Tobacco and Health (PATH) waves 1 to 5. Addict. Behav. Rep. 2023;18 doi: 10.1016/j.abrep.2023.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindson N., Theodoulou A., Ordóñez-Mena J.M., Fanshawe T.R., Sutton A.J., Livingstone-Banks J., Hajizadeh A., Zhu S., Aveyard P., Freeman S.C., et al. Pharmacological and electronic cigarette interventions for smoking cessation in adults: component network meta-analyses. Cochrane Database Syst. Rev. 2023;2023 doi: 10.1002/14651858.CD015226.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann-Boyce J., Chepkin S.C., Ye W., Bullen C., Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst. Rev. 2018;5 doi: 10.1002/14651858.CD000146.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tattan-Birch H., Hartmann-Boyce J., Kock L., Simonavicius E., Brose L., Jackson S., Shahab L., Brown J. Heated tobacco products for smoking cessation and reducing smoking prevalence. Cochrane Database Syst. Rev. 2022;1 doi: 10.1002/14651858.CD013790.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindson-Hawley N., Hartmann-Boyce J., Fanshawe T.R., Begh R., Farley A., Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst. Rev. 2016;10 doi: 10.1002/14651858.CD005231.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann V., Jepson C., Rukstalis M., Perkins K., Audrain-McGovern J., Lerman C. Subjective effects of an initial dose of nicotine nasal spray predict treatment outcome. Psychopharmacology. 2004;172:271–276. doi: 10.1007/s00213-003-1659-8. [DOI] [PubMed] [Google Scholar]
- 17.Phillips C., Pechmann C., Calder D., Prochaska J.J. Understanding Hesitation to Use Nicotine Replacement Therapy: A Content Analysis of Posts in Online Tobacco-Cessation Support Groups. Am. J. Health Promot. 2023;37:30–38. doi: 10.1177/08901171221113835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trigger S., Xu X., Malarcher A., Salazar E., Shin H., Babb S. Trends in Over-the-Counter Nicotine Replacement Therapy Sales, U.S., 2017‒2020. Am. J. Prev. Med. 2023;64:650–657. doi: 10.1016/j.amepre.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker N., Howe C., Glover M., McRobbie H., Barnes J., Nosa V., Parag V., Bassett B., Bullen C. Cytisine versus Nicotine for Smoking Cessation. N. Engl. J. Med. 2014;371:2353–2362. doi: 10.1056/NEJMoa1407764. [DOI] [PubMed] [Google Scholar]
- 20.Gonzales D., Rennard S.I., Nides M., Oncken C., Azoulay S., Billing C.B., Watsky E.J., Gong J., Williams K.E., Reeves K.R., Varenicline Phase 3 Study Group Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 21.Morean M.E., Bold K.W. The Modified E-Cigarette Evaluation Questionnaire: Psychometric Evaluation of an Adapted Version of the Modified Cigarette Evaluation Questionnaire for Use With Adults Who Use Electronic Nicotine Delivery Systems. Nicotine Tob. Res. 2022;24:1396–1404. doi: 10.1093/ntr/ntac062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nides M., Danielsson T., Saunders F., Perfekt R., Kapikian R., Solla J., Leischow S.J., Myers A. Efficacy and Safety of a Nicotine Mouth Spray for Smoking Cessation: A Randomized, Multicenter, Controlled Study in a Naturalistic Setting. Nicotine Tob. Res. 2020;22:339–345. doi: 10.1093/ntr/nty246. [DOI] [PubMed] [Google Scholar]
- 23.Rose J.E., Behm F.M., Botts T.L., Botts D.R., Willette P.N., Vocci F., McCarty J. Novel rapid-acting sublingual nicotine tablet as a cigarette substitution strategy. Psychopharmacology. 2022;239:2853–2862. doi: 10.1007/s00213-022-06171-z. [DOI] [PubMed] [Google Scholar]
- 24.Cipolla D., Gonda I. Inhaled nicotine replacement therapy. Asian J. Pharma. Sci. 2015;10:472–480. doi: 10.1016/j.ajps.2015.07.004. [DOI] [Google Scholar]
- 25.Fucito L.M., Bars M.P., Forray A., Rojewski A.M., Shiffman S., Selby P., West R., Foulds J., Toll B.A., Writing Committee for the SRNT Policy and Treatment Networks Addressing the Evidence for FDA Nicotine Replacement Therapy Label Changes: A Policy Statement of the Association for the Treatment of Tobacco Use and Dependence and the Society for Research on Nicotine and Tobacco. Nicotine Tob. Res. 2014;16:909–914. doi: 10.1093/ntr/ntu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FDA Center for Drug Evaluation and Research . 2023. Smoking Cessation and Related Indications: Developing Nicotine Replacement Therapy Drug Products. [Google Scholar]
- 27.Erhabor J., Boakye E., Obisesan O., Osei A.D., Tasdighi E., Mirbolouk H., DeFilippis A.P., Stokes A.C., Hirsch G.A., Benjamin E.J., et al. E-Cigarette Use Among US Adults in the 2021 Behavioral Risk Factor Surveillance System Survey. JAMA Netw. Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.40859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gravely S., Yong H.-H., Reid J.L., East K.A., Liber A.C., Michael Cummings K., Quah A.C.K., Fong G.T., Hammond D. An examination of quitting smoking as a reason for vaping by the type of nicotine vaping device used most often among adults who smoke and vape: Findings from the Canada, England and the United States 2020 ITC Smoking and Vaping Survey. Prev. Med. Rep. 2023;33 doi: 10.1016/j.pmedr.2023.102201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigotti N.A. Electronic Cigarettes for Smoking Cessation — Have We Reached a Tipping Point? N. Engl. J. Med. 2024;390:664–665. doi: 10.1056/NEJMe2314977. [DOI] [PubMed] [Google Scholar]
- 30.Warner K.E., Benowitz N.L., McNeill A., Rigotti N.A. Nicotine e-cigarettes as a tool for smoking cessation. Nat. Med. 2023;29:520–524. doi: 10.1038/s41591-022-02201-7. [DOI] [PubMed] [Google Scholar]
- 31.Lindson N., Butler A.R., McRobbie H., Bullen C., Hajek P., Begh R., Theodoulou A., Notley C., Rigotti N.A., Turner T., et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2024;1 doi: 10.1002/14651858.CD010216.pub8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mok Y., Jeon J., Levy D.T., Meza R. Associations Between E-cigarette Use and E-cigarette Flavors With Cigarette Smoking Quit Attempts and Quit Success: Evidence From a U.S. Large, Nationally Representative 2018–2019 Survey. Nicotine Tob. Res. 2023;25:541–552. doi: 10.1093/ntr/ntac241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farsalinos K., Russell C., Polosa R., Poulas K., Lagoumintzis G., Barbouni A. Patterns of flavored e-cigarette use among adult vapers in the USA: an online cross-sectional survey of 69,233 participants. Harm Reduct. J. 2023;20:147. doi: 10.1186/s12954-023-00876-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Hourani M., Shihadeh A., Talih S., Eissenberg T., CSTP Nicotine Flux Work Group Comparison of Nicotine Emissions Rate, “Nicotine Flux”, from Heated, Electronic, and Combustible Tobacco Products: data, trends and recommendations for regulation. Tob. Control. 2022;32:1. doi: 10.1136/tobaccocontrol-2021-056850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldenson N.I., Ding Y., Prakash S., Hatcher C., Augustson E.M., Shiffman S. Differences in Switching Away From Smoking Among Adult Smokers Using JUUL Products in Regions With Different Maximum Nicotine Concentrations: North America and the United Kingdom. Nicotine Tob. Res. 2021;23:1821–1830. doi: 10.1093/ntr/ntab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter M.J., Wahlquist A.E., Dahne J., Gray K.M., Cummings K.M., Warren G., Wagener T.L., Goniewicz M.L., Smith T.T. Effect of unguided e-cigarette provision on uptake, use, and smoking cessation among adults who smoke in the USA: a naturalistic, randomised, controlled clinical trial. EClinicalMedicine. 2023;63 doi: 10.1016/j.eclinm.2023.102142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klonizakis M., Gumber A., McIntosh E., Brose L.S. Medium- and longer-term cardiovascular effects of e-cigarettes in adults making a stop-smoking attempt: a randomized controlled trial. BMC Med. 2022;20:276. doi: 10.1186/s12916-022-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajat C., Stein E., Shantikumar S., Niaura R., Ferrara P., Polosa R. A scoping review of studies on the health impact of electronic nicotine delivery systems. Intern. Emerg. Med. 2022;17:241–268. doi: 10.1007/s11739-021-02835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller C.R., Sutanto E., Smith D.M., Hitchman S.C., Gravely S., Yong H.-H., Borland R., O’Connor R.J., Cummings K.M., Fong G.T., et al. Characterizing Heated Tobacco Product Use Among Adult Cigarette Smokers and Nicotine Vaping Product Users in the 2018 ITC Four Country Smoking & Vaping Survey. Nicotine Tob. Res. 2022;24:493–502. doi: 10.1093/ntr/ntab217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caponnetto P., Campagna D., Maglia M., Benfatto F., Emma R., Caruso M., Caci G., Busà B., Pennisi A., Ceracchi M., et al. Comparing the Effectiveness, Tolerability, and Acceptability of Heated Tobacco Products and Refillable Electronic Cigarettes for Cigarette Substitution (CEASEFIRE): Randomized Controlled Trial. JMIR Public Health Surveill. 2023;9 doi: 10.2196/42628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Back S., Masser A.E., Rutqvist L.E., Lindholm J. Harmful and potentially harmful constituents (HPHCs) in two novel nicotine pouch products in comparison with regular smokeless tobacco products and pharmaceutical nicotine replacement therapy products (NRTs) BMC Chem. 2023;17:9. doi: 10.1186/s13065-023-00918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker E., McCaffrey S., Lewis J., Vansickel A., Larson E., Sarkar M. Characterization of Ad Libitum Use Behavior of On! Nicotine Pouches. Am. J. Health Behav. 2023;47:428–449. doi: 10.5993/AJHB.47.3.1. [DOI] [PubMed] [Google Scholar]
- 43.Bordia T., Hrachova M., Chin M., McIntosh J.M., Quik M. Varenicline Is a Potent Partial Agonist at α6β2∗ Nicotinic Acetylcholine Receptors in Rat and Monkey Striatum. J. Pharmacol. Exp. Ther. 2012;342:327–334. doi: 10.1124/jpet.112.194852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Ciano P., Guranda M., Lagzdins D., Tyndale R.F., Gamaleddin I., Selby P., Boileau I., Le Foll B. Varenicline-Induced Elevation of Dopamine in Smokers: A Preliminary [11C]-(+)-PHNO PET Study. Neuropsychopharmacol. 2016;41:1513–1520. doi: 10.1038/npp.2015.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldstein N., Carty J.R.E., Betley J.N. Specificity of Varenicline in Blocking Mesolimbic Circuit Activation to Natural and Drug Rewards. Neuroscience. 2022;483:40–51. doi: 10.1016/j.neuroscience.2021.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tonkin S.S., Colder C., Mahoney M.C., Swan G.E., Cinciripini P., Schnoll R., George T.P., Tyndale R.F., Hawk L.W. Evaluating Treatment Mechanisms of Varenicline: Mediation by Affect and Craving. Nicotine Tob. Res. 2022;24:1803–1810. doi: 10.1093/ntr/ntac138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Courtney R.J., McRobbie H., Tutka P., Weaver N.A., Petrie D., Mendelsohn C.P., Shakeshaft A., Talukder S., Macdonald C., Thomas D., et al. Effect of Cytisine vs Varenicline on Smoking Cessation. JAMA. 2021;326:1–10. doi: 10.1001/jama.2021.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker N., Smith B., Barnes J., Verbiest M., Parag V., Pokhrel S., Wharakura M.K., Lees T., Cubillos Gutierrez H., Jones B., Bullen C. Cytisine versus varenicline for smoking cessation in New Zealand indigenous Māori: a randomized controlled trial. Addiction. 2021;116:2847–2858. doi: 10.1111/add.15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gotti C., Clementi F. Cytisine and cytisine derivatives. More than smoking cessation aids. Pharmacol. Res. 2021;170 doi: 10.1016/j.phrs.2021.105700. [DOI] [PubMed] [Google Scholar]
- 50.Ofori S., Lu C., Olasupo O.O., Dennis B.B., Fairbairn N., Devereaux P.J., Mbuagbaw L. Cytisine for smoking cessation: A systematic review and meta-analysis. Drug Alcohol Depend. 2023;251 doi: 10.1016/j.drugalcdep.2023.110936. [DOI] [PubMed] [Google Scholar]
- 51.Rigotti N.A., Benowitz N.L., Prochaska J., Leischow S., Nides M., Blumenstein B., Clarke A., Cain D., Jacobs C. Cytisinicline for Smoking Cessation: A Randomized Clinical Trial. JAMA. 2023;330:152–160. doi: 10.1001/jama.2023.10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stuebler A.G., Jansen M. Bupropion Inhibits Serotonin Type 3AB Heteromeric Channels at Clinically Relevant Concentrations. Mol. Pharmacol. 2020;97:171–179. doi: 10.1124/mol.119.118349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierce J.P., Benmarhnia T., Chen R., White M., Abrams D.B., Ambrose B.K., Blanco C., Borek N., Choi K., Coleman B., et al. Role of e-cigarettes and pharmacotherapy during attempts to quit cigarette smoking: The PATH Study 2013-16. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beard E., Bruguera C., McNeill A., Brown J., West R. Association of amount and duration of NRT use in smokers with cigarette consumption and motivation to stop smoking: A national survey of smokers in England. Addict. Behav. 2015;40:33–38. doi: 10.1016/j.addbeh.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Blevins C.E., Farris S.G., Brown R.A., Strong D.R., Abrantes A.M. The Role of Self-Efficacy, Adaptive Coping, and Smoking Urges in Long-term Cessation Outcomes. Addict. Disord. Their Treat. 2016;15:183–189. doi: 10.1097/ADT.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mersha A.G., Eftekhari P., Bovill M., Tollosa D.N., Gould G.S. Evaluating level of adherence to nicotine replacement therapy and its impact on smoking cessation: a systematic review and meta-analysis. Arch. Public Health. 2021;79:26. doi: 10.1186/s13690-021-00550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gravely S., Meng G., Hammond D., Hyland A., Michael Cummings K., Borland R., Kasza K.A., Yong H.-H., Thompson M.E., Quah A.C.K., et al. Differences in cigarette smoking quit attempts and cessation between adults who did and did not take up nicotine vaping: Findings from the ITC four country smoking and vaping surveys. Addict. Behav. 2022;132 doi: 10.1016/j.addbeh.2022.107339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang R.J., Bhadriraju S., Glantz S.A. E-Cigarette Use and Adult Cigarette Smoking Cessation: A Meta-Analysis. Am. J. Public Health. 2021;111:230–246. doi: 10.2105/AJPH.2020.305999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veldhuizen S., Behal A., Zawertailo L., Selby P. Adequacy of nicotine replacement and success quitting tobacco in clinical populations: An observational study. Drug Alcohol Depend. 2023;244 doi: 10.1016/j.drugalcdep.2023.109796. [DOI] [PubMed] [Google Scholar]
- 60.Foulds J., Cobb C.O., Yen M.-S., Veldheer S., Brosnan P., Yingst J., Hrabovsky S., Lopez A.A., Allen S.I., Bullen C., et al. Effect of Electronic Nicotine Delivery Systems on Cigarette Abstinence in Smokers With No Plans to Quit: Exploratory Analysis of a Randomized Placebo-Controlled Trial. Nicotine Tob. Res. 2022;24:955–961. doi: 10.1093/ntr/ntab247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lancaster T., Stead L.F. Individual behavioural counselling for smoking cessation. Cochrane Database Syst. Rev. 2017;3 doi: 10.1002/14651858.CD001292.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Livingstone-Banks J., Fanshawe T.R., Thomas K.H., Theodoulou A., Hajizadeh A., Hartman L., Lindson N. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev. 2023;5 doi: 10.1002/14651858.CD006103.pub9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fang Y.E., Zhang Z., Wang R., Yang B., Chen C., Nisa C., Tong X., Yan L.L. Effectiveness of eHealth Smoking Cessation Interventions: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2023;25 doi: 10.2196/45111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marler J.D., Fujii C.A., Utley M.T., Balbierz D.J., Galanko J.A., Utley D.S. Long-Term Outcomes of a Comprehensive Mobile Smoking Cessation Program With Nicotine Replacement Therapy in Adult Smokers: Pilot Randomized Controlled Trial. JMIR Mhealth Uhealth. 2023;11 doi: 10.2196/48157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Styklunas G.M., Shahid N.N., Park E.R., Haberer J.E., Rigotti N.A., Howard S.E., Kruse G.R. A qualitative analysis of nicotine replacement therapy uptake, consistent use, and persistence among primary care patients who smoke. Drug Alcohol Depend. Rep. 2022;2 doi: 10.1016/j.dadr.2021.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones G., McIntosh E., Brose L.S., Klonizakis M. Participant Experiences of a Quit Smoking Attempt Through Either Nicotine Replacement Therapy (NRT) Methods or the Use of an E-cigarette. J. Addict. Med. 2022;16:272–277. doi: 10.1097/ADM.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 67.Kim S., Shiffman S., Sembower M.A. US adult smokers’ perceived relative risk on ENDS and its effects on their transitions between cigarettes and ENDS. BMC Publ. Health. 2022;22:1771. doi: 10.1186/s12889-022-14168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee S.C., Maglalang D.D., Avila J.C., Leavens E.L.S., Nollen N.L., Pulvers K., Ahluwalia J.S. Change in E-cigarette risk perception and smoking behavior of Black and Latinx individuals who smoke. Drug Alcohol Depend. 2023;245 doi: 10.1016/j.drugalcdep.2023.109824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinberg M.B., Bover Manderski M.T., Wackowski O.A., Singh B., Strasser A.A., Delnevo C.D. Nicotine Risk Misperception Among US Physicians. J. Gen. Intern. Med. 2021;36:3888–3890. doi: 10.1007/s11606-020-06172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davis J.M., Masclans L., Rose J.E. Adaptive Smoking Cessation Using Precessation Varenicline or Nicotine Patch: A Randomized Clinical Trial. JAMA Netw. Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.32214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rose J.E., Behm F.M. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. Am. J. Psychiatry. 2013;170:860–867. doi: 10.1176/appi.ajp.2013.12070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hajek P., McRobbie H., Myers Smith K., Phillips A., Cornwall D., Dhanji A.-R. Increasing Varenicline Dose in Smokers Who Do Not Respond to the Standard Dosage: A Randomized Clinical Trial. JAMA Intern. Med. 2015;175:266–271. doi: 10.1001/jamainternmed.2014.6916. [DOI] [PubMed] [Google Scholar]
- 73.Rose J.E., Frisbee S., Campbell D., Salley A., Claerhout S., Davis J.M. Smoking reduction using electronic nicotine delivery systems in combination with nicotine skin patches. Psychopharmacology (Berl) 2023;240:1901–1909. doi: 10.1007/s00213-023-06401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.King A., Vena A., De Wit H., Grant J.E., Cao D. Effect of Combination Treatment With Varenicline and Nicotine Patch on Smoking Cessation Among Smokers Who Drink Heavily: A Randomized Clinical Trial. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baker T.B., Piper M.E., Smith S.S., Bolt D.M., Stein J.H., Fiore M.C. Effects of Combined Varenicline With Nicotine Patch and of Extended Treatment Duration on Smoking Cessation: A Randomized Clinical Trial. JAMA. 2021;326:1485–1493. doi: 10.1001/jama.2021.15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang P.-H., Chiang C.-H., Ho W.-C., Wu P.-Z., Tsai J.-S., Guo F.-R. Combination therapy of varenicline with nicotine replacement therapy is better than varenicline alone: a systematic review and meta-analysis of randomized controlled trials. BMC Publ. Health. 2015;15:689. doi: 10.1186/s12889-015-2055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tattan-Birch H., Kock L., Brown J., Beard E., Bauld L., West R., Shahab L. E-cigarettes to Augment Stop Smoking In-person Support and Treatment With Varenicline (E-ASSIST): A Pragmatic Randomized Controlled Trial. Nicotine Tob. Res. 2023;25:395–403. doi: 10.1093/ntr/ntac149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caponnetto P., Spicuzza L., Campagna D., Ahluwalia J.S., Russell C., Maglia M., Riela P.M., Longo C.F., Caci G., Quattropani M.C., et al. Varenicline for smoking cessation in individuals who smoke cigarettes and use electronic cigarettes: a double-blind, randomised, placebo-controlled phase 3 trial. eClinicalMedicine. 2023;66 doi: 10.1016/j.eclinm.2023.102316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.King B.A., Toll B.A. Commentary on Wackowski et al. .: Opportunities and Considerations for Addressing Misperceptions About the Relative Risks of Tobacco Products among Adult Smokers. Addiction. 2023;118:1892–1894. doi: 10.1111/add.16296. [DOI] [PubMed] [Google Scholar]
- 80.Caponnetto P., Campagna D., Ahluwalia J.S., Russell C., Maglia M., Riela P.M., Longo C.F., Busa B., Polosa R. Varenicline and counseling for vaping cessation: a double-blind, randomized, parallel-group, placebo-controlled trial. BMC Med. 2023;21:220. doi: 10.1186/s12916-023-02919-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rigotti N.A., Benowitz N.L., Prochaska J.J., Cain D.F., Ball J., Clarke A., Blumenstein B.A., Jacobs C. Cytisinicline for Vaping Cessation in Adults Using Nicotine E-Cigarettes: The ORCA-V1 Randomized Clinical Trial. JAMA Intern. Med. 2024;184:922–930. doi: 10.1001/jamainternmed.2024.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Graham A.L., Cha S., Jacobs M.A., Amato M.S., Funsten A.L., Edwards G., Papandonatos G.D. A Vaping Cessation Text Message Program for Adolescent E-Cigarette Users: A Randomized Clinical Trial. JAMA. 2024;332:713–721. doi: 10.1001/jama.2024.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional resources
- 83.Business Wire Qnovia, Inc. Announces Positive Results from First-In-Human Pharmacokinetic and Safety Study of Lead Asset QN-01, a Prescription Inhaled Smoking Cessation Therapy. 2023. https://www.businesswire.com/news/home/20231129757219/en/Qnovia-Inc.-announces-positive-results-from-first-in-human-pharmacokinetic-and-safety-study-of-lead-asset-QN-01-a-prescription-inhaled-smoking-cessation-therapy
- 84.Johnston I. Financial Times; 2021. Upstart E-Cigarette Makers Push for NHS Licences Ahead of Big Tobacco.https://www.ft.com/content/9a5a8205-30d1-4e1d-be33-cb6c81bb03e9 [Google Scholar]
- 85.Cochrane About Us. 2024. https://www.cochrane.org/about-us
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.