Abstract
Strophariarugosoannulata is an important edible mushroom in China, but green mold disease has caused significant production and economic losses. In this study, two new pathogens Trichodermastrophariensis and T.viridistromatis were identified as the causal agents of this disease. During October-November 2023, six strains of the fungal pathogen were isolated from infected fruiting bodies of S.rugosoannulata and identified based on morphological characteristics and molecular phylogenetic analyses of internal transcribed spacer (nrITS), the second largest RNA polymerase II subunit (rpb2) and the partial translation elongation factor 1-alpha (tef1-α) region. The representative isolates of the pathogenic green mold Trichoderma species were used to perform a pathogenicity test with spore suspensions, resulting in symptoms similar to those observed in the cultivated field. The same pathogens were successfully re-isolated, thereby fulfilling Koch’s postulates. Detailed morphological descriptions, illustrations, culture characteristics, and comparisons with morphologically similar and closely related species are provided.
Key words: Ascomycetes, novel taxa, pathogen, phylogeny, taxonomy
Introduction
Strophariarugosoannulata (Wine-cap mushroom), a renowned edible mushroom, also known as Daqiugaigu in Chinese, has been widely cultivated in Poland, Germany, Russia and the United States (Huang et al. 2023). China imported a strain of S.rugosoannulata from Poland in the 1980s and began widespread cultivation in the 1990s (Yan et al. 2020). In recent years, S.rugosoannulata has been rapidly promoted and widely cultivated throughout China (Gu et al. 2024). With the increasing scale of cultivation, the annual yield of S.rugosoannulata in China has exceeded 210,000 tons per year (Huang et al. 2023). However, the emergence of various diseases during the cultivation of S.rugosoannulata has driven researchers to intensify their efforts to optimize its growth conditions. Our investigation observed green mold disease on the soil surface and fruiting bodies of S.rugosoannulata from three different localities. This disease incidence can lead to mushroom rot and a decline in yield and quality. The dedication of researchers to addressing this issue is a reassuring sign for the future of S.rugosoannulata cultivation.
Green mold disease is a major prevalent disease that frequently arises during mushroom development and is characterized by green, villiform mycelia on the surface (Li et al. 2013). Trichoderma Pers. (Hypocreales, Ascomycota) is a saprobic fungus found in soil, healthy plants, wood, and other fungi and plays a crucial role as the causative agent of green mold disease. Trichoderma species are widely used to combat fungal pathogens (Hasan et al. 2012; Liu et al. 2012; Li et al. 2013; Abo-Elyousr et al. 2014; Poveda et al. 2019), produce antibiotics, enzymes, and biofuel (Degenkolb et al. 2008; Jun et al. 2011; Wijayawardene et al. 2022). Additionally, Trichoderma species contribute to the bioremediation of xenobiotic compounds in water and soil (Katayama and Matsumura 1993; Harman et al. 2004; Ezzi and Lynch 2005). Currently, Trichoderma comprises more than 500 species globally, based on the literature search (Jaklitsch 2009; Jaklitsch and Voglmayr 2015; Jambhulkar et al. 2024), legitimate names in the Mycobank (https://www.mycobank.org.) and in the Species Fungorum database (www.speciesfungorum.org; accessed on 23 October 2024).
Trichoderma has two types of species with differing ascospore colours, namely hyaline and green ascospores. Chaverri and Samuels (2004) pioneered comprehensive research on green-spored Trichoderma species, providing foundational insight into their taxonomy and systematics. Subsequently, Jaklitsch and Voglmayr (2015) proposed a comprehensive classification primarily based on molecular phylogenetic analyses rather than the color of ascospores, dividing them into six subclades: Ceramicum, Chlorosporum, Harzianum, Helium, Spinulosum, and Strictipile. However, other researchers have not recognized this classification, largely due to the inconsistencies between molecular sequence data and morphological characteristics, as highlighted by Chen and Zhuang (2017). Bustamante et al. (2021) used multi-locus phylogenetic analyses alongside four DNA-based approaches to accurately delimit species within the Trichoderma Harzianum lineage, including most green-spored species.
The present study was conducted based on the pathogen of the green mold disease, aiming to characterize and identify the isolates. Six isolates were isolated from soil samples and fruiting bodies of S.rugosoannulata cultivated fields in three different regions of Guizhou Province, China. The study described two new species and compared their morphological characteristics among closely related species. A combined dataset of ITS, rpb2, and tef1-α was used for the thorough phylogenetic analyses, ensuring the reliability of the results.
Materials and methods
Pathogen collection, isolation, and maintenance
Infected fruiting bodies of S.rugosoannulata were collected from mushroom-cultivated fields at Baiyun and Shuicheng counties (23°4'23.6352"N, 120°37'39.7812"E and 24°55'39.936"N, 121°11'30.264"E), Guizhou Province, China in October-November 2023. Field photographs of the fresh specimens were taken with a Canon EOS 1200D (Canon, Japan) or Sony DSC-W830 (Sony, Japan) camera. The specimens were packed in aluminium foil and transferred to the Plant Pathology Laboratory at Guizhou University for isolation. Fungal pathogens on infected fruiting bodies were isolated using the spread plate and tissue isolation method following Wang et al. (2019). Purified cultures were incubated on potato dextrose agar (PDA), malt extract agar (MEA), and synthetic low nutrient agar (SNA) plates at 25, 30, and 35 °C. The holotype specimen was deposited in the Herbarium of the Department of Plant Pathology, Agricultural College, Guizhou University (HGUP). All single ex-type strains were deposited in the Culture Collection of the Guizhou University, China (GUCC) Department of Plant Pathology at Agriculture College and maintained in 25% (v/v) glycerol at –80 °C for long-term preservation (Zeng et al. 2022). Index Fungorum numbers were registered for the new taxa (https://www.indexfungorum.org/names/Names.asp).
Pathogenicity assays
A pathogenicity test was conducted by inoculating fungal mycelial blocks and spore suspensions from six strains isolated from Baiyun, Shuicheng, and Anshun counties onto the soil surface and fruiting bodies of S.rugosoannulata, following the updated protocol of Tian et al. (2017). All strains were incubated at 25 °C for 10 days. Control checks (CK) included PDA blocks and distilled water, replacing the mycelial blocks and spore suspensions. Photographs of the inoculated soils were taken after one, seven, and 10 days to monitor the development of any green mycelia. After the 10-day incubation, fungal pathogens were re-examined and re-isolated from the diseased areas to fulfill Koch’s postulates, ensuring accurate identification of pathogenicity (Zhang et al. 2015; Xie et al. 2024). The experiment was repeated three times to validate the results and account for variability.
Morphological studies
Micro-morphological observations were performed from culture photographs of fresh stromata, which were taken using an ultra-depth field stereomicroscope (digital microscope system Keyence VHX-7000) to illustrate the macrostructures. Sections were made using a stereomicroscope (Leica DM2500) and mounted in water or a rehydrated 5% KOH solution. The cultures were incubated at 25 °C in darkness (Põldmaa 2011; Wei et al. 2024). Approximately 30 morphological measurements of new species were made for each feature using the ZEN 3.0 (blue edition) (Jena, Germany) software (Zeiss Scope 5 with color camera AxioCam 208) with differential interference contrast (DIC) optics to observe the morphological characteristics (Jaklitsch and Voglmayr 2015; Fu et al. 2024; Zeng et al. 2024). Colony characteristics, i.e., color and texture on PDA (Potato dextrose agar; 200 g potatoes, 20 g dextrose, 20 g agar per L), MEA (malt extract agar; 30 g malt extract, 5 g mycological peptone, 15 g agar per L) and synthetic low nutrient agar (SNA) plates at 25, 30 and 35 °C were observed and noted over 14 days.
Molecular studies
DNA extraction, Polymerase Chain Reaction (PCR) and sequencing
The genomic DNA was extracted from the colony of the isolates cultured at 25 °C, PDA for seven days using a CwBiotech Plant Genomic DNA Kit (Changping, Beijing, China) following the manufacturer’s protocol.
The internal transcribed spacer (nrITS), the second largest RNA polymerase II subunit (rpb2) and the partial translation elongation factor 1-alpha (tef1-α) regions were amplified using the primer pairs ITS5/ITS4, EF1-728F/TEF1LLErev, and fRPB2-5F/fRPB2-7cR, respectively (White et al. 1990; Carbone and Kohn 1999; Liu et al. 1999; Jaklitsch et al. 2005). A 25 mL reaction mixture containing 1.6 mL dNTP mix (2.5 mM/mL), 0.2 mL Taq polymerase (5 U/mL), 2 mL polymerase buffer (10 /mL), 1 ml forward and reverse primers (10 mM/mL), and 1 mL DNA template was used for PCR experiments. Amplifications were carried out in a T100TM Thermal Cycler (BIO-RAD), which was configured for an initial denaturation at 95 °C for 3 minutes, followed by 34 cycles of 1 minute at 95 °C, 30 seconds at 55 °C, 1-minute extension at 72 °C, and a final extension at 72 °C for 10 minutes. Sangon Biotech (Shanghai) Co., Ltd. sequenced PCR products using the same PCR primers used in amplification operations. The newly generated sequences were checked with BioEdit v.7.2.5 (Hall 1999) and deposited in the NCBI GenBank nucleotide database for future reference.
The amplified sequences were subjected to BLASTn searches in the GenBank nucleotide database for comparison. Subsequently, closely related sequences of the taxa exhibiting zero E-values were retrieved from the database to generate the dataset. Besides, the sequences used by earlier studies on Trichoderma (Zeng et al. 2022) were also obtained from the database to prepare the final dataset (Table 1).
Table 1.
Names, strain numbers, locations, and corresponding GenBank accession numbers of the taxa used in the phylogenetic analysis.
| Species | Strain | Geographic origin | GenBank Accession Numbers | ||
|---|---|---|---|---|---|
| ITS | rpb2 | tef1-α | |||
| T.achlamydosporum | YMF 1.06226 | China | MN977791 | MT052180 | MT070156 |
| T.aerugineum | CBS 120541 T | Austria | FJ860720 | FJ860516 | FJ860608 |
| T.afarasin | DIS 314F | Cameroon | FJ442259 | FJ442778 | FJ463400 |
| T.afroharzianum | CBS 466.94 | Netherlands | KP009262 | KP009150 | KP008851 |
| T.aggregatum | HMAS 248863 | China | KY687946 | KY688001 | KY688062 |
| T.aggressivum | CBS 100525 | United Kingdom | - | AF545541 | AF534614 |
| T.alni | CBS 120633 T | United Kingdom | EU518651 | EU498349 | EU498312 |
| T.alpinum | HMAS 248821 T | China | KY687906 | KY687958 | KY688012 |
| T.amazonicum | IB 95 | Peru | - | HM142368 | HM142377 |
| T.anaharzianum | YMF 1.00383 | China | MH113931 | MH158995 | MH183182 |
| T.asiaticum | YMF 1.00352 | China | MH113930 | MH158994 | MH183183 |
| T.atrobrunneum | S3 | Italy | - | KJ665241 | KJ665376 |
| T.atrogelatinosum | CBS 237.63 T | New Zealand | MH858272 | KJ842201 | KJ871083 |
| T.attinorum | LESF 236 | USA | - | KT278971 | KT279039 |
| T.aureoviride | CPK 2848 | Germany | FJ860733 | FJ860523 | FJ860615 |
| T.azevedoi | CEN1422 T | Brazil | MK714902 | MK696821 | MK696660 |
| T.bannaense | HMAS 248840 T | China | KY687923 | KY687979 | KY688037 |
| T.breve | HMAS 248844 T | China | KY687927 | KY687983 | KY688045 |
| T.brevicrassum | HMAS 248871 T | China | KY687954 | KY688008 | KY688064 |
| T.britannicum | CBS 253.62 T | United Kingdom | MH858149 | KF134787 | KF134796 |
| T.brunneoviride | CBS 121130 | Germany | EU518659 | EU498357 | EU498316 |
| T.byssinum | HMAS 248838 T | China | KY687921 | KY687977 | KY688035 |
| T.catoptron | GJS 02-76 T | Sri Lanka | AY737766 | AY391900 | AY391963 |
| T.ceraceum | GJS 95-159 T | North Carolina | AF275332 | AF545508 | AF534603 |
| T.ceramicum | CBS 114576 T | Austria | FJ860743 | FJ860531 | FJ860628 |
| T.ceratophylli | YMF 1.04621 | China | MK327581 | MK327580 | MK327579 |
| T.cerinum | S357 | France | - | KF134788 | KF134797 |
| T.chlamydosporicum | HMAS 248850 | China | KY687933 | KY687989 | KY688052 |
| T.chlorosporum | GJS 88-33 T | USA | - | AY391903 | AY391966 |
| T.christiani | CBS 132572 T | Spain | - | KJ665244 | KJ665439 |
| T.chromospermum | HMAS 252535 | China | KF923304 | KF923315 | KF923292 |
| T.cinnamomeum | GJS 97-237 | USA | AY737759 | AY391920 | AY391979 |
| T.compactum | CBS 121218 T | China | - | KF134789 | KF134798 |
| T.concentricum | HMAS 248833 T | China | KY687915 | KY687971 | KY688027 |
| T.corneum | GJS 97-82 ET | Thailand | - | KJ665252 | KJ665455 |
| T.costaricense | PC 21 T | Costa Rica | AY737754 | AY391921 | AY391980 |
| T.cremeoides | S112 T | Italy | - | KJ665253 | KJ665456 |
| T.cremeum | GJS 91-125 T | USA | AY737760 | AF545511 | AF534598 |
| T.cuneisporum | GJS 91-93 T | USA | AY737763 | AF545512 | AF534600 |
| T.dacrymycellum | WU 29044 | Germany | FJ860749 | FJ860533 | FJ860633 |
| T.danicum | CBS 121273 T | Denmark | FJ860750 | FJ860534 | FJ860634 |
| T.epimyces | CBS 120534 T | Austria | EU518663 | EU498360 | EU498320 |
| T.estonicum | GJS 96-129 T | Estonia | AY737767 | AF545514 | AF534604 |
| T.ganodermatis | HMAS 248856 | China | KY687939 | KY687995 | KY688060 |
| T.gelatinosum | GJS 88-17 | France | AY737775 | AF545516 | AF534579 |
| T.gliocladium | CBS 130009 T | Italy | MH865622 | KJ665271 | KJ665502 |
| T.guizhouense | S278 | Croatia | - | KF134791 | KF134799 |
| T.hainanense | HMAS 248837 T | China | KY687920 | KY687976 | KY688033 |
| T.harzianum | CBS 226.95 T | Austria | AY605713 | AF545549 | AF534621 |
| T.hausknechtii | CBS 133493 T | France | - | KJ665276 | KJ665515 |
| T.helicolixii | CBS 133499 T | Greece | - | KJ665278 | KJ665517 |
| T.helicum | DAOM 230021 | Austria | - | DQ087239 | KJ871125 |
| T.hirsutum | HMAS 248834 T | China | KY687916 | KY687972 | KY688029 |
| T.hunanense | HMAS 248841 T | China | KY687924 | KY687980 | KY688039 |
| T.hymenopellicola | GUCC202008 | China | MZ330754 | ON088663 | ON102007 |
| T.hymenopellicola | GUCC202009 | China | MZ330755 | ON088664 | ON102008 |
| T.hymenopellicola | GUCC202010 | China | MZ330756 | ON088661 | ON102005 |
| T.hymenopellicola | GUCCTB626 | China | ON074580 | ON088662 | ON102006 |
| T.hymenopellicola | GUCCTB625 | China | ON074583 | - | ON102011 |
| T.inaequilaterale | YMF 1.06203 | China | MN977795 | MT052186 | MT070152 |
| T.ingratum | HMAS 248822 T | China | KY687917 | KY687973 | KY688018 |
| T.inhamatum | CBS 273.78 T | Colombia | - | FJ442725 | AF348099 |
| T.italicum | CBS 132567 T | Italy | - | KJ665282 | KJ665525 |
| T.jaklitschii | CP61-2 T | Peru | - | MW480149 | MW480140 |
| T.lentiforme | DIS 94D | Peru | - | FJ442749 | FJ463379 |
| T.lentinulae | CGMCC 3.19847 T | China | - | MN605867 | MN605878 |
| T.liberatum | HMAS 248831 T | China | KY687913 | KY687969 | KY688025 |
| T.linzhiense | HMAS 248846 T | China | KY687929 | KY687985 | KY688047 |
| T.lixii | CBS 110080 T | USA | AF443920 | KJ665290 | FJ716622 |
| T.longibrachiatum | CBS 816.68 T | Austria | Z31019 | DQ087242 | EU401591 |
| T.longifialidicum | LESF 552 | USA | - | KT278955 | KT279020 |
| T.longipile | DAOM 177227 T | Austria | AY865630 | AF545550 | AF534622 |
| T.longisporum | HMAS 248843 | China | KY687926 | KY687982 | KY688043 |
| T.lycogaloides | WU 32096 T | French Guiana | - | KF134792 | KF134800 |
| T.parepimyces | CBS 122769 T | Austria | FJ860800 | FJ860562 | FJ860664 |
| T.parestonicum | CBS 120636 T | Austria | FJ860803 | FJ860565 | FJ860667 |
| T.peberdyi | CEN1426 T | Brazil | MK714906 | MK696825 | MK696664 |
| T.peruvianum | CP15-2 T | Peru | - | MW480153 | MW480145 |
| T.perviride | HMAS 273786 T | China | - | KX026962 | KX026954 |
| T.phyllostachydis | CBS 114071 T | Austria | FJ860809 | FJ860570 | FJ860673 |
| T.pinicola | SFC20130926-S233 T | South Korea | MH050354 | MH025993 | MH025981 |
| T.pleuroti | CBS 124387 T | USA | - | HM142372 | HM142382 |
| T.pleuroticola | CBS 124383 T | USA | - | HM142371 | HM142381 |
| T.polypori | HMAS 248855 T | China | KY687938 | KY687994 | KY688058 |
| T.priscilae | CBS 131487 T | Austria | - | KJ665333 | KJ665691 |
| T.propepolypori | YMF 1.06224 | China | MN977789 | MT052181 | MT070158 |
| T.pseudoasiaticum | YMF 1.06200 T | China | MN977792 | MT052183 | MT070155 |
| T.pseudocandidum | PC 59 T | Costa Rica | AY737757 | AY391899 | AY737742 |
| T.pseudodensum | HMAS 248828 T | China | KY687910 | KY687967 | KY688023 |
| T.pseudogelatinosum | CNU N309 T | South Korea | - | HM920173 | HM920202 |
| T.purpureum | HMAS 273787 T | China | - | KX026961 | KX026953 |
| T.pyramidale | CBS 135574 T | Italy | - | KJ665334 | KJ665699 |
| T.rifaii | DIS 337F ET | Panama | - | FJ442720 | FJ463321 |
| T.rosulatum | HMAS 252548 | China | KF729995 | KF730005 | KF729984 |
| T.rufobrunneum | HMAS 266614 T | China | KF729998 | KF730010 | KF729989 |
| T.rugulosum | SFC20180301-1 T | South Korea | MH050353 | MH025986 | MH025984 |
| T.shennongjianum | HMAS 245009 | China | - | KT735259 | KT735253 |
| T.silvae-virgineae | CBS 120922 | Austria | - | FJ860587 | FJ860696 |
| T.simile | YMF 1.06201 | China | MN977793 | MT052184 | MT070154 |
| T.simmonsii | S7 | Italy | - | KJ665337 | KJ665719 |
| T.simplex | HMAS 248842 T | China | KY687925 | KY687981 | KY688041 |
| T.sinuosum | CPK 1595 | Austria | FJ860838 | FJ179619 | FJ860697 |
| T.solum | HMAS 248848 T | China | KY687931 | KY687987 | KY688050 |
| T.spinulosum | CBS 311.50 T | Austria | FJ860844 | FJ860591 | FJ860701 |
| T.spirale | DAOM 183974 T | Thailand | EU280068 | AF545553 | EU280049 |
| T.stipitatum | HMAS 266612 | China | KF730002 | KF730011 | KF729990 |
| T.stramineum | GJS 02-84 T | Sri Lanka | AY737765 | AY391945 | AY391999 |
| T.strictipile | CPK 1601 | Austria | - | FJ860594 | FJ860704 |
| T.strophariensis | GUCC TB1117 T | China | PP920011 | PP954941 | PP954947 |
| T.strophariensis | GUCC TB1118 | China | PP920012 | PP954942 | PP954948 |
| T.strophariensis | GUCC TB1119 | China | PP920013 | PP954943 | PP954949 |
| T.subazureum | YMF 1.06207 | China | MN977799 | MT052190 | MT070148 |
| T.subuliforme | YMF 1.06204 | China | MN977796 | MT052187 | MT070151 |
| T.sulawesense | GJS 85-228 | USA | - | AY391954 | AY392002 |
| T.surrotundum | GJS 88-73 T | USA | AY737769 | AF545540 | AF534594 |
| T.tawa | GJS 97-174 T | Thailand | AY737756 | AY391956 | AY392004 |
| T.tenue | HMAS 273785 T | China | - | KX026960 | KX026952 |
| T.thailandicum | GJS 97-61 T | Thailand | AY737772 | AY391957 | AY392005 |
| T.thelephoricola | CBS 120925 | Austria | FJ860858 | FJ860600 | FJ860711 |
| T.tibetense | HMAS 245010 | China | - | KT735261 | KT735254 |
| T.tomentosum | CBS 120637 | Austria | - | FJ860532 | FJ860629 |
| T.tropicosinense | HMAS 252546 | China | KF923302 | KF923313 | KF923286 |
| T.undatipile | HMAS 248854 | China | KY687937 | KY687993 | KY688056 |
| T.velutinum | CPK 298 T | Nepal | - | KF134794 | KJ665769 |
| T.vermifimicola | CGMCC 3.19694 T | China | MN594473 | MN605871 | MN605882 |
| T.virens | DAOM 167652 T | USA | EU330955 | AF545547 | AF534619 |
| T.virescentiflavum | PC 278 | Costa Rica | AY737768 | AY391959 | AY392007 |
| T.viridistromatis | GUCC TB1120 T | China | PP922277 | PP954944 | PP954950 |
| T.viridistromatis | GUCC TB1121 | China | PP926290 | PP954945 | PP954951 |
| T.viridistromatis | GUCC TB1122 | China | PP922285 | PP954946 | PP954952 |
| T.xixiacum | CGMCC 3.19697 T | China | MN594476 | MN605874 | MN605885 |
| T.zayuense | HMAS 248835 T | China | KY687918 | KY687974 | KY688031 |
| T.zelobreve | CGMCC 3.19695 T | China | MN594474 | MN605872 | MN605883 |
| T.zeloharzianum | YMF 1.00268 | China | MH113932 | MH158996 | MH183181 |
Note: Newly sequenced strains are shown in bold. T denotes type cultures.
Dataset representation
Sequences of the closely related taxa with zero E-value were searched from the BLASTn analyses in the NCBI GenBank nucleotide database. A preliminary BLAST search with the newly amplified sequences of the collected specimens showed the highest sequence similarity with the members of the Trichoderma Pers. Hence, a dataset was prepared based on the highest-scored hits of the BLAST search plus the datasets used in the earlier studies on Trichoderma (Zeng et al. 2022).
Sequence alignment and phylogenetic analyses
The newly generated reverse and forward sequences were reassembled manually using BioEdit version 7.0.5.3 (Hall 1999) and were aligned with MAFFT v.7.427 (Katoh et al. 2019) in an online platform (https://www.ebi.ac.uk/Tools/msa/mafft/). The aligned sequences were imported to MEGA v.7.0 (Kumar et al. 2016) for manual improvement and trimming of both ends.
A quick phylogenetic analyses of DNA fragments (ITS, rpb2 and tef1-α) from 128 strains were performed with alignments and associated data matrices, including six isolates in this study (GUCC TB1117, GUCC TB1118, GUCC TB1119, GUCC TB1120, GUCC TB1121 and GUCC TB1122) and 122 reference strains (Table 1) by using offline software ‘One-click Fungal Phylogenetic Tool’ (OFPT-https://ofpt.guhongxin.com) following its default protocol (Zeng et al. 2023). The final Maximum likelihood analysis was performed with RAxML-HPC2 v. 8.2.12 (Stamatakis 2014) on the CIPRES Science Gateway platform using the GTR+I+G model with 1,000 bootstrap replicates and Bayesian analyses were conducted with MrBayes v.3.2.2 (Ronquist et al. 2012) using MCMC methods (Geyer 1991) under a GTR+I+G model. Markov chains were run for 2 × 106 generations, saving a tree every 100th generation with all the remaining parameters set to default. Bayesian analyses reached a standard deviation of split frequency of 0.0048 at the end of the specified number of generations. For both analyses, the initial 25% of trees recovered (10,000 trees) were excluded as the burn-in, while the remaining 30,002 trees were utilized to estimate the posterior probabilities for the group. ML bootstrap values (MLBS) ≥ 70% and Bayesian posterior probabilities (PP) values ≥ 0.95 are displayed in the phylogenetic tree. The resulting trees were visualized in FigTree v1.4.3 (Rambaut 2016).
Results
Pathogenicity tests
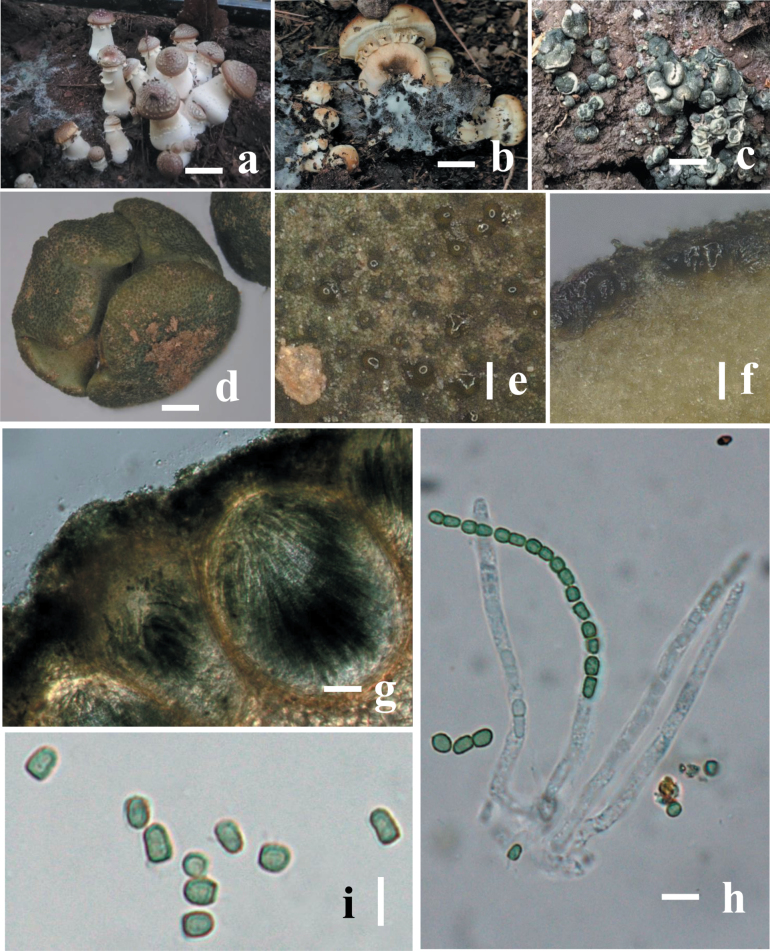
Both soil inoculating groups of covering mycelial blocks and soil mixed with spore suspension of isolates GUCC TB1117 and GUCC TB1120 exhibited similar symptoms of green mold disease in the field after seven days (Fig. 1g), while the control group did not have (Fig. 1d). The green mycelia can be observed on the surface of the mushroom tray after 3–5 days and spread fast, covering the whole surface of the substrate and turning green within 10 days (Fig. 1e, f). The rate of isolates GUCC TB1117 and GUCC TB1120 infecting mushroom tray is about 50%, similar to its incidence in the field. The same fungal pathogen had been observed and re-isolated from these symptoms, which fulfills Koch’s postulates (Fig. 1).
Figure 1.
Field symptoms of green mold disease on Strophariarugosoannulata and pathogenicity tests of isolates GUCC TB1117 and GUCC TB1120 with spore suspension a healthy fruiting bodies of S.rugosoannulatab field symptoms of green mold disease on S.rugosoannulatac large stroma of the pathogen T.strophariensis (GUCC TB1117) d control, no disease after seven days of inoculation with distilled water e–g pathogenicity tests after spraying with 0.5 mL spore suspension (1 × 106 conidia mL–1) e, f hyphal blocks and pathogen stroma (F = yellow arrow) appear on the surface of the soil after five days of inoculation g whole rotten fruiting bodies after seven days of inoculation h, i rotten fruiting bodies of S.rugosoannulata in the field with T.viridistromatis (GUCC TB1120) j Aggregated stroma of the pathogen T.viridistromatis (GUCC TB1120) with typical green symptoms k, l yellow arrows showing pathogen hyphal blocks and stroma appear on the surface of the soil after five days of inoculation. Scale bars: 20 mm (a–e); 10 mm (f); 20 mm (g); 10 mm (h); 20 mm (I, j); 10 mm (k, l).
Phylogenetic analyses
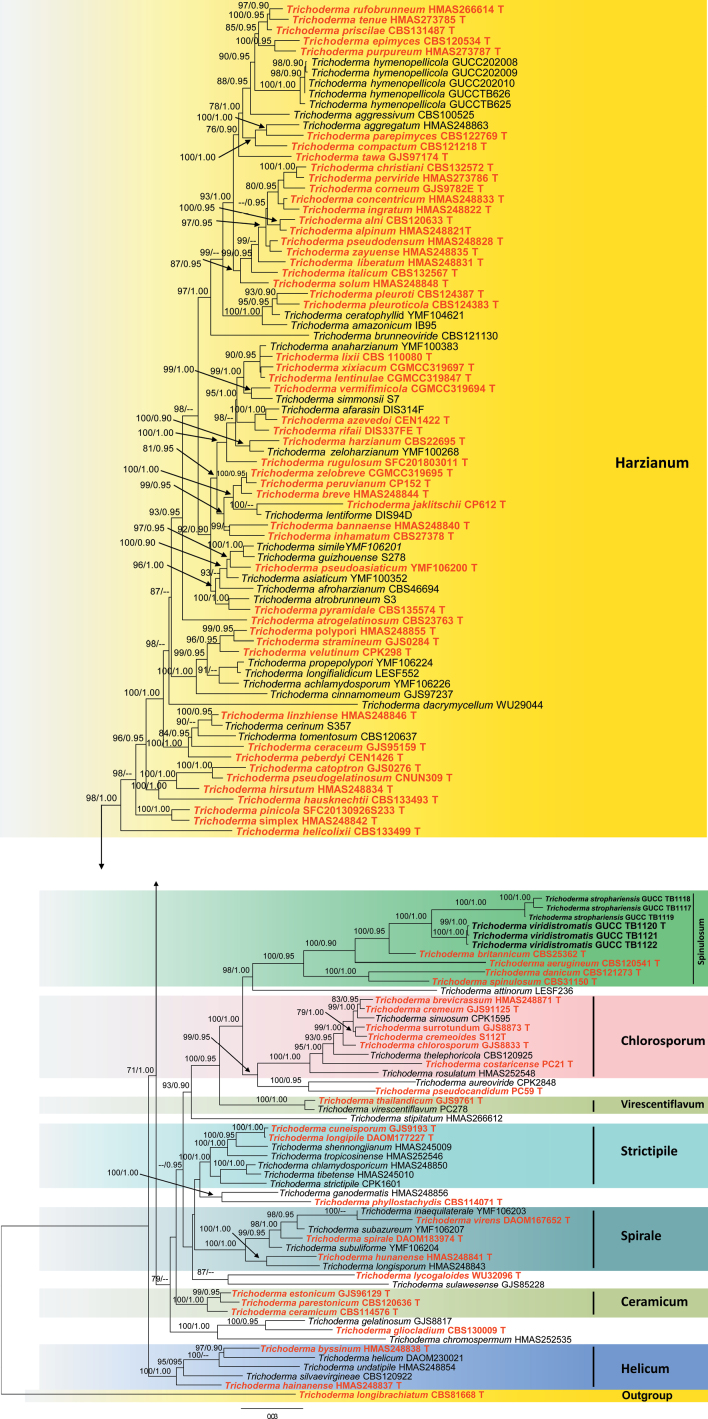
The phylogenetic analyses were conducted using a combined dataset of nrITS, rpb2, and tef1-α sequences. A total of 128 sequences were aligned, and this resulted in a dataset consisting of 2934 nucleotides; after the ends of the individual alignments were trimmed, the size of the aligned dataset was as nrITS 610 bp, rpb2 was 1080 bp, and tef1-α was 1244 bp respectively. The best-fit substitution model of each gene is ITS (TIM2+F+R4), rpb2 (TIM3e+I+G4) and tef1-α (TIM+F+R4). The RAxML analysis of the combined dataset yielded a best-scoring tree with a final ML optimization likelihood value of –37957.575772. Estimated base frequencies are as follows: A = 0.233134, C = 0.285526, G = 0.253003, and T = 0.228336; substitution rates AC = 1.134637, AG = 4.477934, AT = 1.149518, CG = 1.048786, CT = 6.335323, and GT = 1.000000; proportion of invariable sites I = 0.544721; and gamma distribution shape parameter a = 0.951765. The Bayesian analysis ran 29,64000 generations before the average standard deviation for split frequencies reached 0.00998. The analysis generated 59,282 trees, from which 44,462 were sampled after burn-in, and the 99% credible set contains 35,309 trees. Our new strains belong to a distinct clade that is genetically distant from T.britannicum, T.aerugineum, T.danicum, and T.spinulosum and is divided into four subclades represented by our newly generated strains (Fig. 2). DNA base pair differences also supported the phylogenetic placements of these novel taxa (Table 2).
Figure 2.
A phylogram was constructed using ML analysis, utilizing a combined ITS, rpb2, and tef1-α sequences dataset. The green-spored T.longibrachiatum (CBS81668) was used as the outgroup taxon following Zeng et al. (2022). The tree with the highest score according to RAxML, with a final probability value of -InL = 37957.575772, is displayed. Maximum Likelihood (ML) values equal to or greater than 70% and Bayesian Inference (BI) values equal to or greater than 0.90 are given above the nodes (ML values on the left side of ‘/’ in regular font and BI values on right side of ‘/’ in italics). Type strain sequences are indicated in red bold, while newly generated sequences are shown in black bold. Strain numbers for the sequences are shown in the tree following the taxon name. ‘T’ denotes ex-holotype strains.
Table 2.
The DNA base differences of our isolates and related taxa in different loci.
| Species | Strain number | ITS (1–610 bp) | rpb2 (611–1690 bp) | tef1-α (1691-2934 bp) |
|---|---|---|---|---|
| Trichodermastrophariensis | GUCC24-0002 | 0 | 0 | 0 |
| Trichodermastrophariensis | GUCC24-0003 | 0 | 0 | 0 |
| Trichodermastrophariensis | GUCC24-0004 | 0 | 0 | 0 |
| Trichodermabritannicum | CBS 25362 | 28 (gaps: 4) | 48 (gap: 0) | 64 (gap: 25) |
| Trichodermaaerugineum | CBS 120541 | 16 (gaps: 9) | 78 (gap: 0) | 67 (gap: 11) |
| Trichodermadanicum | CBS 121273 | 25 (gaps: 8) | 99 (gaps: 0) | 105 (gaps: 3) |
| Trichodermaspinulosum | CBS 31150 | 21 (gaps: 8) | 82 (gaps: 0) | 108 (gap: 7) |
| Trichodermaviridistromatis | GUCC24-0005 | 0 | 0 | 0 |
| Trichodermaviridistromatis | GUCC24-0006 | 0 | 0 | 0 |
| Trichodermaviridistromatis | GUCC24-0007 | 0 | 0 | 0 |
| Trichodermabritannicum | CBS 25362 | 17 (gaps: 5) | 20 (gap: 0) | 55 (gaps: 21) |
| Trichodermaaerugineum | CBS 120541 | 10 (gaps:10) | 79 (gap: 0) | 78 (gaps: 11) |
| Trichodermadanicum | CBS 121273 | 25 (gaps: 8) | 95 (gap: 0) | 113 (gap: 0) |
| Trichodermaspinulosum | CBS 31150 | 22 (gaps: 8) | 73 (gaps: 0) | 109 (gaps: 7) |
Taxonomy
. Trichoderma strophariensis
E. Tarafder & F.H. Tian sp. nov.
22D20AFB-BC41-5495-B40A-EB53EFFBE5CB
Fungal Names: FN 902311
Figure 3.
Morphology of Trichodermastrophariensis (HGUP 24-0001, GUCC 24-0002) a, b disease in the field habitat c fresh stromata on natural habitat d dry stromata e ostiolar dots on stromata surface f cortical and subcortical tissues in section g ascomatal tissue in section h asci with ascospores i ascospores. Scale bars: 10 mm (a, b); 20 mm (c); 100 mm (d–f); 50 μm (g); 20 μm (h, i).
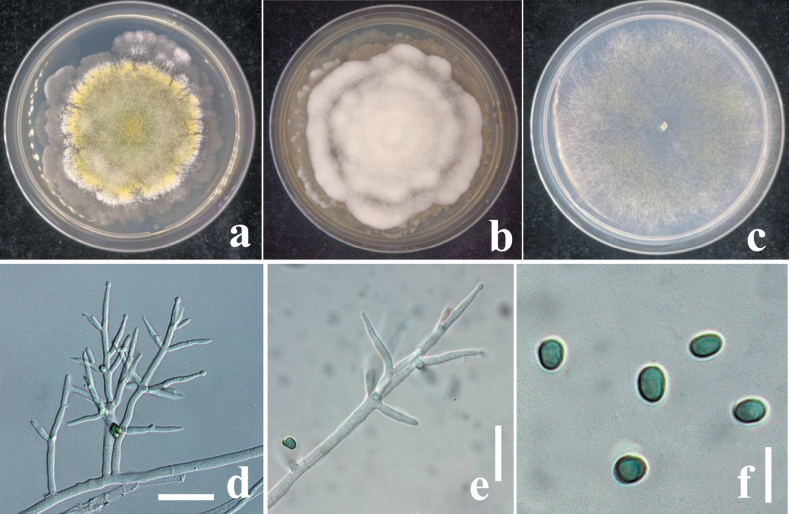
Figure 4.
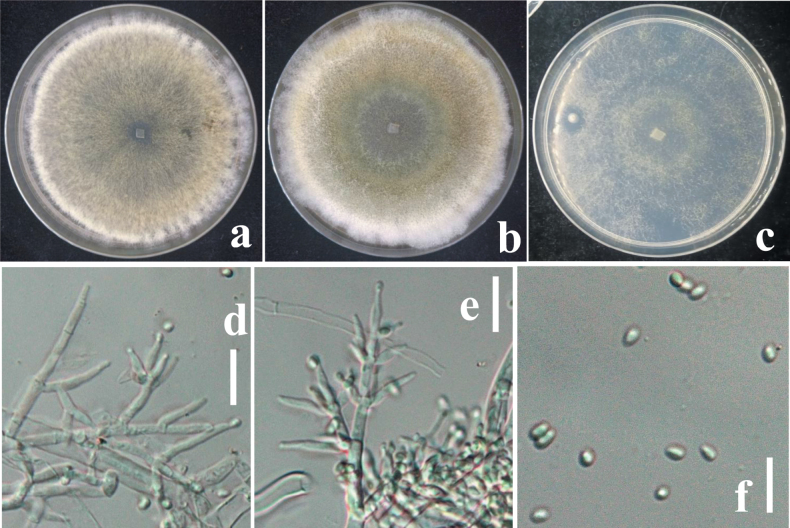
a cultures on MEA (five days) b cultures on PDA (five days) c cultures on SNA (4 days) d conidiophores e phialides f conidia. Scale bars: 10 μm (d, e); 5 μm (f).
Diagnosis.
Trichodermastrophariensis differs from T.britannicum by smaller stromata (0.9–2.2 × 0.8–2 mm) with dark green surface, margin free; surface finely rugose or tubercular, brownish between black ascomata; ostiolar dots absent, inconspicuous or convex to distinctly papillate measuring (27–)35–64(–90) mm diam. Additionally, it is easily distinguished from T.viridistromatis by its relatively larger ascospores (8.4–16.9 × 5.5–8.1 µm) and conidia (8.5–25.5 × 5.7–17.9 μm). Phylogenetically, T.strophariensis forms a distinct clade and is closely related to T.viridistromatis, T.britannicum, and T.aerugineum with 100% ML and 0.90 BYPP statistical support (Fig. 1).
Holotype.
HGUP 24-0001.
Etymology.
The specific epithet ‘strophariensis’ refers to the occurrence of the new taxon in cultivated mushrooms Strophariarugosoannulata.
Description.
Stromata, when fresh 1–14 mm in diameter, 1–11 mm thick (n = 10), solitary to sometimes aggregated, discoid or undulate, with brownish margin and pale red, depressed center when young, becoming reddish with rugose surface when mature. Attached to the host by hyphae, easily detached; sides often attenuated downward, surrounded at the base by white cottony mycelium when young. Surface finely rugose, tubercular, brownish between black ascomata; Ostiolar dots are convex to umbilicate, greenish, overall colors light green, darker green when dry, surface and spores green when mature. Ostiole 14–21 μm wide at apex, 41–59 μm high (n = 30). Ascomata (139–)175–295(–347) × (113–)151–248(–290) μm (n = 20), flask-shaped or sub-globose, crowded. Peridium 18–28 μm thick at the base and sides (n = 40), light brown. Asci (67–)110–146(–207) × (3.7–)5.8–7.7(–9.4) μm, stipe (3–)7–11(–18) μm long (n = 50), containing 16-ascospores, apex slightly thickened, hyaline, cylindrical. Ascospores (8.4–)9.2–11.6(–16.9) × (5.5–)6.6–7.8(–8.1) μm, l/w (1.2–)1.4–1.6(–2.1) (n = 90), green, verruculose; sub-globose, oblong, elongated, thick-walled.
Culture characteristics.
Optimal growth at 25 °C on all media, poor and limited growth at 30 °C, no growth at 35 °C.
On MEA and PDA growth is slow, colony creamy white, finely farinose by scant effuse conidiation; on PDA reverse brownish, surface turning greenish-brown. On MEA at 25 °C after five days colony radius 5–7 mm; colony circular, dense, thick, first whitish, becoming zonate after a few weeks, turning olive-green to brown with yellow greenish, farinose center; conidiation effuse, on short odd verticillium like conidiophores. On SNA colony radius at 25 °C after 2 weeks 6–9 mm; colony dense, hyaline, turning greenish or olivaceous from conidia. Conidiation following growth, effuse, on aerial hyphae and short odd verticillium-like conidiophores, spreading from the plug. Conidiophores simple, 1–4 level are branched and tapered at the tips, bearing few asymmetric side branches, terminated by solitary phialides of 2–3 divergent phialides. Phialides (10.5–)37–44(–55) × (1.5–)2.5–11(–12.5) μm (n = 50), mostly gregarious, cylindrical, less commonly subfusiform, often thickest near the base. Conidia (8.5–)12.5–16.4(–25.5) × (5.7–)6.5–10.7(–17.9) μm (n = 70), one-celled, variable shape and size, typically oblong and pale olive green when fully mature, sub-globose, oval or ellipsoid and hyaline when immature, straight or slightly curved, sides sometimes pinched, smooth; base often truncate, thick-walled.
Habitat.
On mushroom cultivated field, associated with Strophariarugosoannulata.
Distribution.
China, Guizhou Province, Guiyang City, and Liupanshui City; Guizhou City in Anshun Province.
Material examined.
China • Guizhou, Liupanshui City, Shuicheng District, 23°55'39.36"N, 120°11'30.64"E, on soil surfaces of Strophariarugosoannulata cultivated field, 16-November-2023, E. Tarafder and F.H. Tian (HGUP 24-0001, holotype); ex-type living cultures GUCC TB1117, GUCC TB1118 and GUCC TB1119.
GenBank accession numbers.
GUCC TB1117 (ITS: PP920011; rpb2: PP954941; tef1-α: PP954947); GUCC TB1118 (ITS: PP920012; rpb2: PP954942; tef1-α: PP954948); GUCC TB1119 (ITS: PP920013; rpb2: PP954943; tef1-α: PP954949).
Notes.
Morphologically, our new isolates are most similar to T.danicum in the size of stromata (5–20 mm) but can be distinguished by its generally smaller ascospores and conidia (Table 3); the presence of deeper color of stromata and ascospores, less pigment on media, and faster growth rate on PDA and SNA. However, our new isolates differ from T.britannicum by smaller stromata (0.9–2.2 mm) with dark green surfaces (Jaklitsch, 2009). In addition, it differs from other new species (T.viridistromatis) in producing cylindrical, less commonly subfusiform phialides (10.5–55 × 1.5–12.5 μm) and larger conidia (8.5–25.5 × 5.7–17.9 μm), typically oblong, subglobose, oval, sometimes ellipsoid and pale olive green after maturity. Phylogenetically, our isolate (HGUP 24-0001) forms an independent clade and clustering with Trichodermabritannicum, T.aerugineum, T.danicum, T.viridistromatis, and T.spinulosum within the Spinulosum lineage with 100% ML and 1.00 BYPP statistical support (Fig. 2). It exhibits 4% sequence differences (28/610 nucleotides, four gaps) in the ITS region, 4% differences (48/1080 nucleotides, no gaps) in the rpb2 gene, and 5% differences (64/1244 nucleotides, twenty-five gaps) in tef1-α gene when compared with T.britannicum. Additionally, the differences between our isolate with T.viridistromatis are 4% (29/610 nucleotides, four gaps) in the ITS region, 4% (46/1080 nucleotides, no gaps) differences in the rpb2 gene, and 5% (65/1244 nucleotides, twenty-five gaps) differences in the tef1-α gene. In contrast, the differences in our isolate with T.danicum are more than 4% (25/610 nucleotides, eight gaps) in the ITS region, 9% (99/1080 nucleotides, no gaps) in rpb2 gene, and 8% (105/1244 nucleotides, three gaps) in tef1-α gene (Table 2). Therefore, based on both morphological and phylogenetic distinctions, T.strophariensis is introduced as a new species from cultivated mushrooms.
Table 3.
Morphological comparison of Trichodermabritannicum, T.aerugineum, T.strophariensis, T.danicum, T.viridistromatis, and T.spinulosum.
| Taxon (holotype) | Ascospores | Conidia | Substratum | References |
|---|---|---|---|---|
| T.britannicum | 10–16 × 4.5–6.2 μm | 4.7–19.3 × 4–6.2 μm | Decaying wood of broadleaf trees | Jaklitsch et al. 2014 |
| T.aerugineum | 8–12 × 4–6 µm | 3–5 × 2–4 µm | Decaying wood | Chaverri and Samuels (2004) |
| T.strophariensis | 8.4–16.9 × 5.5–8.1 µm | 8.5–25.5 × 5.7–17.9 μm | mushroom species (Stropharia) | This study |
| T.danicum | 3–5 × 2.5–4.4 µm | 3–3.5 × 2.7–3 µm | On pine wood | Jaklitsch 2009 |
| T.viridistromatis | 3.4–5.6 × 2.4–3.3 µm | 2.8–4 × 1.7–3.2 µm | mushroom species (Stropharia) | This study |
| T.spinulosum | 5–7 × 3–4 μm | 3.5–4.7 × 3–3.7 μm | On stems of Chelidoniummajus | Jaklitsch and Voglmayr 2015 |
. Trichoderma viridistromatis
E. Tarafder & F.H. Tian sp. nov.
6C4FE537-E9C8-585A-8384-1E1B8AF4AC09
Fungal Names: FN 902312
Figure 5.
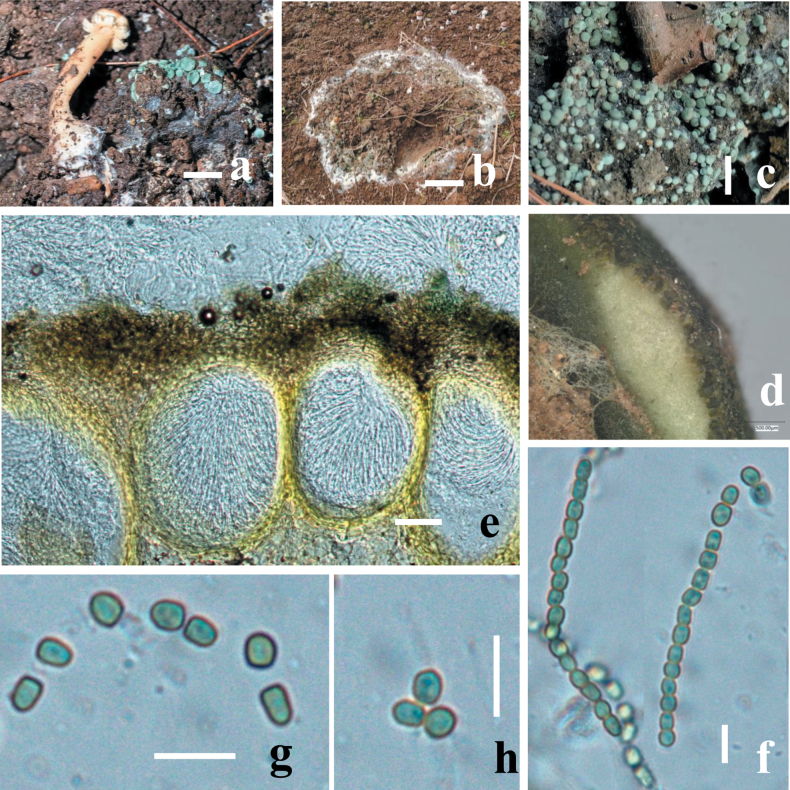
Morphology of Trichodermaviridistromatis (HGUP 24-0004, GUCC 24-0005) a, b diseased in the field, c fresh stromata on natural substrate d cortical and subcortical tissues e ascomatal tissue in section f asci with ascospores g, h ascospores. Scale bars: 10 mm (a–c); 1,000 μm (d); 50 μm (e); 20 μm (f–h).
Figure 6.
a cultures on MEA (five days) b cultures on PDA (five days) c cultures on SNA (four days) d conidiophores e phialides f conidia. Scale bars: 10 μm (d, e); 5 μm (f).
Diagnosis.
Trichodermaviridistromatis differs from T.aerugineum by its smaller stromata (0.5–2 mm diam, to ca. 1 mm thick in T.aerugineum) and bigger phialides measuring 7–23 × 2.4–4 μm in T.aerugineum. In addition, it is easily distinguished from T.strophariensis by its smaller ascospores (3.4–5.6 × 2.4–3.3 µm) and conidia (2.8–4 × 1.7–3.2 μm). Phylogenetically, T.viridistromatis forms a distinct clade and is closely related to T.strophariensis, T.britannicum, and T.aerugineum with 100% ML and 0.90 BYPP statistical support (Fig. 1).
Holotype.
HGUP 24-0004.
Etymology.
The epithet “viridistromatis” refers to an entirely green-colored stroma.
Description.
Stromata, when fresh 1–7 mm in diam., 0.5–2 mm thick (n = 10), mostly gregarious, aggregated, discoid or undulate, becoming pulvinate, compact; outline circular to oblong; margin attached or free, surface smooth when immature without ostiolar dots, with yellowish margin and pale red, depressed center when young, becoming reddish with rugose surface when mature. Outline circular, oblong or irregularly lobed. Surface smooth, tubercular or rugose, when young finely velvety. Ostiolar dots absent, ostiolar openings sometimes visible, (16–)20–30(–32) μm (n = 30) wide, inconspicuous, pale, more distinct and shinier after rehydration. Ostioles (18–)24–30(–45) μm long, plane with the surface, (8–)12–19(–23) μm wide at the apex (n = 30). Ascomata (69–)75–85(–96) × (36–)41–55(–60) μm (n = 30), numerous, 5–7 per mm stroma length, sub-globose or flask-shaped. Peridium (7–)11–19(–22) μm (n = 60) thick at the base and sides; hyaline to pale yellowish. Asci (63–)74–81(–85) × (3.2–)4.2–5(–5.5) μm, stipe (4–)5–11(–14) μm (n = 30) long, containing 16-ascospores, apex not thickened, hyaline, cylindrical. Ascospores (3.4–)3.6–4.3(–5.6) × (2.4–)2.8–3.1(–3.3) μm, l/w 1–1.1(–1.2) (n = 34), hyaline, verruculose, single-celled, non-septate, sub-globose, oblong or slightly tapered downwards, thick-walled.
Culture characteristics.
Optimal growth at 25 °C on all media, poor and limited growth at 30 °C, no growth at 35 °C. Although MEA exhibited good growth, precultures were made on it.
On MEA and PDA, growth is slow, colony is creamy white, finely farinose by scant effuse conidiation; on PDA, reverse brownish, surface turning greenish-brown. On MEA at 25 °C after five days colony radius 5–7 mm; colony circular, dense, thick, first whitish, becoming zonate after a few weeks, turning olive-green to brown with yellow-greenish, farinose center; conidiation effuse, on short odd verticillium like conidiophores. On SNA colony radius at 25 °C after 2 weeks 6–9 mm; colony dense, hyaline, turning greenish or olivaceous from conidia. Conidiation following growth, effuse, on aerial hyphae and short odd verticillium-like conidiophores, spreading from the plug. Conidiophores simple, 1–4 level, are branched and tapered at the tips, bearing few asymmetric side branches, terminated by solitary phialides of 2–3 divergent phialides. Phialides (5.5–)7–10(–14) × (1.6–)2.5–2.9(–3.5) μm (n = 32), mostly gregarious, lageniform, less commonly subfusiform, not thickest near the base. Conidia (2.8–)3.1–3.7(–4) × (1.7–)2.2–2.7(–3.2) μm (n = 70), variable shape and size, typically oblong and pale yellowish green when fully mature, oval, ellipsoid and hyaline when immature, straight or slightly curved, sides sometimes pinched, smooth; base often truncate.
Habitat.
On mushroom cultivated field, associated with Strophariarugosoannulata.
Distribution.
China, Guizhou Province, Guiyang City, and Liupanshui City; Guizhou City in Anshun Province.
Material examined.
China • Guizhou, Liupanshui City, Shuicheng District, 24°55'39.936"N, 121°11'30.264"E, on soil surfaces of Strophariarugosoannulata cultivated field, 16-November-2023, E. Tarafder and F.H. Tian (HGUP 24-0004, holotype); ex-type living cultures GUCC TB1120, GUCC TB1121 and GUCC TB1122.
GenBank accession numbers.
GUCC TB1120 (ITS: PP922277; rpb2: PP954944; tef1-α: PP954950); GUCC TB1121 (ITS: PP926290; rpb2: PP954945; tef1-α: PP954951); GUCC TB1122 (ITS: PP922285; rpb2: PP954946; tef1-α: PP954952)
Notes.
Morphologically, our newly described taxon Trichodermaviridistromatis shares common characteristics with T.aerugineum (CBS120541) and T.britannicum, a species previously isolated from dead stems and leaves of Calamagrostisepigejos. However, T.viridistromatis differs from T.aerugineum by having smaller stromata (0.5–2 mm in diameter, compared to ca. 1 mm thick in T.aerugineum) and larger phialides (7–23 × 2.4–4 μm in T.aerugineum) and ascospores (8–12 × 4–6 µm; Table 4) (Chaverri and Samuels 2004). Additionally, it can be distinguished from T.strophariensis by its larger stromata (1–14 mm in diameter, 1–11 mm thick in T.strophariensis) and significantly larger subglobose to elongated ascospores (8.4–16.9 × 5.5–8.1 µm). In comparison, T.britannicum has discoid, convex to turbinate stromata surrounded by light brown radial mycelium and much larger one-celled ascospores (10–16 × 4.5–6.2 μm; Table 4) (Jaklitsch et al. 2014). The phylogenetic positions of the new taxon (Fig. 2) demonstrated that Trichodermaviridistromatis is closely related to T.strophariensis, T.britannicum, and T.aerugineum, with strong statistical support (Fig. 2). However, our isolate differs from T.britannicum with 3% (17/610 nucleotides, with five gaps) in ITS region, 2% (20/1080 nucleotides, no gaps) in rpb2 gene, and 4% (55/1244 nucleotides, twenty-one gaps) in tef1-α gene. Moreover, the difference in our collections with T.aerugineum is more than 2% (10/610 nucleotides, ten gaps) in the ITS region, 7% (79/1080 nucleotides, no gaps) in the rpb2 gene, and 6% (78/1244 nucleotides, eleven gaps) in tef1-α gene (Table 2). Additionally, the differences between our isolate with T.strophariensis are 4% (29/610 nucleotides, four gaps) in ITS region, 4% (46/1080 nucleotides, no gaps) differences in rpb2 gene, and 5% (65/1244 nucleotides, twenty-five gaps) differences in tef1-α gene also supported T.viridistromatis to be a distinct species compared to T.strophariensis and T.britannicum respectively.
Table 4.
Morphological comparison of Trichodermabritannicum, T.aerugineum, T.viridistromatis, T.spinulosum, and T.strophariensis.
| Taxon (holotype) | Ascospores | Conidia | Substratum | References |
|---|---|---|---|---|
| T.britannicum | 10–16 × 4.5–6.2 μm | 4.7–19.3 × 4–6.2 μm | Decaying wood of broadleaf trees | Jaklitsch et al. 2014 |
| T.aerugineum | 8–12 × 4–6 µm | 3–5 × 2–4 µm | Decaying wood | Chaverri and Samuels (2004) |
| T.viridistromatis | 3.4–5.6 × 2.4–3.3 µm | 2.8–4 × 1.7–3.2 μm | mushroom species (Stropharia) | This study |
| T.spinulosum | 5–7 × 3–4 µm | 3.5–4.7 × 3–3.7 µm | On stems of Chelidoniummajus | Jaklitsch and Voglmayr 2015 |
| T.strophariensis | 8.4–16.9 × 5.5–8.1 µm | 8.5–25.5 × 5.7–17.9 μm | mushroom species (Stropharia) | This study |
Discussion
Green mold is a prevalent disease in mushroom cultivation that disrupts the average growth of mushroom fruiting bodies or mycelium and inhibits the average growth of mushrooms (Zeng et al. 2022). The discovery of two new Trichoderma species causing green mold disease significantly advances our understanding of fungal pathogens in mushroom cultivation. This finding underscores the urgent need for effective disease management strategies in agriculture. The pathogenicity of HGUP 24-0001 and HGUP 24-0004 on Strophariarugosoannulata was confirmed in controlled field tests, where both strains caused symptoms consistent with green mold disease. The rapid development of green mycelia covering the mushroom trays fulfilled Koch’s postulates. In this study, the rapid colonization of mushroom trays by green mycelia is a clear indicator of the aggressive interaction between the pathogens and the host, a situation of intense concern, leading to significant damage to the mushroom fruiting bodies. The occurrence of mold diseases affecting S.rugosoannulata, highlights the significant economic losses due to fungal infections in mushroom cultivation (Huang et al. 2023). The infection rate of both isolates in the mushroom trays mirrors their incidence in the field, indicating a potentially significant agricultural impact. A detailed observation of symptoms, from initial mycelial growth to full substrate colonization, provides a comprehensive timeline of disease progression and is crucial for effective disease management in mushroom cultivation (Zhang et al. 2022).
Morphological analysis of the newly identified Trichoderma species revealed distinct characteristics. The typical symptoms of green mold disease were greenish mycelial growth and rotting of the fruiting bodies of the mushrooms. Moreover, molecular phylogenetic analyses of the nuclear ribosomal internal transcribed spacer (nrITS) region, the second largest subunit of RNA polymerase II (rpb2), the partial translation elongation factor 1-alpha (tef1-α) provided conclusive evidence for the delineation of the two new Trichoderma species, and these isolates were separated from previously identified and described species of T.britannicum, T.aerugineum, T.danicum, and T.spinulosum and properly placed within the distinct clades (Fig. 2). This molecular approach not only confirmed the novelty of the species but also highlighted the genetic diversity within the genus Trichoderma. This study successfully identified and described two new species of T.strophariensis and T.viridistromatis as the causal agents of green mold disease in Strophariarugosoannulata in Guizhou Province, China.
The discovery of these new pathogens emphasizes the need for continuous monitoring and research on fungal diseases affecting economically important mushrooms. Integrating morphological and molecular identification techniques provides a robust framework for identifying and characterizing new fungal pathogens, ultimately improving disease management practices. Future studies should continue to explore the agricultural and biotechnological potential of these and other Trichoderma species, contributing to a deeper understanding and sustainable management of fungal pathogens in agriculture. This new pathogen can infect the mycelia of S.rugosoannulata at an early stage and the entire fruit body at maturity, making it a challenging competitor in the field. However, our significant findings also reveal an unexpected diversity of Trichoderma in China, highlighting the need for further research and inspiring future investigations.
Supplementary Material
Acknowledgements
Samantha C. Karunarathna thanks the “Yunnan Revitalization Talents Support Plan” (High-End Foreign Experts Program), the National Natural Science Foundation of China (NSFC 32260004), and the Key Laboratory of Yunnan Provincial Department of Education of the Deep-Time Evolution on Biodiversity from the Origin of the Pearl River for their support. The authors extend their appreciation to the Researchers Supporting Project (number RSP2024R56), King Saud University, Riyadh, Saudi Arabia.
Citation
Tarafder E, Wenjun Z, Karunarathna SC, Elgorban AM, Huilian M, Nan W, Zeng X, Yong W, Tian F-H (2024) Unveiling two new species of Trichoderma (Hypocreales, Hypocreaceae) that cause green mold disease on Stropharia rugosoannulata from Guizhou Province, China. MycoKeys 110: 361–383. https://doi.org/10.3897/mycokeys.110.134154
Additional information
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
No ethical statement was reported.
Funding
This research was funded by Guizhou Provincial Basic Research Program (Natural Science) ZK[2023] general 087 and the National Natural Science Foundation of China, grant number NSFC 32000013 & 32260044; Guizhou Provincial Support Fund of Science and Technology, grant number Support of QKH [2021] General 199; Guizhou Department, grant number Qianjiaoji [2022] 071; Guizhou Province Edible Fungi Industry Technology System (GZMARS-SYJ-2024-2026); Researchers Supporting Project (number RSP2024R56), King Saud University, Riyadh, Saudi Arabia.
Author contributions
Conceptualization, ET., FT; Data curation, ET., ZW., MH, FT.; Formal analysis, ET., FT., XZ., SCK.; Investigation, ET., ZW., FT.; Methodology, ET., ZW., SCK., WN., YW.; Project administration and resources, FT.; Software, ET., SCK., AME, MH, XZ.; Supervision, FT.; Writing original draft, ET.; Writing review and editing, ET., SCK., AME., WN., YW., XZ.; Funding acquisition, FT., XZ.; The first draft of the manuscript was written by ET and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Author ORCIDs
Entaj Tarafder https://orcid.org/0000-0002-3680-3433
Zhang Wenjun https://orcid.org/0009-0005-8310-0537
Samantha C. Karunarathna https://orcid.org/0000-0001-7080-0781
Abdallah M. Elgorban https://orcid.org/0000-0003-3664-7853
Man Huilian https://orcid.org/0009-0006-7894-1873
Wu Nan https://orcid.org/0009-0006-1192-4171
Xiangyu Zeng https://orcid.org/0000-0003-1341-1004
Wang Yong https://orcid.org/0000-0003-3831-2117
Feng-Hua Tian https://orcid.org/0000-0002-6962-9531
Data availability
Sequence data generated for the present study have been deposited in GenBank with the accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, GUCC TB1117 (ITS: PP920011; rpb2: PP954941; tef1-α: PP954947); GUCC TB1118 (ITS: PP920012; rpb2: PP954942; tef1-α: PP954948); GUCC TB1119 (ITS: PP920013; rpb2: PP954943; tef1-α: PP954949); GUCC TB1120 (ITS: PP922277; rpb2: PP954944; tef1-α: PP954950); GUCC TB1121 (ITS: PP926290; rpb2: PP954945; tef1-α: PP954951); GUCC TB1122 (ITS: PP922285; rpb2: PP954946; tef1-α: PP954952). All of the data that support the findings of this study are available in the main text.
References
- Abo-Elyousr KA, Abdel-Hafez SI, Abdel-Rahim IR. (2014) Isolation of Trichoderma and evaluation of their antagonistic potential against Alternariaporri. Journal of Phytopathology 162(9): 567–574. 10.1111/jph.12228 [DOI]
- Bustamante DE, Calderon MS, Leiva S, Mendoza JE, Arce M, Oliva M. (2021) Three new species of Trichoderma in the Harzianum and Longibrachiatum lineages from Peruvian cacao crop soils based on an integrative approach. Mycologia 113(5): 1056–1072. 10.1080/00275514.2021.1917243 [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91(3): 553–556. 10.1080/00275514.1999.12061051 [DOI] [Google Scholar]
- Chaverri P, Samuels GJ. (2004) Hypocrea/Trichoderma (Ascomycota, Hypocreales, Hypocreaceae): Species with green ascospores. Studies in Mycology 48: 1–116. [Google Scholar]
- Chen K, Zhuang WY. (2017) Discovery from a large-scaled survey of Trichoderma in soil of China. Scientific Reports 7(1): 9090. 10.1038/s41598-017-07807-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenkolb T, Dhren HV, Nielsen KF, Samuels GJ, Brückner H. (2008) Recent advances and future prospects in peptaibiotics, hydrophobin, and mycotoxin research, and their importance for chemotaxonomy of Trichoderma and Hypocrea. Chemistry & Biodiversity 5(5): 671–680. 10.1002/cbdv.200890064 [DOI] [PubMed]
- Ezzi MI, Lynch JM. (2005) Biodegradation of cyanide by Trichoderma spp. and Fusarium spp. Enzyme and Microbial Technology 36(7): 849–854. 10.1016/j.enzmictec.2004.03.030 [DOI] [Google Scholar]
- Fu S, Sun JE, Tarafder E, Wijayawardene NN, Hu Y, Wang Y, Li Y. (2024) Pezizomycotina species associated with rotten plant materials in Guizhou Province, China. MycoKeys 106: 265–285. 10.3897/mycokeys.106.125920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer CJ. (1991) Markov chain Monte Carlo maximum likelihood. In: Keramidas EM. (Ed.) Computing Science and Statistics: Proceedings of the 23rd Symposium on the Interface.Interface Foundation, Fairfax Station, 156–163.
- Gu M, Chen Q, Zhang Y, Zhao Y, Wang L, Wu X, Zhao M, Gao W. (2024) Evaluation of Genetic Diversity and Agronomic Traits of Germplasm Resources of Strophariarugosoannulata. Horticulturae 10(3): 213. 10.3390/horticulturae10030213 [DOI]
- Hall TA. (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. 10.1021/bk-1999-0734.ch008 [DOI] [Google Scholar]
- Harman GE, Lorito M, Lynch JM. (2004) Uses of Trichoderma spp. to alleviate or remediate soil and water pollution. Advances in Applied Microbiology 56: 313–330. 10.1016/S0065-2164(04)56010-0 [DOI] [PubMed] [Google Scholar]
- Hasan MM, Rahman S, Kim GH, Abdallah E, Oh DH. (2012) Antagonistic potentiality of Trichodermaharzianum towards seed-borne fungal pathogens of winter wheat cv. Protiva in vitro and in vivo. Journal of Microbiology and Biotechnology 22(5): 585–591. 10.4014/jmb.1107.07063 [DOI] [PubMed] [Google Scholar]
- Huang L, He C, Si C, Shi H, Duan J. (2023) Nutritional, Bioactive, and Flavor Components of Giant Stropharia (Strophariarugoso-annulata): A review. Journal of Fungi 9(8): 792. 10.3390/jof9080792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM. (2009) European species of Hypocrea Part I. The green-spored species. Studies in Mycology 63: 1–91. 10.3114/sim.2009.63.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. (2015) Biodiversity of Trichoderma (Hypocreaceae) in southern Europe and Macaronesia. Studies in Mycology 80(1): 1–87. 10.1016/j.simyco.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. (2005) Hypocreavoglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia 97(6): 1365–1378. 10.1080/15572536.2006.11832743 [DOI] [PubMed]
- Jaklitsch WM, Lechat C, Voglmayr H. (2014) The rise and fall of Sarawakus (Hypocreaceae, Ascomycota). Mycologia 106(1): 133–144. 10.3852/13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambhulkar PP, Singh B, Raja M, Ismaiel A, Lakshman DK, Tomar M, Sharma P. (2024) Genetic diversity and antagonistic properties of Trichoderma strains from the crop rhizospheres in southern Rajasthan, India. Scientific Reports 14(1): 8610. 10.1038/s41598-024-58302-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun H, Kieselbach T, Jönsson LJ. (2011) Enzyme production by filamentous fungi: Analysis of the secretome of Trichodermareesei grown on unconventional carbon source. Microbial Cell Factories 10(1): 1–10. 10.1186/1475-2859-10-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama A, Matsumura F. (1993) Degradation of organochlorine pesticides, particularly endosulfan, by Trichodermaharzianum. Environmental Toxicology and Chemistry 12(6): 1059–1065. 10.1002/etc.5620120612 [DOI]
- Katoh K, Rozewicki J, Yamada KD. (2019) MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20(4): 1160–1166. 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7): 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QR, Tan P, Jiang YL, Hyde KD, Mckenzie EH, Bahkali AH, Wang Y. (2013) A novel Trichoderma species isolated from soil in Guizhou, T.guizhouense. Mycological Progress 12(2): 167–172. 10.1007/s11557-012-0821-2 [DOI]
- Liu YJ, Whelen S, Hall BD. (1999) Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 16(12): 1799–1808. 10.1093/oxfordjournals.molbev.a026092 [DOI] [PubMed] [Google Scholar]
- Liu M, Liu J, Wang WM. (2012) Isolation and functional analysis of Thmfs1, the first major facilitator superfamily transporter from the biocontrol fungus Trichodermaharzianum. Biotechnology Letters 34(10): 1857–1862. 10.1007/s10529-012-0972-x [DOI] [PubMed]
- Põldmaa K. (2011) Tropical species of Cladobotryum and Hypomyces producing red pigments. Studies in Mycology 68: 1–34. 10.3114/sim.2011.68.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poveda J, Hermosa R, Monte E, Nicolás C. (2019) Trichodermaharzianum favours the access of arbuscular mycorrhizal fungi to non-host Brassicaceae roots and increases plant productivity. Scientific Reports 9(1): 11650. 10.1038/s41598-019-48269-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A. (2016) FigTree version 1.4.3 [Online]. https://github.com/rambaut/figtree/releases/tag/v1.4.3 [accessed May 2024]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2014) RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9): 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian FH, Li CT, Li Y. (2017) First report of Penicilliumbrevicompactum causing blue mold disease of Grifolafrondosa in China. Plant Disease 101(8): 1549–1549. 10.1094/PDIS-09-16-1301-PDN [DOI] [Google Scholar]
- Wang GZ, Luo Y, Li JL, Kadri P, Bian YB, Zhou Y. (2019) Characteristics of cob-web disease in fruiting bodies of Auriculariacornea and physiological feature and control strategy of the pathogenic fungus Cladobotryumcubitense. Mycosystema 38: 341–348. 10.13346/j.mycosystema.180270 [DOI]
- Wei GY, Mo WI, Wen JT, Chang LF, Chen YS, Yang Q, Wang Y. (2024) A new species and one new record of Sporocadaceae in Guizhou Province, China. Current Research in Environmental & Applied Mycology 14(1): 157–166. [Journal of Fungal Biology] [Google Scholar]
- White TJ, Bruns TD, Lee SB, Taylor JW. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: A guide to methods and applications. Academic Press, New York, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Wijayawardene NN, Hyde KD, Dai DQ, Sánchez-García M, Goto BT, Saxena RK, Erdoğdu M, Selçuk F, Rajeshkumar KC, Aptroot A, Błaszkowski J, Boonyuen N, da Silva GA, de Souza FA, Dong W, Ertz D, Haelewaters D, Jones EBG, Karunarathna SC, Kirk PM, Kukwa M, Kumla J, Leontyev DV, Lumbsch HT, Maharachchikumbura SSN, Marguno F, Martínez-Rodríguez P, Mešić A, Monteiro JS, Oehl F, Pawłowska J, Pem D, Pfliegler WP, Phillips AJL, Pošta A, He MQ, Li JX, Raza M, Sruthi OP, Suetrong S, Suwannarach N, Tedersoo L, Thiyagaraja V, Tibpromma S, Tkalčec Z, Tokarev YS, Wanasinghe DN, Wijesundara DSA, Wimalaseana SDMK, Madrid H, Zhang GQ, Gao Y, Sánchez-Castro I, Tang LZ, Stadler M, Yurkov A, Thines M. (2022) Outline of Fungi and fungus-like taxa – 2021. Mycosphere 13(1): 53–453. 10.5943/mycosphere/13/1/2 [DOI] [Google Scholar]
- Xie J, Lu S, Tarafder E, Pan Y, Peng K, Zeng X, Tian F. (2024) Taxonomy, biological characterization and fungicide sensitivity assays of Hypomycescornea sp. nov. causing cobweb disease on Auriculariacornea. Fungal Biology 128(1): 1616–1625. 10.1016/j.funbio.2023.12.007 [DOI] [PubMed]
- Yan QX, Huang MX, Sun P, Cheng SX, Zhang Q, Dai H. (2020) Steroids, fatty acids and ceramide from the mushroom Strophariarugosoannulata Farlow apud Murrill. Biochemical Systematics and Ecology 88: 103963. 10.1016/j.bse.2019.103963 [DOI]
- Zeng XY, Yuan XX, Peng KQ, Pan YT, Tan TJ, Wu N, Tian FH. (2022) Taxonomy and control of Trichodermahymenopellicola sp. nov. responsible for the first green mold disease on Hymenopellisraphanipes. Frontiers in Microbiology 13: 991987. 10.3389/fmicb.2022.991987 [DOI] [PMC free article] [PubMed]
- Zeng XY, Tan TJ, Tian FH, Wang Y, Wen TC. (2023) OFPT: A one-stop software for fungal phylogeny. Mycosphere 14(1): 1730–1741. 10.5943/mycosphere/14/1/20 [DOI] [Google Scholar]
- Zeng M, Zhao Q, Gentekaki E, Su H. (2024) Pseudoplectaniaglobospora (Sarcosomataceae, Pezizales), a new species from Yunnan, China. Current Research in Environmental & Applied Mycology 14(1): 63–70. [Journal of Fungal Biology] [Google Scholar]
- Zhang QH, Wang W, Li CH, Wen ZQ. (2015) Biological characteristics of Hypomycesaurantius parasitic on Hypsizygusmarmoreus. Mycosystema 34: 350–356. 10.13346/j.mycosystema.140248 [DOI]
- Zhang GZ, Yang HT, Zhang XJ, Zhou FY, Wu XQ, Xie XY, Zhao XY, Zhou HZ. (2022) Five new species of Trichoderma from moist soils in China. MycoKeys 87: 133–157. 10.3897/mycokeys.87.76085 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data generated for the present study have been deposited in GenBank with the accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, GUCC TB1117 (ITS: PP920011; rpb2: PP954941; tef1-α: PP954947); GUCC TB1118 (ITS: PP920012; rpb2: PP954942; tef1-α: PP954948); GUCC TB1119 (ITS: PP920013; rpb2: PP954943; tef1-α: PP954949); GUCC TB1120 (ITS: PP922277; rpb2: PP954944; tef1-α: PP954950); GUCC TB1121 (ITS: PP926290; rpb2: PP954945; tef1-α: PP954951); GUCC TB1122 (ITS: PP922285; rpb2: PP954946; tef1-α: PP954952). All of the data that support the findings of this study are available in the main text.