Abstract
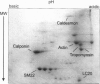
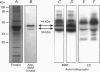
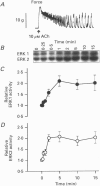
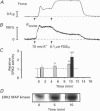
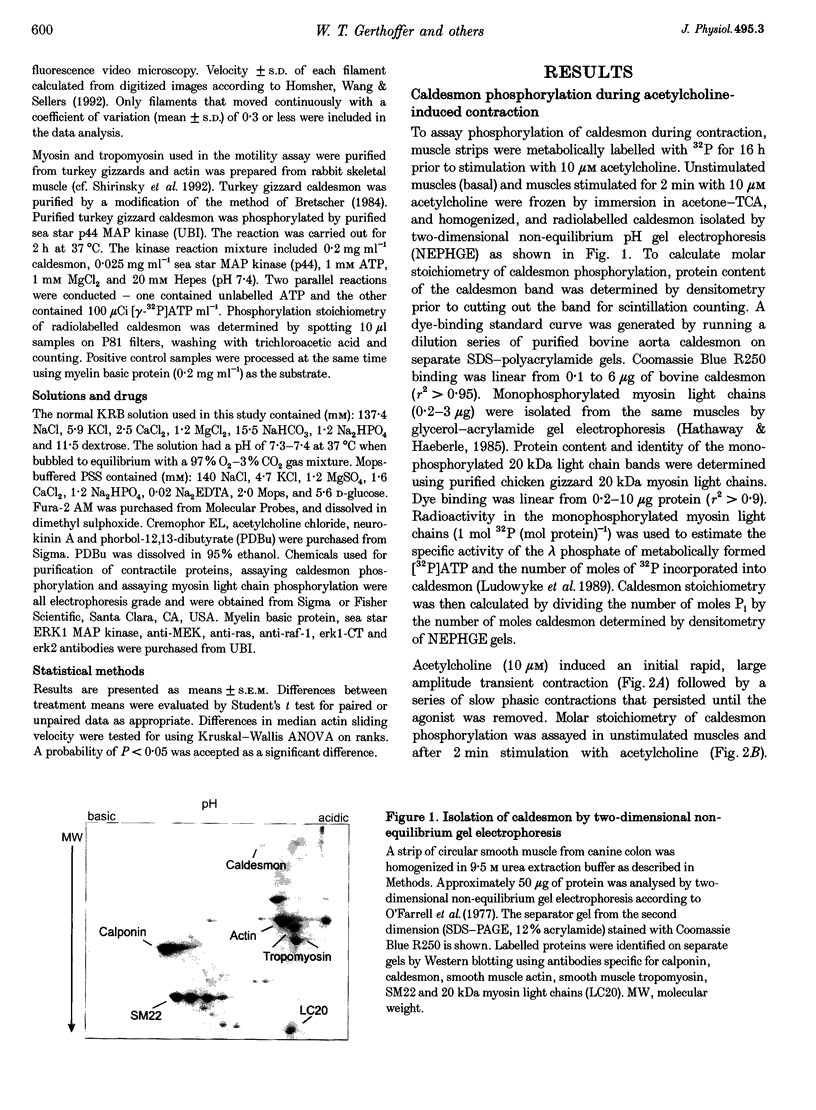
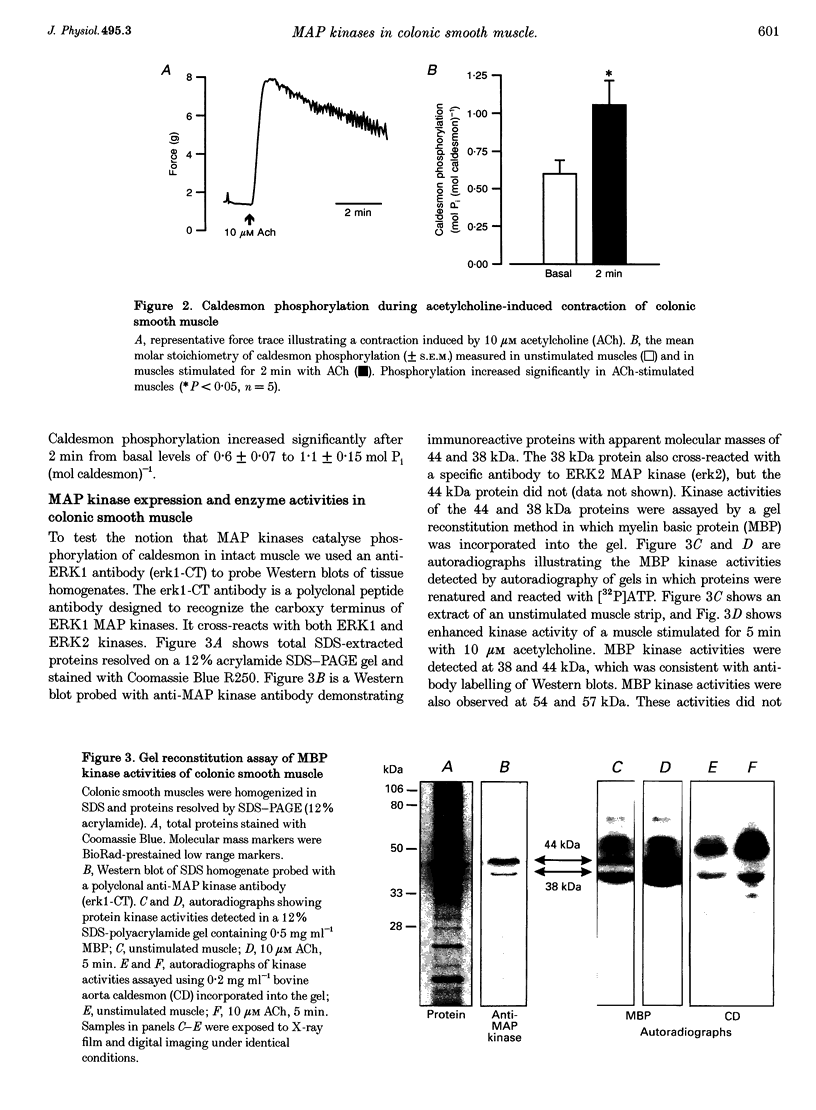
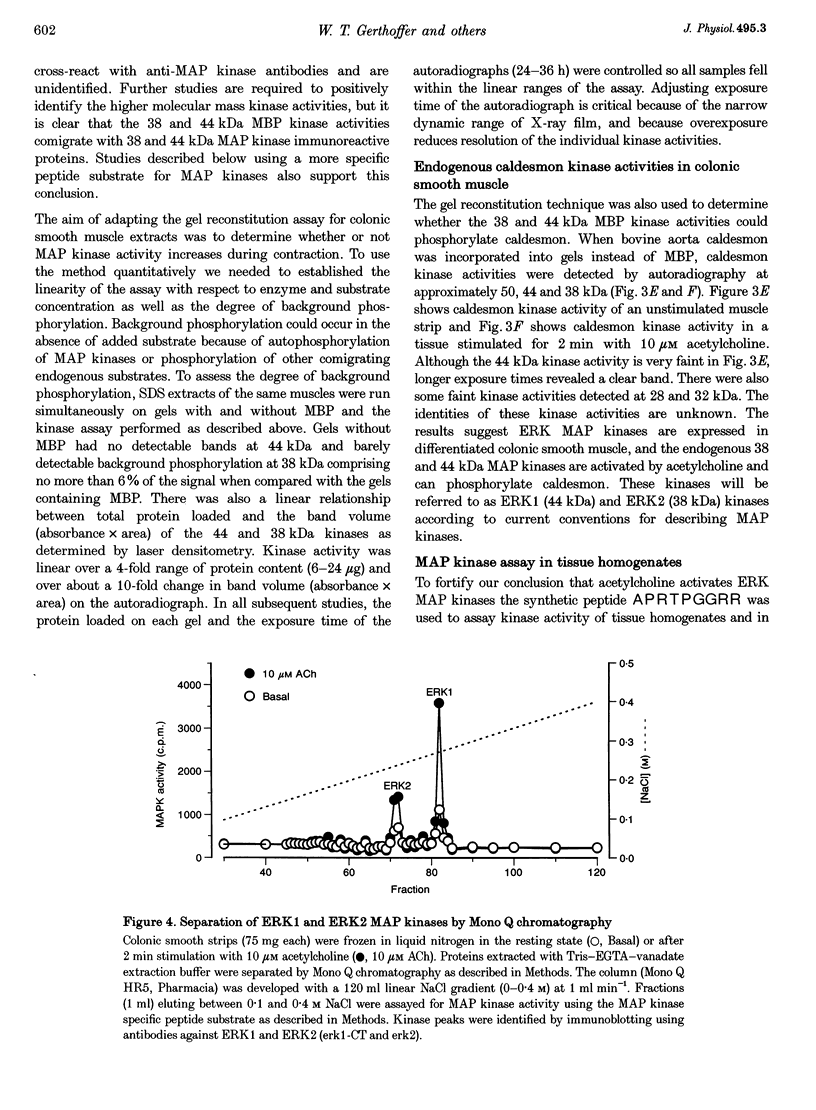
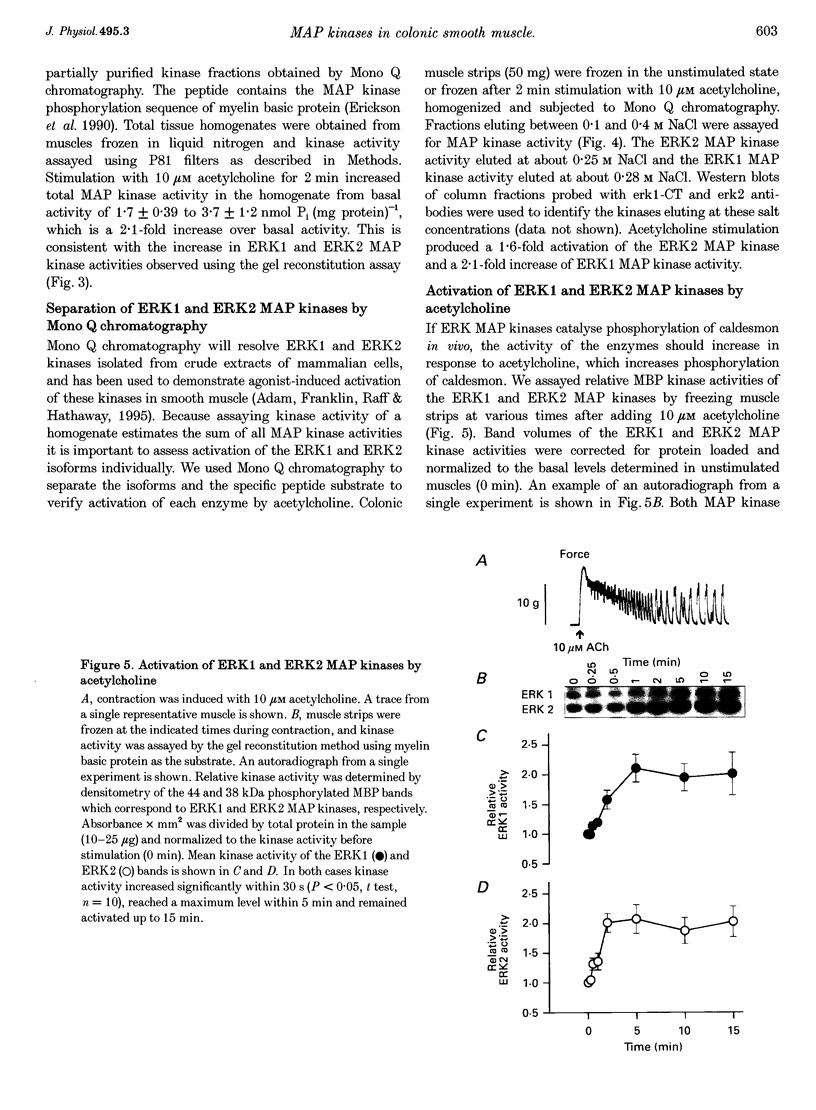
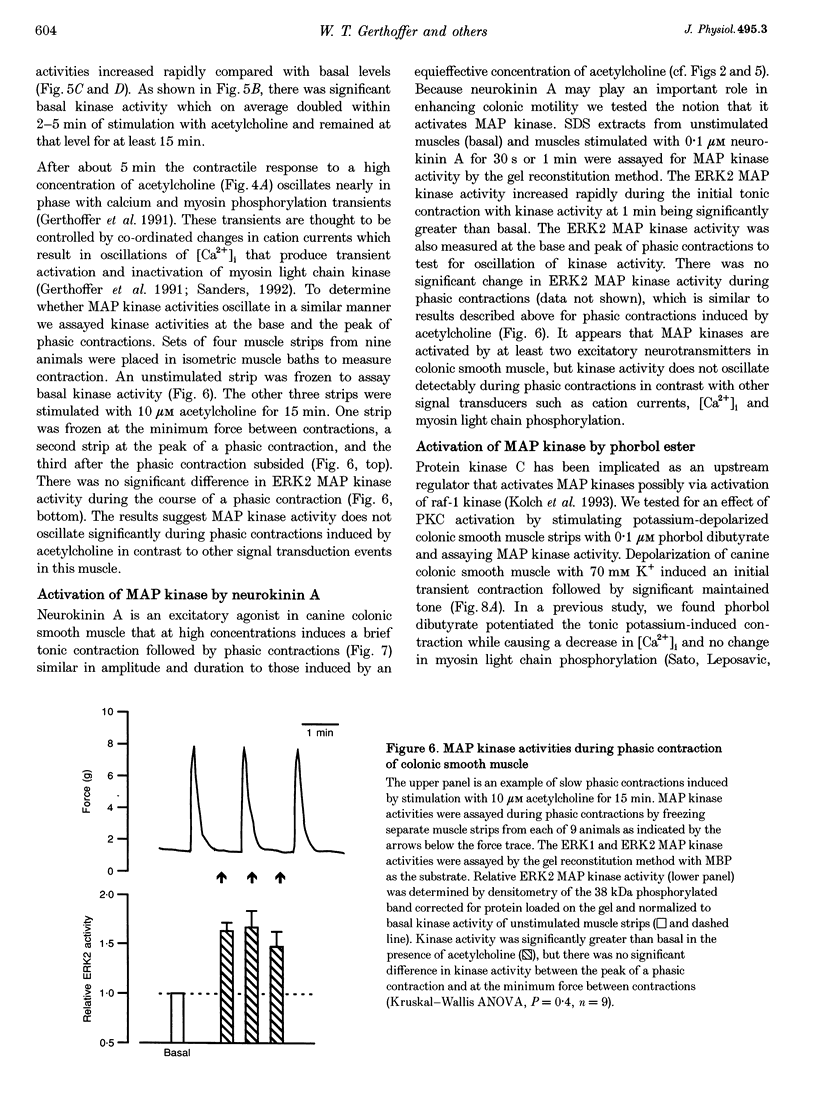
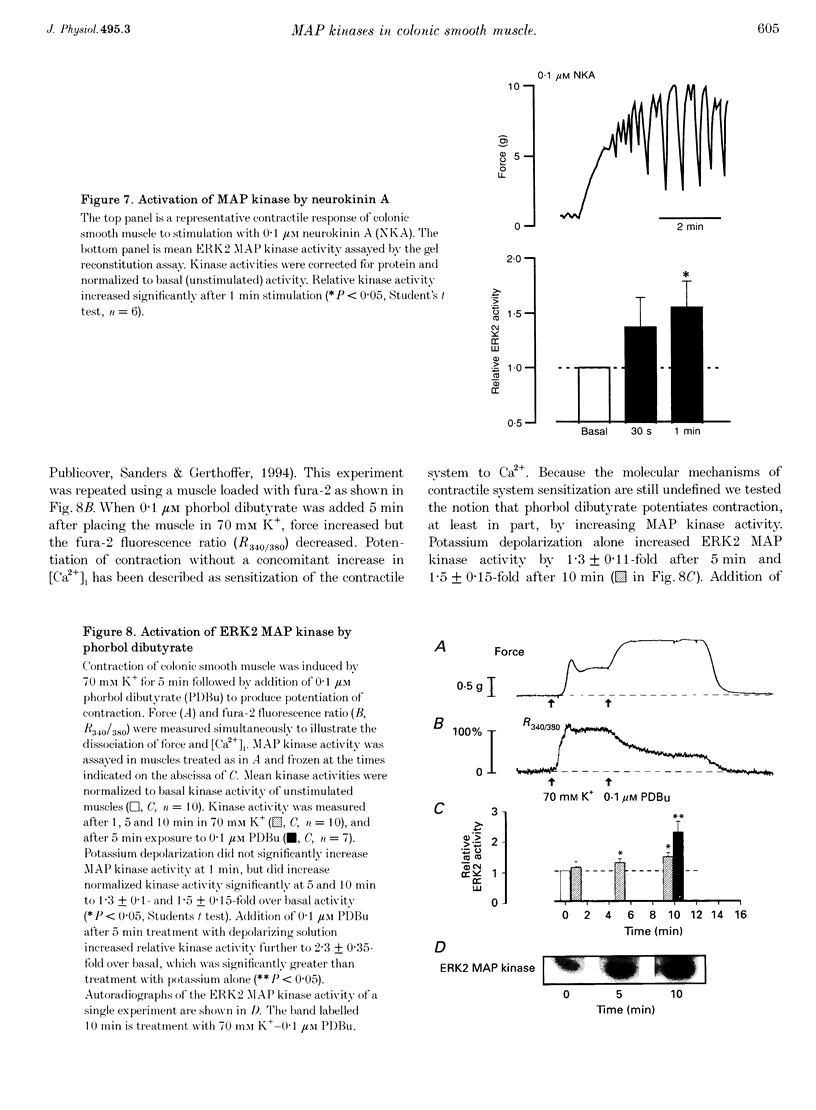
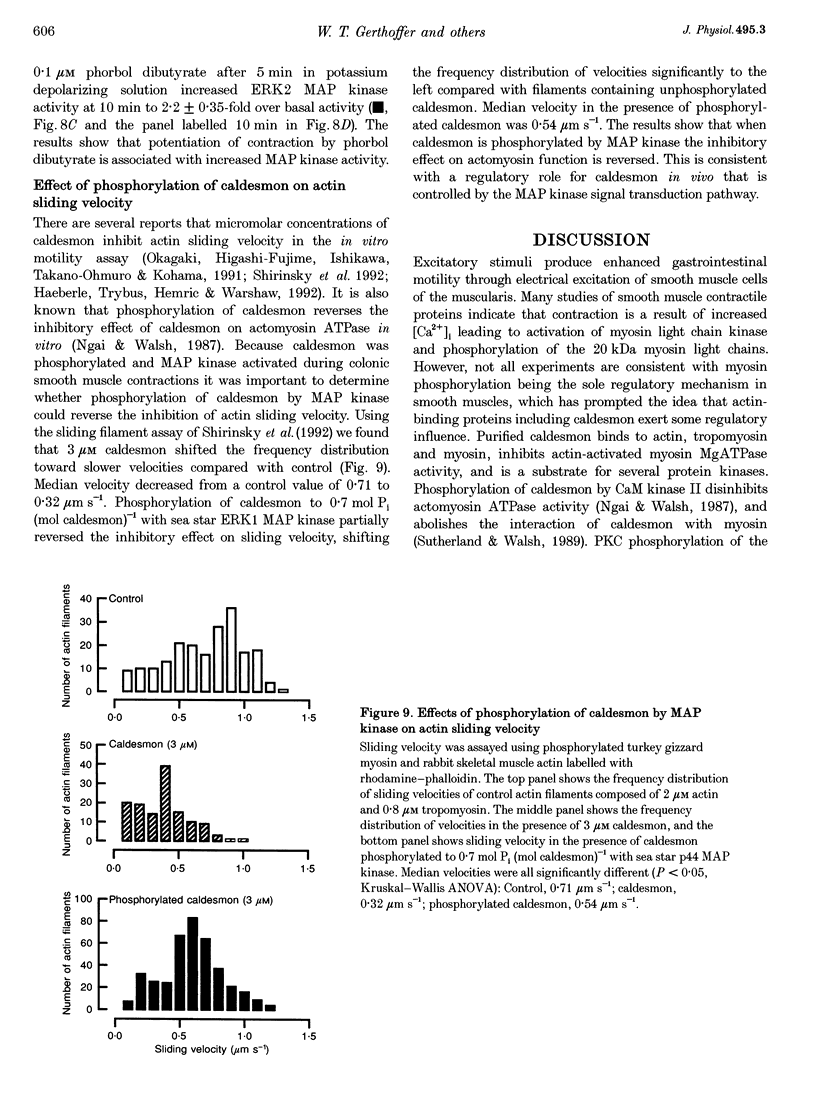
1. Phosphorylation of caldesmon was assayed in canine colonic circular smooth muscle strips labelled with 32P and stimulated with 10 microM acetylcholine. Caldesmon was isolated by two-dimensional non-equilibrium pH gel electrophoresis. Stimulation with acetylcholine increased caldesmon phosphorylation significantly from a basal level of 0.6 +/- 0.07 to 1.1 +/- 0.15 mol P1 (mol caldesmon)-1 after 2 min. 2. MAP kinase activities were measured in SDS extracts of muscle by a gel reconstitution method using myelin basic protein. Myelin basic protein kinase activities were observed at 38, 44, 50 and 57 kDa by the gel reconstitution method. Endogenous caldesmon kinase activities were also identified by the gel reconstitution method at 38, 44 and 50 kDa. The 38 and 44 kDa kinases comigrated with proteins labelled by anti-ERK1 MAP kinase antibodies on Western blots. Both 38 and 44 kDa MBP kinase activities increased significantly during contractions induced by 10 microM acetylcholine, 0.1 microM neurokinin A and 70 mM potassium. 3. Phorbol dibutyrate (0.1 microM) potentiated activation of MAP kinases and contraction of depolarized muscles while producing a decrease in fura-2 fluorescence ratio. This suggests that protein kinase C activation is coupled to MAP kinase activity in colonic smooth muscle. 4. MAP kinases isolated form muscle homogenates by Mono Q chromatography were assayed using the specific MAP kinase substrate peptide APRTPGGRR. Stimulation of muscles for 2 min with 10 microM acetylcholine activated both ERK1 and ERK2 MAP kinase activities 2-fold. 5. To determine the effects of caldesmon phosphorylation by MAP kinase on the cross-bridge cycle, actin sliding velocity was measured with an in vitro motility assay. Unphosphorylated turkey gizzard caldesmon (3 microM) significantly reduced mean sliding velocity. Phosphorylation of caldesmon with sea star ERK1 MAP kinase reversed the inhibitory effect of caldesmon on sliding velocity. The results are consistent with a protein kinase cascade being activated by contractile agonists in gastrointestinal smooth muscle which activates ERK MAP kinases leading to phosphorylation of caldesmon. Phosphorylation of caldesmon in vivo may reverse inhibitory influences of caldesmon on cross-bridge cycling.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam L. P., Franklin M. T., Raff G. J., Hathaway D. R. Activation of mitogen-activated protein kinase in porcine carotid arteries. Circ Res. 1995 Feb;76(2):183–190. doi: 10.1161/01.res.76.2.183. [DOI] [PubMed] [Google Scholar]
- Adam L. P., Gapinski C. J., Hathaway D. R. Phosphorylation sequences in h-caldesmon from phorbol ester-stimulated canine aortas. FEBS Lett. 1992 May 18;302(3):223–226. doi: 10.1016/0014-5793(92)80446-n. [DOI] [PubMed] [Google Scholar]
- Adam L. P., Haeberle J. R., Hathaway D. R. Phosphorylation of caldesmon in arterial smooth muscle. J Biol Chem. 1989 May 5;264(13):7698–7703. [PubMed] [Google Scholar]
- Adam L. P., Hathaway D. R. Identification of mitogen-activated protein kinase phosphorylation sequences in mammalian h-Caldesmon. FEBS Lett. 1993 May 3;322(1):56–60. doi: 10.1016/0014-5793(93)81110-l. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Childs T. J., Watson M. H., Sanghera J. S., Campbell D. L., Pelech S. L., Mak A. S. Phosphorylation of smooth muscle caldesmon by mitogen-activated protein (MAP) kinase and expression of MAP kinase in differentiated smooth muscle cells. J Biol Chem. 1992 Nov 15;267(32):22853–22859. [PubMed] [Google Scholar]
- Clark T., Ngai P. K., Sutherland C., Gröschel-Stewart U., Walsh M. P. Vascular smooth muscle caldesmon. J Biol Chem. 1986 Jun 15;261(17):8028–8035. [PubMed] [Google Scholar]
- Erickson A. K., Payne D. M., Martino P. A., Rossomando A. J., Shabanowitz J., Weber M. J., Hunt D. F., Sturgill T. W. Identification by mass spectrometry of threonine 97 in bovine myelin basic protein as a specific phosphorylation site for mitogen-activated protein kinase. J Biol Chem. 1990 Nov 15;265(32):19728–19735. [PubMed] [Google Scholar]
- Geahlen R. L., Anostario M., Jr, Low P. S., Harrison M. L. Detection of protein kinase activity in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1986 Feb 15;153(1):151–158. doi: 10.1016/0003-2697(86)90074-6. [DOI] [PubMed] [Google Scholar]
- Gerthoffer W. T., Murphey K. A., Mangini J., Boman S., Lattanzio F. A., Jr Myosin phosphorylation and calcium in tonic and phasic contractions of colonic smooth muscle. Am J Physiol. 1991 Jun;260(6 Pt 1):G958–G964. doi: 10.1152/ajpgi.1991.260.6.G958. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Raden D. L., Rigby M. R., Davis R. J. Heterogeneous expression of four MAP kinase isoforms in human tissues. FEBS Lett. 1992 Jun 15;304(2-3):170–178. doi: 10.1016/0014-5793(92)80612-k. [DOI] [PubMed] [Google Scholar]
- Haeberle J. R., Trybus K. M., Hemric M. E., Warshaw D. M. The effects of smooth muscle caldesmon on actin filament motility. J Biol Chem. 1992 Nov 15;267(32):23001–23006. [PubMed] [Google Scholar]
- Hathaway D. R., Haeberle J. R. A radioimmunoblotting method for measuring myosin light chain phosphorylation levels in smooth muscle. Am J Physiol. 1985 Sep;249(3 Pt 1):C345–C351. doi: 10.1152/ajpcell.1985.249.3.C345. [DOI] [PubMed] [Google Scholar]
- Homsher E., Wang F., Sellers J. R. Factors affecting movement of F-actin filaments propelled by skeletal muscle heavy meromyosin. Am J Physiol. 1992 Mar;262(3 Pt 1):C714–C723. doi: 10.1152/ajpcell.1992.262.3.C714. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Reardon S. Binding of caldesmon to smooth muscle myosin. J Biol Chem. 1988 Mar 5;263(7):3055–3058. [PubMed] [Google Scholar]
- Katayama E., Ikebe M. Mode of caldesmon binding to smooth muscle thin filament: possible projection of the amino-terminal of caldesmon from native thin filament. Biophys J. 1995 Jun;68(6):2419–2428. doi: 10.1016/S0006-3495(95)80424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Kochs G., Hummel R., Vahidi H., Mischak H., Finkenzeller G., Marmé D., Rapp U. R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993 Jul 15;364(6434):249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- Lee H. K., Bayguinov O., Sanders K. M. Role of nonselective cation current in muscarinic responses of canine colonic muscle. Am J Physiol. 1993 Dec;265(6 Pt 1):C1463–C1471. doi: 10.1152/ajpcell.1993.265.6.C1463. [DOI] [PubMed] [Google Scholar]
- Ludowyke R. I., Peleg I., Beaven M. A., Adelstein R. S. Antigen-induced secretion of histamine and the phosphorylation of myosin by protein kinase C in rat basophilic leukemia cells. J Biol Chem. 1989 Jul 25;264(21):12492–12501. [PubMed] [Google Scholar]
- Mabuchi K., Lin J. J., Wang C. L. Electron microscopic images suggest both ends of caldesmon interact with actin filaments. J Muscle Res Cell Motil. 1993 Feb;14(1):54–64. doi: 10.1007/BF00132180. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Carpenter M., Smillie L. B., Wang J. H. Phosphorylation of caldesmon by p34cdc2 kinase. Identification of phosphorylation sites. J Biol Chem. 1991 Oct 25;266(30):19971–19975. [PubMed] [Google Scholar]
- Mak A. S., Watson M. H., Litwin C. M., Wang J. H. Phosphorylation of caldesmon by cdc2 kinase. J Biol Chem. 1991 Apr 15;266(11):6678–6681. [PubMed] [Google Scholar]
- Moreland R. S., Pott J. W., Cilea J., Moreland S. Regulation of a smooth muscle contraction: a hypothesis based on skinned fiber studies. Adv Exp Med Biol. 1991;304:61–75. doi: 10.1007/978-1-4684-6003-2_7. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. The effects of phosphorylation of smooth-muscle caldesmon. Biochem J. 1987 Jun 1;244(2):417–425. doi: 10.1042/bj2440417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Okagaki T., Higashi-Fujime S., Ishikawa R., Takano-Ohmuro H., Kohama K. In vitro movement of actin filaments on gizzard smooth muscle myosin: requirement of phosphorylation of myosin light chain and effects of tropomyosin and caldesmon. J Biochem. 1991 Jun;109(6):858–866. doi: 10.1093/oxfordjournals.jbchem.a123471. [DOI] [PubMed] [Google Scholar]
- Park S., Rasmussen H. Carbachol-induced protein phosphorylation changes in bovine tracheal smooth muscle. J Biol Chem. 1986 Nov 25;261(33):15734–15739. [PubMed] [Google Scholar]
- Sanders K. M. Ionic mechanisms of electrical rhythmicity in gastrointestinal smooth muscles. Annu Rev Physiol. 1992;54:439–453. doi: 10.1146/annurev.ph.54.030192.002255. [DOI] [PubMed] [Google Scholar]
- Sato K., Leposavic R., Publicover N. G., Sanders K. M., Gerthoffer W. T. Sensitization of the contractile system of canine colonic smooth muscle by agonists and phorbol ester. J Physiol. 1994 Dec 15;481(Pt 3):677–688. doi: 10.1113/jphysiol.1994.sp020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Woo G. C., Sutherland C., Walsh M. P. Kinase activity associated with caldesmon is Ca2+/calmodulin-dependent kinase II. Biochem J. 1990 Jun 1;268(2):367–370. doi: 10.1042/bj2680367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirinsky V. P., Biryukov K. G., Hettasch J. M., Sellers J. R. Inhibition of the relative movement of actin and myosin by caldesmon and calponin. J Biol Chem. 1992 Aug 5;267(22):15886–15892. [PubMed] [Google Scholar]
- Stoscheck C. M. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- Sutherland C., Walsh M. P. Phosphorylation of caldesmon prevents its interaction with smooth muscle myosin. J Biol Chem. 1989 Jan 5;264(1):578–583. [PubMed] [Google Scholar]
- Tanaka T., Ohta H., Kanda K., Tanaka T., Hidaka H., Sobue K. Phosphorylation of high-Mr caldesmon by protein kinase C modulates the regulatory function of this protein on the interaction between actin and myosin. Eur J Biochem. 1990 Mar 30;188(3):495–500. doi: 10.1111/j.1432-1033.1990.tb15427.x. [DOI] [PubMed] [Google Scholar]
- Wawrzynow A., Collins J. H., Bogatcheva N. V., Vorotnikov A. V., Gusev N. B. Identification of the site phosphorylated by casein kinase II in smooth muscle caldesmon. FEBS Lett. 1991 Sep 9;289(2):213–216. doi: 10.1016/0014-5793(91)81072-g. [DOI] [PubMed] [Google Scholar]
- Zhang L. B., Buxton I. L. Muscarinic receptors in canine colonic circular smooth muscle. II. Signal transduction pathways. Mol Pharmacol. 1991 Dec;40(6):952–959. [PubMed] [Google Scholar]
- Zhang L., Buxton I. L. Protein kinase regulation of muscarinic receptor signalling in colonic smooth muscle. Br J Pharmacol. 1993 Mar;108(3):613–621. doi: 10.1111/j.1476-5381.1993.tb12850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]