SUMMARY
Tendons are connective tissues with limited healing potential which results in permanently impaired function. Although direct mechanical testing of tendon remains the gold standard for functional analyses, this assay is terminal and tracking healing over time requires the use of many animals. An alternative method for quantifying tendon function is gait analysis, which is non-terminal and enables longitudinal tracking of the same animal. To date, commercial systems used to analyze gait are mostly applied to study neurobehavior, and applications for tendon research has been limited. Since these systems typically output many parameters, it is challenging to know which parameter is most relevant for a specific injury model. To address this challenge, we used a well-established rodent locomotion system (CatWalk XT) to measure longitudinal gait parameters in sham and Achilles tendon-injured mice (from PREOP to 56 days post-injury) and identified relevant and reproducible parameters specifically associated with Achilles tendon injury and healing. Micro computed tomography (micro-CT) and mechanical testing also confirmed persistent tendon impairment in the same animals at the terminal timepoint. Collectively, our results provide a useful reference and recommendation of CatWalk gait parameters and the control comparisons that both conserve the use of animals while maintaining high reproducibility standards for Achilles tendon injury studies.
Keywords: Mouse gait, tendon injury, tendon healing, gait biomechanics, Catwalk XT
INTRODUCTION
Tendons are dense, fibrous connective tissues that attach muscles to bones to transduce force and allow bodily movements. Although tendon injuries are extremely common (making up 30-50% of sport-related injuries), tendons heal by disorganized, fibrotic scar tissue that does not restore full function. For the Achilles tendon, patients at 12 months post-treatment still display functional deficits compared to the uninjured limb, regardless of surgical or non-surgical intervention. This poor innate healing is often accompanied by chronic inflammation and pain which significantly decreases quality of life (1, 2).
The mouse has emerged as an important model to investigate basic cell and molecular mechanisms of mammalian tendon healing, as genetic tools are widely available for mouse and the anatomic structure and patterning of tendons closely resemble that of humans (3). The gold standard metric of functional tendon healing remains tensile testing of dissected tendon tissues, however other assessments such as limb gait can also serve as useful indicators for functional restoration. Key advantages of gait function include its non-invasive and non-terminal nature and the ability to repeatedly measure parameters in the same animal over time, thus improving reproducibility since pre-operative baseline measurements can be acquired and fewer animals are needed. Gait function may also be more relevant clinically, as evaluation of tendon healing in human patients frequently relies on general limb functional measures, like self-reported assessments of physical activity, as well as physical performance tests that evaluate jumping, strength and muscular endurance (1), since direct tensile testing of tendons is not possible in humans.
In rodents, gait outcome parameters are typically obtained by collecting and analyzing pawprints using paint (4), running wheels, or custom gait arenas. Commercially available systems such as Digigait, MouseWalker, Treadscan and Catwalk XT (5, 6) are also now widely used, particularly in the field of neurobehavior. Commercial systems are advantageous from a rigor and reproducibility standpoint in that studies can be readily compared across different labs. While the Digigait System has been used to test tendon healing outcomes in both mouse and rat and parameters associated with specific tendon injuries have been reported (1, 7–12), there is little information on the other systems, particularly in the context of tendon healing. One widely used system originally developed for spinal injury is CatWalk XT (Noldus Information Technologies). Unlike Digigait which relies on treadmill running, CatWalk is based on free movement of rodents. Paw placement is captured by LED lights emitted in a glass walkway such that pawprints are illuminated by light refraction where the paw touches the glass. These illuminated footprints are captured by a high-speed digital camera and the information is processed by an algorithm that generates gait parameters for each limb. The Catwalk XT system provides several advantages compared to its counterparts; for instance, animal stress is minimized by allowing the animal to ambulate freely and a red ceiling light is used. Furthermore, the data output better recapitulates natural walking habits without potential artifacts resulting from forced movement and algorithmic calculations.
Although Catwalk XT is one of the most widely used commercial rodent gait systems, there are few studies reporting its use for tendon research. As of December 2023, a PubMed literature search for “Catwalk XT AND tendon” yielded only 8 research articles. The Achilles tendon was the focus of 5 out of 8 articles, and partial transection injury was the most commonly studied (13–16). Other tendons examined in the remaining 3 articles included rotator cuff and flexor digitorum longus (17–19). In general, most of these studies reported a limited number of parameters (in some cases only a single parameter was reported), and how these parameters were selected was unclear. Since there is evidence that presenting one or a few parameters does not provide a reliable, accurate description of animal gait (5), we focused on comprehensively defining functional gait parameters specifically associated with Achilles tendon injury and healing using the CatWalk XT system. Since full transection of the Achilles tendon is one of the most commonly applied injury models in the field and is widely accessible as no special equipment and limited surgical expertise is required (20–25), we applied these injuries to mice and identified all of the Catwalk XT hindlimb paw statistics indicating either transiently or persistently impaired function and identified the most robust controls for Achilles tendon gait analyses using this system. Collectively, these results form the basis of our recommendation to the tendon injury field for Achilles tendon-relevant gait analysis using CatWalk XT.
MATERIALS AND METHODS
Mouse Achilles tendon injury model
20 wild-type C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA), housed in a pathogen-free barrier facility at the Institute of Comparative Medicine at Columbia University Medical Center and aged to 4 months old when musculoskeletal development is largely complete (26). To account for sex and weight differences that are known to affect gait studies (27), equal numbers of male and female mice were distributed to two different groups undergoing different surgeries: sham injury (10 mice) and Achilles tendon transection injury (10 mice). For these mice, we found that while males were significantly heavier than females, the combined weights of sham vs injured mice were not different (appendix 1). Before surgery, animals were injected intra-peritoneally with extended-release buprenorphine for post-operative pain management, according to the manufacturer’s instructions (Ethiqa XR, Fidelis Pharmaceutical’s LLC). Sham injuries were carried out on the right hindlimb by transecting only the skin adjacent to the Achilles tendon which was then closed by one simple continuous suture (6-0 non-absorbable polypropylene). Achilles tendon transection surgeries were carried out on a separate cohort of animals on the right hindlimb, without repair. Following tendon transection, skin was closed as in the sham animals. All animal procedures were carried out in accordance with the Institutional Animal Care and Use Committee guidelines at Columbia University (AC-ABN0552 – approval date: April 2023).
CatWalk XT Gait Analyses
Mouse gait was captured pre- and post-operatively using the CatWalk XT system (Noldus Information Technologies). Briefly, mice were allowed to freely ambulate in a corridor on a glass plate and illuminated paw prints were captured with a high-speed digital camera. Mice were gaited before the surgery (pre-operative, PREOP), and at 3-, 14-, 28-, and 56-days post-injury (3-56 DPI) to capture baseline gait characteristics before tendon injury and during various stages of tendon healing and remodeling. These timepoints were selected based on our prior research in this model characterizing the phases of inflammation, cell recruitment, and matrix deposition (8). The following settings were used for all animals at all timepoints: 99% allowed maximum speed variation, 1 s minimum run duration, 20 s maximum run duration, 3-5 minimum compliant runs to acquire, 12.4 dB camera gain, 0.1 green intensity threshold, 17.7 V ceiling light and 18 V walkway light. After image acquisition, each video was analyzed individually to classify any paw placement that wasn’t automatically identified previously through the system algorithm. After exporting the data, all 3-5 compliant run parameters were averaged. For the purposes of these studies, we only focused our analyses on the right (injured) and left (uninjured) hindlimbs of each mouse. The parameters listed by the Catwalk XT software as “base of support”, “step sequence” and “other statistics” were not analyzed. However, all the parameters listed as “paw statistics” were examined, except for the toe spread values, which were not consistently recorded. Representative images of sham and injured mouse hindlimbs at 3 DPI collected by CatWalk is shown in figure 1. Analysis of average speeds across timepoints for sham and injured mice showed almost no change in average speed (appendix 2).
Figure 1.
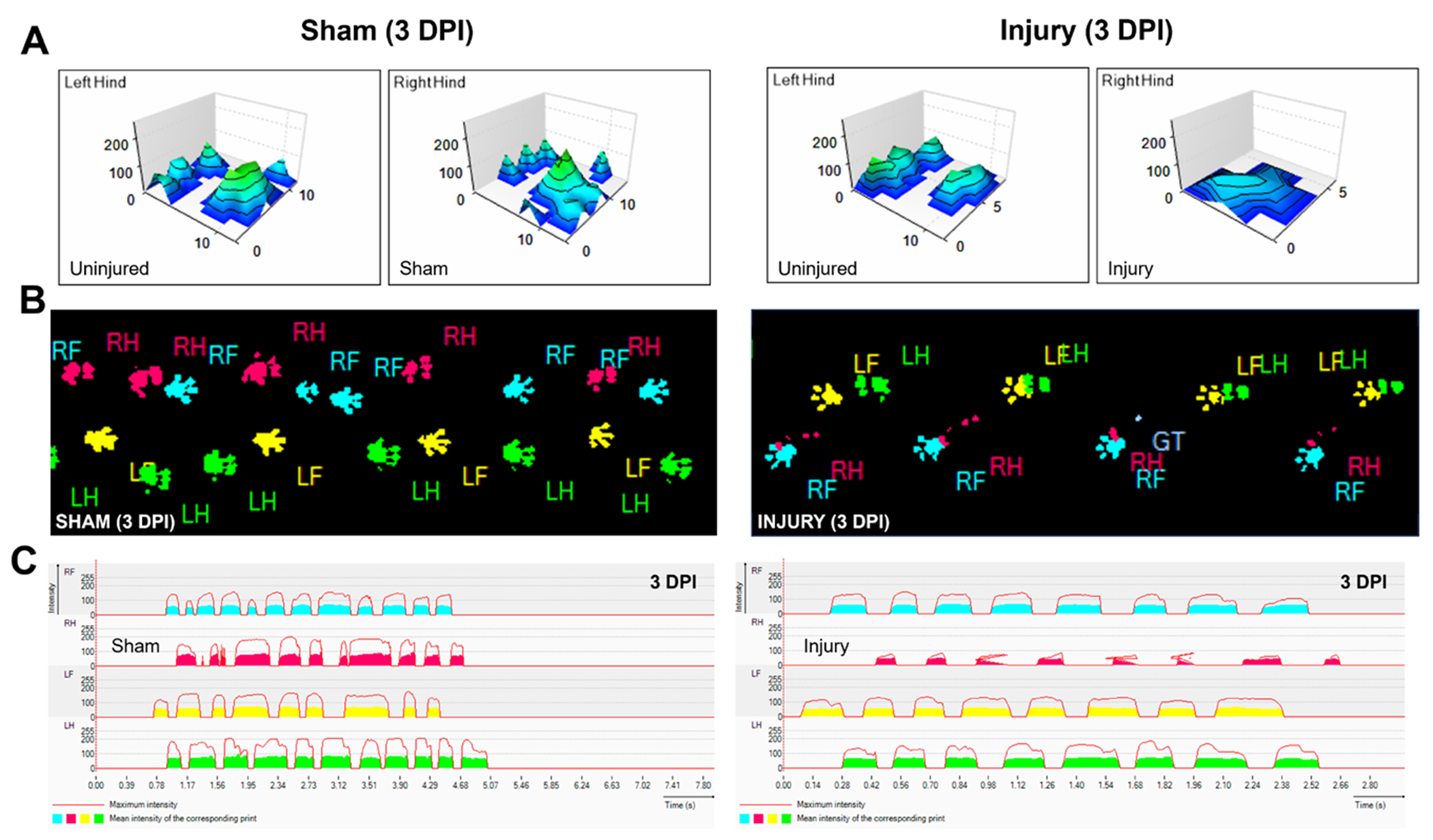
Representative CatWalk XT gait data.
(A) Representative 3-dimensional paw density visualization; (B) Paw print images; (C) Waveforms for Achilles tendon sham and injured hindlimbs.
Micro-CT
At 56 DPI, mice were sacrificed, and the Achilles tendons dissected for microcomputed tomography (micro-CT) imaging, keeping the calcaneus bone and most of the gastrocnemius muscle intact. Specimens were maintained in PBS at 4 °C until ready for testing the same day. Micro-CT scans were acquired as previously described (27), using the Bruker micro-CT instrument at 55 kVp, with an A1 0.25 filter and 6.4 uM resolution with a 0.6° rotation step. The images were processed and reconstructed with the following softwares: SkyScan NRecon, SkyScan Data-Viewer, Micro-CT CT-Analyser (Ctan) to obtain the minimum cross-sectional tendon area.
Tensile testing
After micro-CT imaging, the gastrocnemius muscle was removed to perform tensile testing as previously described, using a custom 3D printed fixture for gripping the calcaneus bone which was mounted onto a ElectroForce 3200 mechanical tester (TA Instruments) (28). Tendons were pre-conditioned for 5 cycles between 0.05 N and 2 N, held for 120 s followed by a ramp to failure at 1% strain/s. Load-deformation curves were generated with Microsoft Excel (version 16.59) to determine the maximum force achieved and stiffness (linear region). Then, stress-strain curves were generated to obtain maximum stress, as well as the Young’s modulus as previously described (28).
Statistics
Gait
For all gait parameters, we completed ROUT outlier tests (Q = 1%) and Shapiro-Wilks normality tests to remove prominent outliers and ensure a normal distribution. Significant differences were detected using ANOVA/mixed effect analyses followed by Sidak’s or Dunnett post-hoc testing with correction to compare groups. For all comparisons (except sham vs injured hindlimb measurements), repeated and paired comparisons were used.
Micro-CT and tensile testing
After performing ROUT outlier tests and Shapiro-Wilks normality tests as described above, one way ANOVA tests with Tukey’s post-hoc comparisons were used to compare uninjured, sham, and injured tendons. Uninjured tendons were collected equally from sham and injured animals. For all analyses, significance was set at p < 0.05. All tests were completed using the GraphPad Prism software (version 9.5.1).
RESULTS
Identification of relevant gait parameters for Achilles Tendon injury
To identify relevant gait parameters, we initially focused on parameters that were consistently changed in a single direction (increased or decreased) for injured hindlimbs over time relative to pre-operative controls (PREOP) (figure 2). Parameters that displayed increased values at one timepoint and decreased values at another timepoint were not assessed further even if differences were statistically significant. Out of the six parameters identified meeting these criteria, three showed only transient changes at 3 DPI (print area, swing, and swing speed) before returning to baseline PREOP values from 14 DPI onward (figure 2). The remaining three parameters (mean intensity, max intensity, and duty cycle) showed consistent and persistent deficits compared to PREOP at almost every timepoint post-injury (figure 2). Analysis of the same parameters in sham-injured animals showed either no change (swing, swing speed, duty cycle) or inconsistent changes (print area, mean intensity, and max intensity) compared to PREOP. The inconsistent changes observed were typically in the opposite direction observed in tendon-injured hindlimbs and were inconsistent across timepoints (figure 3).
Figure 2.
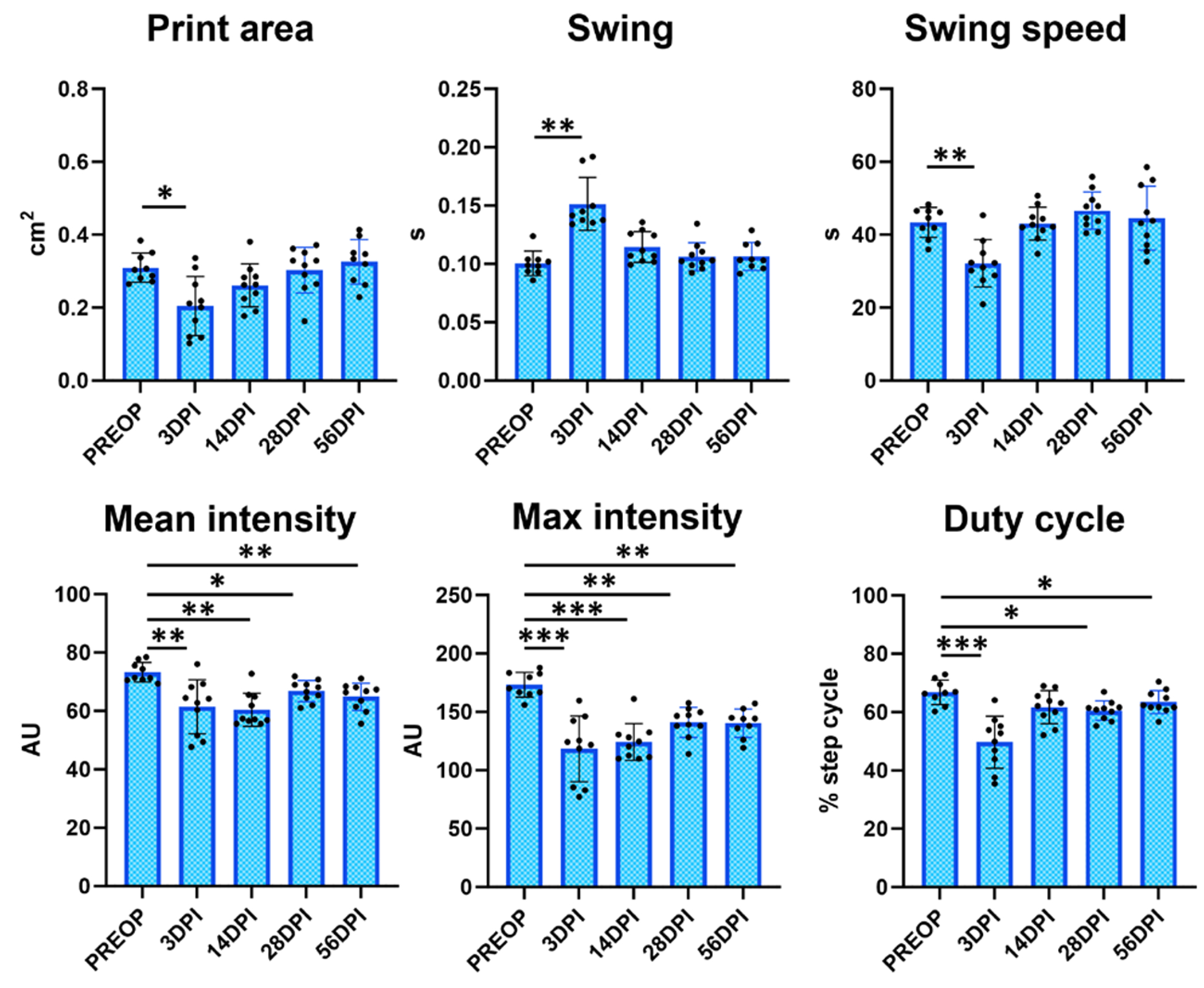
Gait parameters associated with Achilles tendon transection injury compared to pre-operative values. Analysis of the injured hindlimb compared to its own pre-operative values identified several gait parameters that were significantly changed with injury.
n = 9-10 mice; *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 3.
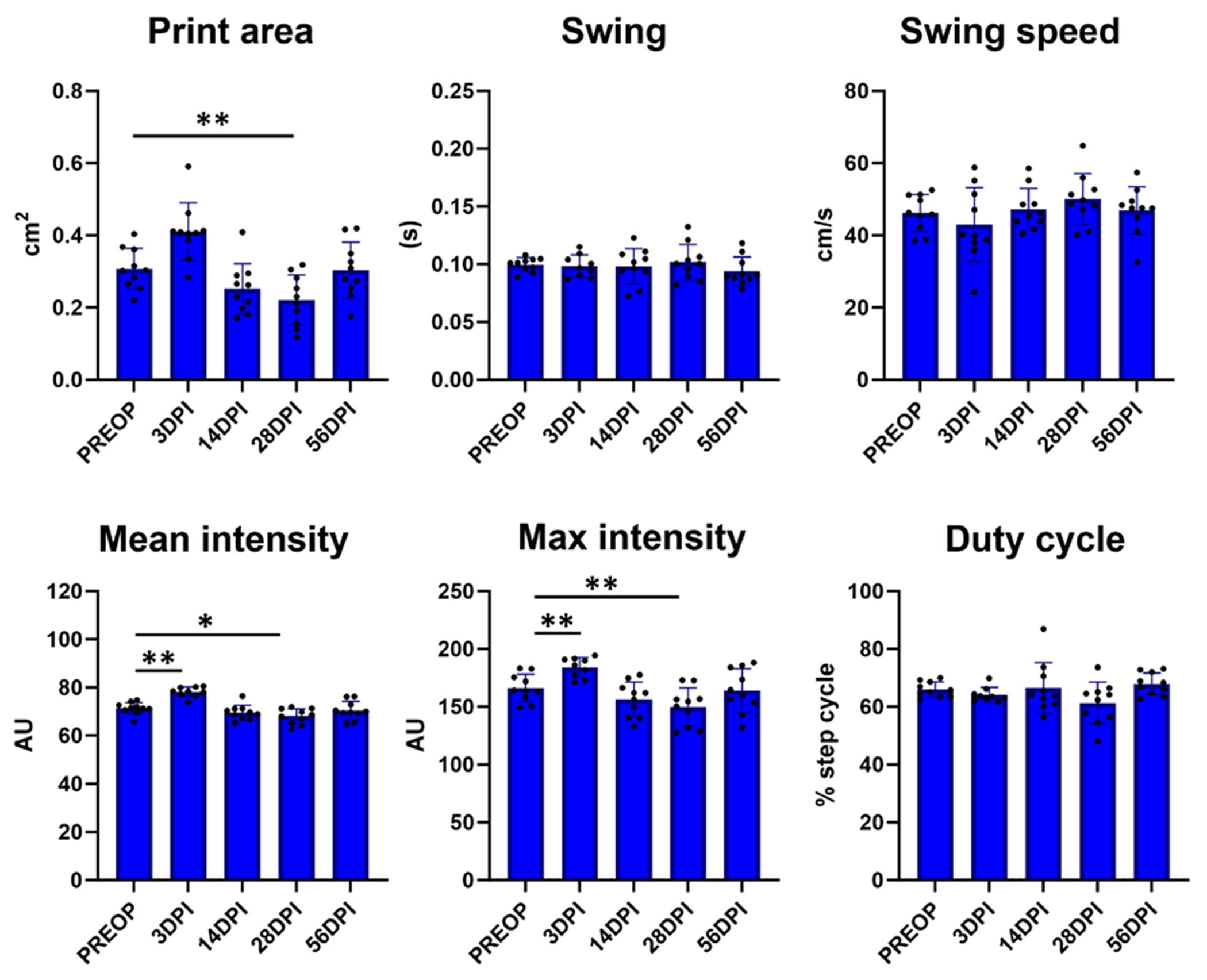
Analysis of injury gait parameters for sham hindlimbs compared to pre-operative values.
Analysis of the gait parameters identified in figure 2 for sham hindlimbs compared to its own pre-operative values showed some significant changes but in variable direction (higher or lower depending on timepoint). n = 9-10 mice; *p < 0.05; **p < 0.01.
We next compared tendon-injured hindlimbs to their contralateral uninjured hindlimbs over time. As expected, no differences were observed between hindlimbs at PREOP for any parameter (figure 4). While some parameters only showed transient changes at early stages (max contact, swing, swing speed, and duty cycle), the parameters mean and max intensity both showed consistently impaired values at all post-injury timepoints (figure 4). Importantly, there were no differences observed for sham-injured hindlimbs and their contralateral uninjured hindlimbs for any of these parameters (figure 5).
Figure 4.
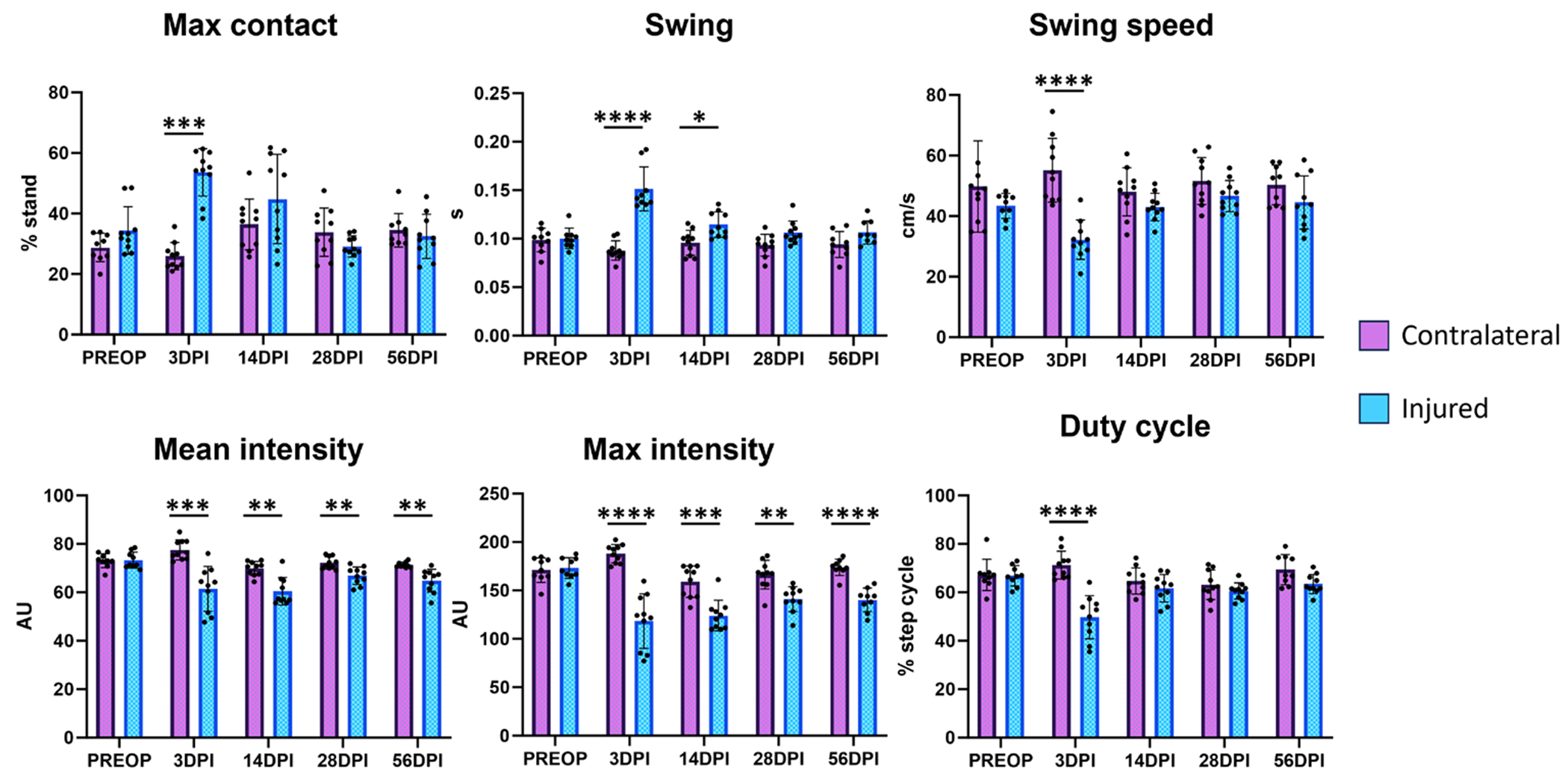
Gait parameters associated with Achilles tendon transection injury compared to contralateral hindlimb values. Analysis of the injured hindlimb compared to its own uninjured contralateral hindlimb identified several gait parameters that were significantly changed with injury.
n = 9-10 mice; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Figure 5.
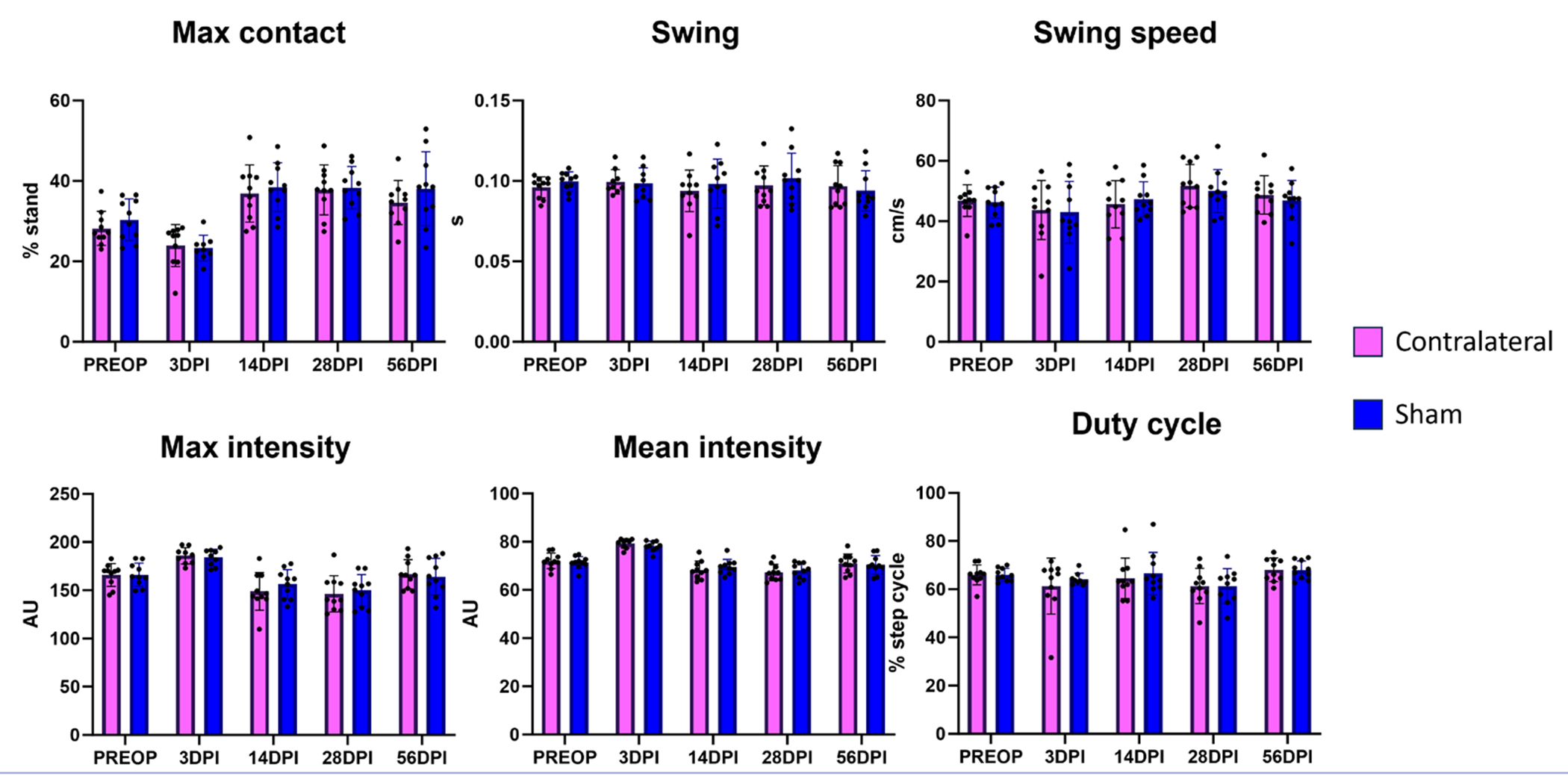
Analysis of injury gait parameters for sham hindlimbs compared to contralateral hindlimb values. Analysis of the gait parameters identified in Figure 4 for sham hindlimbs compared to their own uninjured contralateral hindlimbs showed no significant differences at any timepoint.
n = 9-10 mice.
Finally, we directly compared sham-injured and tendon-injured hindlimbs and again found no differences in PREOP values. We also identified a subset of transiently affected parameters (print area, swing, single stance, duty cycle) and more consistently impaired parameters (mean and max intensity). However, significant differences were not detected at 28 DPI (figure 6). A full list of parameters identified (beyond those represented in the figures) is listed in table I.
Figure 6.
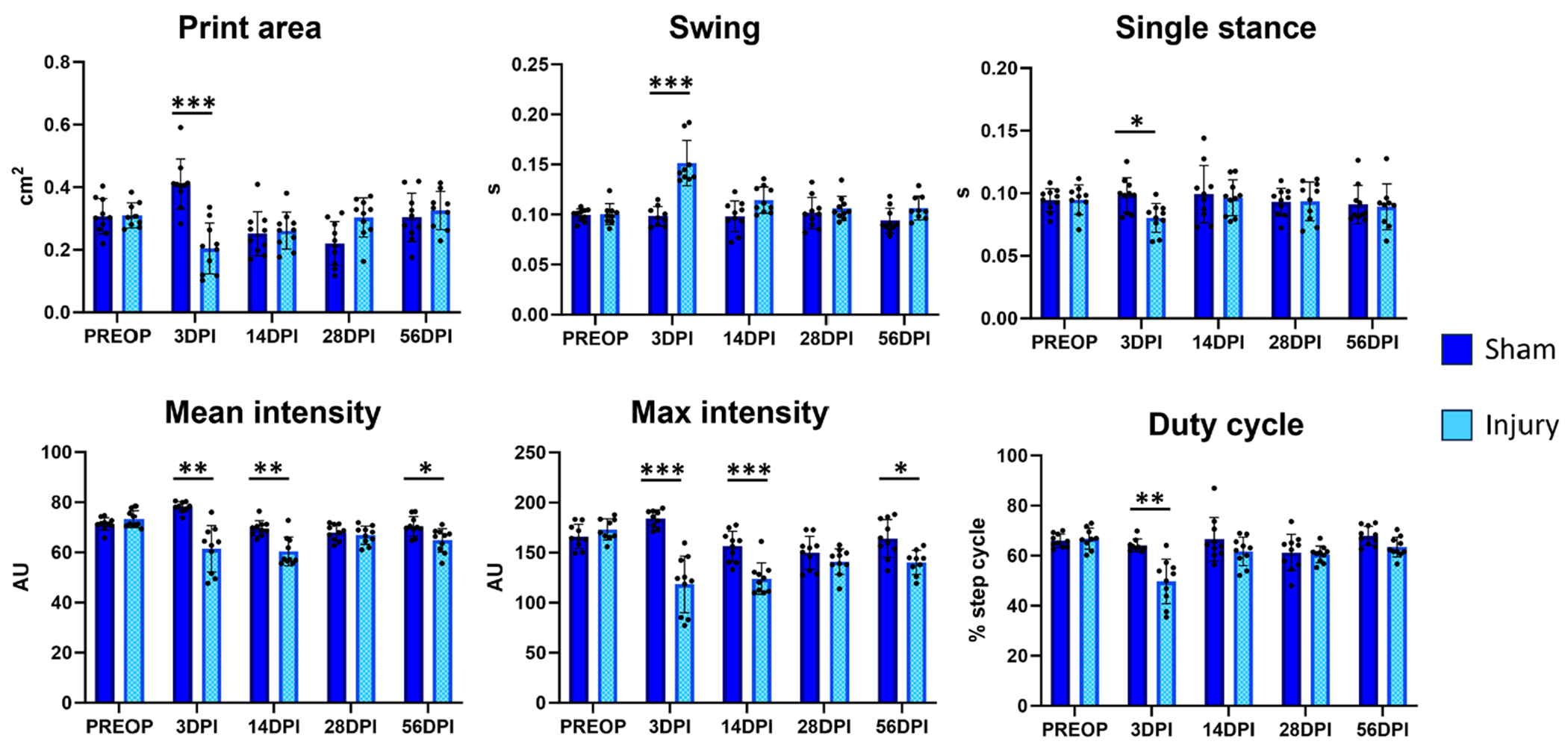
Gait parameters associated with Achilles tendon transection injury compared to sham injured hindlimbs. Analysis of the injured hindlimb compared to sham injuries performed on a separate cohort of mice identified several gait parameters that were significantly changed with injury.
n = 9-10 mice; *p < 0.05; **p < 0.01; ***p < 0.001.
Table I.
Relevant Catwalk XT parameters to observe functional outcomes of tendon healing following complete Achilles transection without repair.
| PREOP RH compared to POST-OP RH | Injured/sham RH compared to contralateral LH | Injured RH compared to sham RH |
|---|---|---|
| Print area | - | Print area |
| Max contact (% stand) | Max contact (% stand) | - |
| Max intensity at max contact | Max intensity at max contact | - |
| Mean intensity at max contact | Mean intensity at max contact | - |
| Max intensity | Max intensity | Max intensity |
| Mean intensity | Mean intensity | - |
| Mean intensity of the 15 most intense pixels | Mean intensity of the 15 most intense pixels | Mean intensity of the 15 most intense pixels |
| Swing | Swing | Swing |
| Swing speed | Swing Speed | - |
| Duty cycle | Duty cycle | Duty cycle |
| - | Max contact area | - |
| - | Min intensity | - |
| - | Single stance | Single stance |
Tendon mechanical properties are not recovered after injury
To confirm that tendon mechanical properties remained impaired at the end of the study duration (56 DPI), we carried out tensile testing and determined structural and material mechanical properties. Micro-CT imaging showed the presence of heterotopic ossification within both Achilles tendon stubs, as we and others have previously reported (figure 7A). Consistent with prior literature (28), stiffness and modulus were also significantly decreased for injured tendons relative to both sham and uninjured tendons (figure 7B,C). Cross-sectional area was also significantly increased in injured tendons, indicative of fibrotic scar formation. No differences in failure properties (max force and max stress) were observed.
Figure 7.
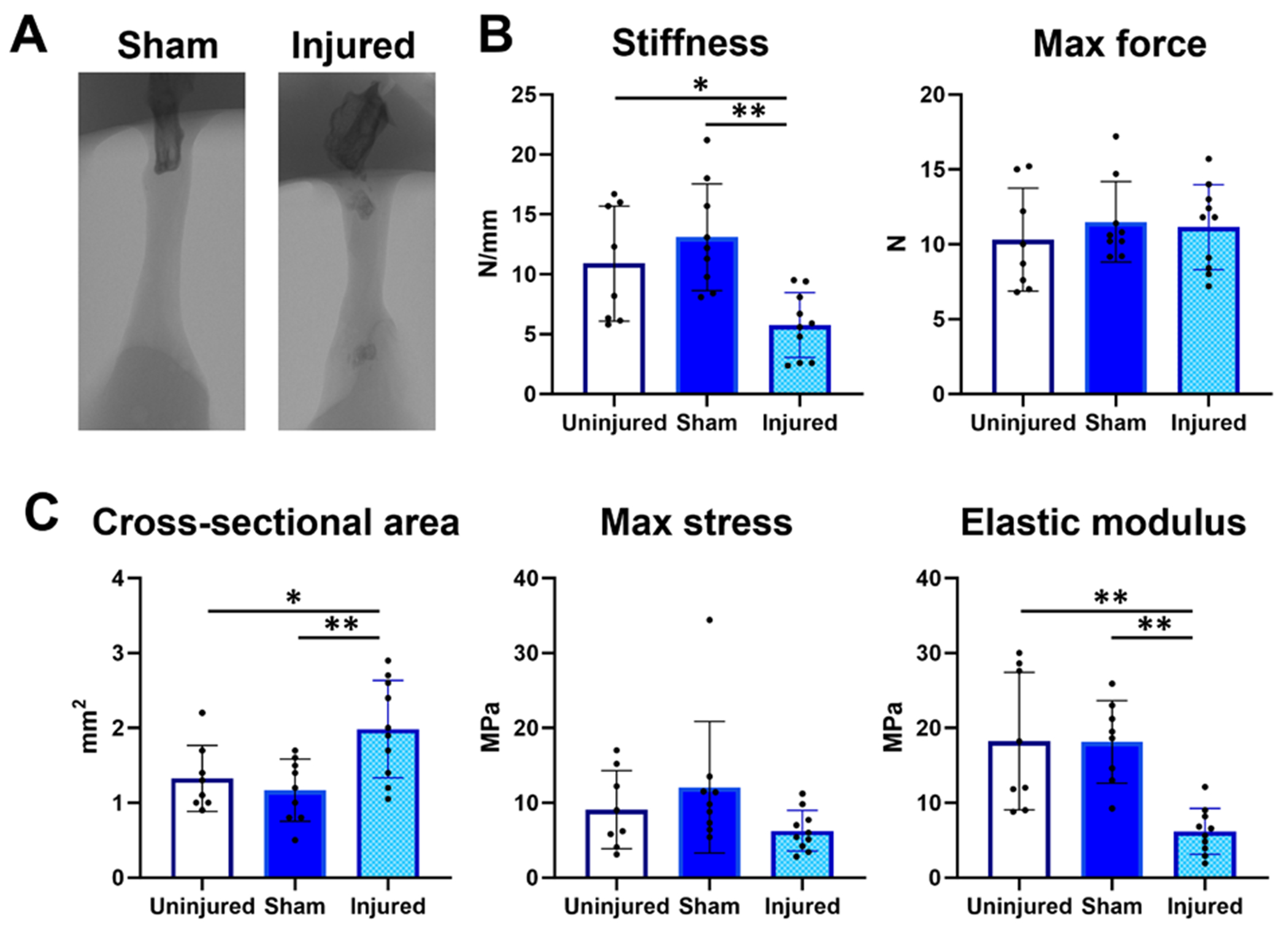
Tensile mechanical properties are impaired at 56 days post-injury.
(A) MicroCT imaging showed the presence of heterotopic ossification in the original Achilles tendon stubs at 56 DPI; (B) Tensile structural properties; (C) material properties show persistent deficits at 56 DPI. n = 9-10 mice; *p < 0.05; **p < 0.01.
DISCUSSION
In this study, we identified useful and reproducible gait parameters associated specifically with Achilles tendon injury and healing using the CatWalk XT system. Overall, performing paired comparisons between the injured and contralateral uninjured hindlimbs yielded the greatest number of detectable and reproducible differences. Importantly, differences in these parameters using the contralateral control limb were highly specific to Achilles tendon injury group and the sham group was largely not affected. Although we found that comparisons to baseline also yielded consistent results in the Achilles tendon injured limb, the sham limbs in this comparison yielded varying and inconsistent results, which can challenge interpretation. Since direct comparisons to sham injury did not produce additional parameters of interest and mechanical properties were also not altered compared to uninjured, we recommend that functional studies focused on Achilles tendon injury and healing can reasonably omit a separate sham control group in the interest of conserving animal use. Comparing different animals also might require more stringent weight and speed normalization to obtain reliable differences (27). Note that our recommendation is limited to the gait and relatively limited mechanical analyses we carried out here, as some biological considerations (related to immune responses for example) might require separate sham rather than uninjured controls. This should be determined in future studies. In general, we found that differences in intensity measurements (such as max and mean intensity, and other related intensity measurements listed in table I), were the most consistently associated with Achilles tendon injury in skeletally mature adult mice. Since intensity measurements depends on animal weight, it is possible that these parameters would not be useful in younger animals below a certain weight threshold, although neurological studies have successfully performed gait analysis in young mice (29, 30). Whether tendon injury-induced gait changes can be detected in young animals must be determined in future studies. In adults, male mice are also typically heavier than female mice (we observed ~5 g difference in C57/Bl6 mice at this age). We intentionally combined male and female mice in our analyses to determine whether statistically significant parameters could still be detected using minimum animals. Although the study was not powered to detect sex differences, when we analyzed the parameters separated by sex (n = 5 per group), we did find that females generally showed greater variation, which could be due to their lower weight and variation in estrus cycle stage, which has been shown to affect mouse physical performance (31). Ongoing studies will increase sample size for both sexes to determine whether any of the parameters may have sex-dependent differences (especially since previous studies showed baseline sex differences in material mechanical properties for the Achilles tendon) (28).
While permanent deficits in tendon function were best represented by the intensity parameters, we also identified several parameters that showed transient differences only at early timepoints (typically 3 DPI). These parameters may be useful in studies where scar-mediated healing is impaired (for example in the presence of genetic mutations or experimental interventions such as prolonged inflammatory challenge). Under those scenarios we could anticipate that deficits in these typically transient parameters would be prolonged into extended timepoints post-injury.
In addition to limitations related to sex, our study also focused solely on hindlimb statistics and did not determine changes in the forelimbs; there may be additional useful parameters associated with compensatory forelimb gait that were missed. We also did not include analyses of “base of support” and “step sequence” which are associated with inter-paw coordination. It is also challenging to separate the effects of pain from the injury vs pure functional considerations. It is possible that some of the changes we identified are more closely associated with painful behaviors, especially in the early stages of healing when inflammation is at its height. Moreover, another limitation of our studies is the lack of habituation to the gait testing facility, and lack of training on the runway prior to our longitudinal gait testing. This creates greater variability within the data since mice are less likely to walk uniformly as they tend to explore their surroundings. To only record uniform walking trajectories (without standing, stopping etc.), lower maximum allowed speed variation settings will be tested in future studies. While gait results were confirmed by mechanical function results, structure was not evaluated despite the importance of structural assessment in tendon healing (32). This will be determined in future studies. Finally, it is important to note that the current study focused on tendon transection injury; other disease conditions such as more subtle tendinopathy models due overuse or fatigue loading (33) will need to be separately validated in terms of gait. Despite these limitations however, these results provide researchers using CatWalk XT a reference to decide which parameters are worth examining to investigate functional Achilles tendon injury outcomes and which controls may be most appropriate.
CONCLUSIONS
In conclusion, these results demonstrate reproducible CatWalk gait parameters that are significantly associated with adult Achilles tendon injury and impaired functional healing.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Mouse NeuroBehavior Core at the Institute for Comparative Medicine at Columbia University Medical Center for assisting with training and usage of the Catwalk XT. We also thank Dr. Iden Kurtaliaj and Dr. Steve Thomopoulos for training and assistance with micro-CT and mechanical testing.
FUNDINGS
This work was supported by NIH/NIAMS funding (R56 AR076984, R01 AR081674) to AH and T32 AR080744 fellowship to EK.
Footnotes
LEVEL OF EVIDENCE: 3
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interests.
DATA AVAILABILITY
Data are available under reasonable request to the corresponding author.
REFERENCES
- 1.Myhrvold SB, Brouwer EF, Andresen TKM, et al. Nonoperative or Surgical Treatment of Acute Achilles’ Tendon Rupture. N Engl J Med. 2022;386(15):1409–20. doi: 10.1056/NEJMoa2108447. [DOI] [PubMed] [Google Scholar]
- 2.Järvinen TAH, Kannus P, Maffulli N, Khan KM. Achilles Tendon Disorders: Etiology and Epidemiology. Foot Ankle Clin. 2005;10(2):255–66. doi: 10.1016/j.fcl.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Watson SS, Riordan TJ, Pryce BA, Schweitzer R. Tendons and muscles of the mouse forelimb during embryonic development. Dev Dyn. 2009;238(3):693–700. doi: 10.1002/dvdy.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herculano AM, Maciel A, Martins ML, et al. Treatment with Conditioned Medium from Tenocytes Primary Cell Cultures Accelerates Histological and Functional Recovery of Achilles Tendon in Tenotomized Mice. Muscles Ligaments Tendons J. 2024;14 (1):5–11. doi: 10.32098/mltj.01.2024.01. [DOI] [Google Scholar]
- 5.Lakes EH, Allen KD. Gait analysis methods for rodent models of arthritic disorders: reviews and recommendations. Osteoarthritis Cartilage. 2016;24(11):1837–49. doi: 10.1016/j.joca.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wertman V, Gromova A, La Spada AR, Cortes CJ. Low-Cost Gait Analysis for Behavioral Phenotyping of Mouse Models of Neuromuscular Disease. J Vis Exp. 2019(149):10.3791/59878. doi: 10.3791/59878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell R, Taub P, Cagle P, Flatow EL, Andarawis-Puri N. Development of a mouse model of supraspinatus tendon insertion site healing. J Orthop Res. 2015;33(1):25–32. doi: 10.1002/jor.22727. [DOI] [PubMed] [Google Scholar]
- 8.Howell K, Chien C, Bell R, et al. Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Sci Rep. 2017;7(1):45238. doi: 10.1038/srep45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Liu X, Davies MR, Horne D, Kim H, Feeley BT. A Mouse Model of Delayed Rotator Cuff Repair Results in Persistent Muscle Atrophy and Fatty Infiltration. Am J Sports Med. 2018;46(12):2981–9. doi: 10.1177/0363546518793403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee C, Liu M, Agha O, Kim HT, Feeley BT, Liu X. Beige FAPs Transplantation Improves Muscle Quality and Shoulder Function After Massive Rotator Cuff Tears. J Orthop Res. 2020;38(5):1159–66. doi: 10.1002/jor.24558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moser HL, Abraham AC, Howell K, et al. Cell lineage tracing and functional assessment of supraspinatus tendon healing in an acute repair murine model. J Orthop Res. 2021;39(8):1789–99. doi: 10.1002/jor.24769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sikes KJ, Andrie KM, McConnell A, et al. Clinical and Histologic Manifestations of a Novel Rectus Femoris Myotendinous Junction Injury in Rats. Muscles Ligaments Tendons J. 2021;11(4):600–13. doi: 10.32098/mltj.04.2021.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aro AA, Simões GF, Esquisatto MA, et al. Arrabidaea chica extract improves gait recovery and changes collagen content during healing of the Achilles tendon. Injury. 2013;44(7):884–92. doi: 10.1016/j.injury.2012.08.055. [DOI] [PubMed] [Google Scholar]
- 14.Guerra Fda R, Vieira CP, dos Santos de Almeida M, et al. Pulsed LLLT improves tendon healing in rats: a biochemical, organizational, and functional evaluation. Lasers Med Sci. 2014;29(2):805–11. doi: 10.1007/s10103-013-1406-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Wang L, Gao Y, et al. Synergistic Therapy of Celecoxib-Loaded Magnetism-Responsive Hydrogel for Tendon Tissue Injuries. Front Bioeng Biotechnol. 2020;8:592068. doi: 10.3389/fbioe.2020.592068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan J, Liu X, Zhou M, et al. Effect of treadmill training on fibrocartilage complex repair in tendon-bone insertion healing in the postinflammatory stage. Bone Joint Res. 2023;12(5):339–51. doi: 10.1302/2046-3758.125.Bjr-2022-0340.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi T, Ochiai N, Sasaki Y, et al. Efficacy of hyaluronic acid or steroid injections for the treatment of a rat model of rotator cuff injury. J Orthop Res. 2015;33(12):1861–7. doi: 10.1002/jor.22976. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki H, Ochiai N, Kenmoku T, et al. Assessment of pain-related behavior and pro-inflammatory cytokine levels in the rat rotator cuff tear model. J Orthop Res. 2014;32(2):286–90. doi: 10.1002/jor.22486. [DOI] [PubMed] [Google Scholar]
- 19.Fritz T, Schäfer J, Scheuer C, et al. Macrophage-activating lipoprotein (MALP)-2 impairs the healing of partial tendon injuries in mice. Ann Anat. 2022;239:151818. doi: 10.1016/j.aanat.2021.151818. [DOI] [PubMed] [Google Scholar]
- 20.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. 2015;33(6):832–9. doi: 10.1002/jor.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang K, Asai S, Hast MW, et al. Tendon mineralization is progressive and associated with deterioration of tendon biomechanical properties, and requires BMP-Smad signaling in the mouse Achilles tendon injury model. Matrix Biol. 2016;52-54:315–24. doi: 10.1016/j.matbio.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorkin M, Huber AK, Hwang C, et al. Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nat Comm. 2020;11(1):722. doi: 10.1038/s41467-019-14172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izumi S, Oichi T, Shetye SS, et al. Inhibition of glucose use improves structural recovery of injured Achilles tendon in mice. J Orthop Res. 2022;40(6):1409–19. doi: 10.1002/jor.25176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagani CA, Bancroft AC, Tower RJ, et al. Discoidin domain receptor 2 regulates aberrant mesenchymal lineage cell fate and matrix organization. Sci Adv. 2022;8(51):eabq6152. doi: 10.1126/sciadv.abq6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little D, Amadio PC, Awad HA, et al. Preclinical tendon and ligament models: Beyond the 3Rs (replacement, reduction, and refinement) to 5W1H (why, who, what, where, when, how). J Orthop Res. 2023;41(10):2133–62. doi: 10.1002/jor.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maupin KA, Childress P, Brinker A, et al. Skeletal adaptations in young male mice after 4 weeks aboard the International Space Station. NPJ Microgravity. 2019;5:21. doi: 10.1038/s41526-019-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitzer C, Kurpiers B, Eltokhi A. Gait performance of adolescent mice assessed by the CatWalk XT depends on age, strain and sex and correlates with speed and body weight. Sci Rep. 2021;11(1):21372. doi: 10.1038/s41598-021-00625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtaliaj I, Golman M, Abraham AC, Thomopoulos S. Biomechanical Testing of Murine Tendons. J Vis Exp. 2019(152):10.3791/60280. doi: 10.3791/60280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jinnou H, Sawada M, Kawase K, et al. Radial Glial Fibers Promote Neuronal Migration and Functional Recovery after Neonatal Brain Injury. Cell Stem Cell. 2018;22(1):128–37.e9. doi: 10.1016/j.stem.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Tsuboi Y, Ito A, Otsuka T, Murakami H, Sawada M, Sawamoto K. Habilitation Improves Mouse Gait Development Following Neonatal Brain Injury. Prog Rehabil Med. 2022;7:20220061. doi: 10.2490/prm.20220061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguiar AS Jr., Speck AE, Amaral IM, Canas PM, Cunha RA. The exercise sex gap and the impact of the estrous cycle on exercise performance in mice. Sci Rep. 2018;8(1):10742. doi: 10.1038/s41598-018-29050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliva F, Maffulli N, Gissi C, et al. Combined ascorbic acid and T(3) produce better healing compared to bone marrow mesenchymal stem cells in an Achilles tendon injury rat model: a proof of concept study. J Orthop Surg Res. 2019;14(1):54. doi: 10.1186/s13018-019-1098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handoyo HR, Fitri LE, Hidayat M, Sardjono TW, Soeharto S. Combined extract (Curcuma longa-Glycyrrhiza glabra) alleviates the inflammations of Achilles tendinopathy in Wistar rats. Eur Rev Med Pharmacol Sci. 2023;27(20):9668–79. doi: 10.26355/eurrev_202310_34138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available under reasonable request to the corresponding author.


