Abstract
Objective
To investigate the role of biglycan (BGN) in colon cancer progression.
Methods
The association between BGN mRNA levels and the survival time of patients with colon cancer was analysed by referencing data from the Gene Expression Omnibus and The Cancer Genome Atlas (TCGA). Gene Set Enrichment Analysis (GSEA) was conducted to explore gene sets and pathways associated with BGN. In vitro analyses were undertaken in HCT116 cells to analyse cell proliferation, migration, invasion, cell cycle control and apoptosis. GSEA, Western blot analysis and small interfering RNAs were used to explore the molecular mechanisms of BGN in colon cancer cells.
Results
Analysis of the pairs of tissues from colon cancer patients showed that BGN protein levels were higher in colon cancer tissues compared with adjacent non-tumour tissues. High BGN levels were significantly associated with shorter survival times. BGN knockdown inhibited cell proliferation, migration and invasion; and induced cell cycle arrest in the G0/G1 phase and promoted apoptosis in HCT116 cells. GSEA found that BGN might affect tumour progression via the mitogen-activated protein kinase (MAPK) signalling pathway.
Conclusion
BGN might promote colon cancer progression via the MAPK signalling pathway and could be a potential target for future novel therapeutic strategies.
Keywords: Colon cancer, biglycan, proliferation, invasion, mitogen-activated protein kinase
Introduction
Colon cancer is one of the most common malignant tumours of the digestive tract, which is characterized by extremely high morbidity and mortality rates, making it the fourth leading cause of cancer-related death worldwide. 1 In recent years, the incidence of colon cancer in China has rapidly increased, severely threatening public health. 2 Although the clinical outcomes for colon cancer patients have significantly improved with the development of new treatments, colon cancer remains a lethal disease, with a 5-year survival rate of only 42.7%. 1 Therefore, in-depth research into the pathogenesis of colon cancer is crucial for the development of effective treatment strategies and the improvement of patient survival.
Biglycan (BGN) is a member of the small leucine-rich proteoglycan (SLRP) family of proteoglycans that are present in the extracellular matrix (ECM), which play roles in immune system regulation, bone growth, muscle development and regeneration, and collagen fibril assembly.3–8 Emerging evidence suggests that BGN plays an important role in various aspects of cancer biology, including cancer cell proliferation, cell cycle, chemotherapy resistance and epithelial–mesenchymal transition.9–12 Previous studies have found that BGN is highly expressed in various tumour tissues, including prostate, ovarian, gastric and colon cancer.13–16 Moreover, a previous study found that BGN was strongly expressed in both mouse and human tumour endothelial cells and that downregulation of BGN inhibited their migration. 17 However, the role and underlying mechanisms of BGN in colon cancer remain to be further elucidated.
The present study aimed to evaluate the relationship between BGN mRNA and protein levels and clinical characteristics of patients with colon cancer. In addition, the effect of BGN on cell proliferation, migration, invasion, cell cycle and apoptosis of colon cancer cells was investigated in vitro. Mechanistically, Gene Set Enrichment Analysis (GSEA) results indicated that BGN was closely related to the mitogen-activated protein kinase (MAPK) signalling pathway. The effect of BGN knockdown on p38 and the ERK1/2 signal pathway was investigated to explore the regulatory mechanism of BGN in colon cancer.
Patients and methods
Bioinformatics analysis
The association between BGN mRNA levels and the survival time of patients with colon cancer was analysed by referencing data from the Gene Expression Omnibus (GEO, GSE20916 and GSE39582; http://www.ncbi.nlm.nih.gov/geo) and The Cancer Genome Atlas (TCGA; http://gdc.cancer.gov). GSEA was conducted to explore gene sets and pathways associated with BGN using data obtained from TCGA.
Patients and tissue samples
A total of 47 pairs of tumour tissues and adjacent normal tissue samples were obtained from consecutive colon cancer patients collected between July 2015 and July 2017 from the Department of Molecular Diagnostics, The Tenth Affiliated Hospital, Southern Medical University, Dongguan, Guangdong Province, China. None of the patients had received radiotherapy or chemotherapy prior to surgery. Tumour specimens were collected from the luminal surface, while paired adjacent normal tissues were obtained from the bowel wall, 2-cm away from the tumour margin. The diagnosis of colon cancer was confirmed through pathological examination.
This study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients. Specimens were handled and anonymized according to ethical and legal standards. This study was approved by the Ethics Committee of the Tenth Affiliated Hospital, Southern Medical University on 2 June 2015 (no. KYKT-2015-004X).
Quantitative real-time polymerase chain reaction
Total cellular RNA was extracted from 50 mg tissue or 1 × 106 cells using TRIzol® reagent (Invitrogen™, Carlsbad, CA, USA) according to the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the SYBR® Green PCR kit (TaKaRa, Dalian, China) in QuantStudio™ 3 (Thermo Fisher Scientific, Waltham, MA, USA). The levels of BGN mRNA were normalized to the levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA and calculated using the 2−ΔΔCt method. The primers used in this qRT-PCR were as follows: human BGN forward: 5′-CAGTGGCTTTGAACCTGGAG-3′; human BGN reverse: 5′-GGGAGGTCTTTGGGGATGC-3′; human GAPDH forward: 5′-GCACCGTCAAGGCTGAGAAC-3′; human GAPDH reverse: 5′-TGGTGAAGACGCCAGTGGA-3′. All primers were designed and synthesized by Sangon Biotech (Shanghai, China). The cycling programme involved preliminary denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing and elongation at 60°C for 1 min.
Immunohistochemistry
Tissue specimens from 93 cases of colon cancer were fixed in 10% formalin and embedded in paraffin for BGN immunohistochemistry. After deparaffinization, antigen retrieval was performed by heating the slides in sodium citrate buffer (pH 6.0) in a microwave (approximately 95–100°C) for 20 minutes. Following hydration and blocking, the sections were incubated overnight at 4°C with a rabbit antihuman antibody for BGN (dilution 1:500; Abcam®, Cambridge, MA, USA). Subsequently, the sections were washed three times with 0.01 M phosphate-buffered saline (PBS, pH 7.2) and incubated with a horseradish peroxidase-conjugated goat antirabbit secondary antibody (1:2000 dilution) for 60 min at room temperature. Lastly, the slides were washed with 0.01 M PBS (pH 7.2) three times and stained with 3, 3'-diaminobenzidine (Beyotime, Shanghai, China). Two pathologists (Y.Z. & G.L.) scored the BGN immunoreactivity based on staining intensity. Staining intensity was defined using the following criteria: absent, 0; slightly yellow, 1; yellow-brown, 2; and brown, 3. The percentage of staining was scored as follows: 0 (0–4%), 1 (5–25%), 2 (26–50%) and 3 (51–100%). The immunoreactivity scores (IRS) of each specimen were the sum of the intensity and percentage scores. Samples were divided into low (IRS ≤ 1) and high (IRS > 1) groups based on BGN immunohistochemistry staining levels.
Cell culture and transfection
The human colon cancer cell line HCT116 was obtained from American Type Culture Collection (RRID: CVCL_0291) and cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco) at 37°C in an atmosphere containing 5% CO2. For BGN downregulation, small interfering RNAs (siRNAs) targeting the BGN coding sequence were synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China) and transfected into HCT116 cells using Lipofectamine 3000 (Invitrogen™). The siRNA sequences were as follows: siBGN-1: F5ʹ-UCUGAAGUCUGUGCCCAAATT-3ʹ, R5ʹ-UUUGGGCACAGACUUCAGATT-3ʹ; siBGN-3: F5ʹ-GCCAUUCAUGAUGAACGAUTT-3ʹ, R5ʹ-AUCGUUCAUCAUGAAUGGCTT-3ʹ.
Western blot analysis
A total of 1 × 106 colon cancer cells were lysed in cold RIPA Lysis and Extraction buffer containing protease inhibitors (Thermo Fisher Scientific). Protein concentrations were determined using the BCA Protein Assay Kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). The extracted proteins were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and then transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking with 5% non-fat milk for 1 h at room temperature, the membranes were incubated overnight at 4°C with rabbit antihuman antibodies for BGN, p38, p-p38, ERK1/2, p-ERK1/2 and α-tubulin (dilution 1:1000; Abcam®). The following day, after three washes in Tris-buffered saline-Tween-20 (TBST; pH 7.5; 20 mmol/l Tris-HCl, 150 mmol/l sodium chloride, 0.1% Tween-20), the membranes were incubated with goat antirabbit secondary antibody (dilution 1:5000; Santa Cruz, Dallas, Texas, USA) for 1 h at room temperature. They were then washed three times with TBST (pH 7.5). Finally, the targeted proteins were detected using enhanced chemiluminescence (Millipore).
Cell viability
Cell viability of HCT116 cells was assessed using the Cell Counting Kit-8 (CCK-8; Nanjing KeyGen Biotech Co., Ltd.). BGN-knockdown HCT116 cells were seeded at a density of 1 × 104 cells/well in 96-well plates and cultured in an incubator for the indicated times (0, 24, 48 and 72 h). Subsequently, 10 µl of CCK-8 solution was added to each well, followed by a 2-h incubation period. The absorbance at 450 nm was measured using a spectrophotometer (Thermo Fisher Scientific).
Scratch wound healing assay
The transfected HCT116 cells were seeded into 6-well plates and allowed to grow until nearly confluent. A 10-µl pipette tip was used to create a scratch in the cell monolayer. To prevent cell division, a serum-free medium with 4 µg/ml mitomycin (Sigma-Aldrich, St Louis, MO, USA) was added. Images were captured at 0 and 24 h and the data are presented as the relative distance between the two edges.
Cell invasion assay
Cell invasion assays were carried out using Matrigel® transwell® inserts (BD Biosciences, San Jose, CA, USA). Serum-free medium was added to the upper chamber, while the lower chamber contained growth medium. After 24 h of incubation, the membrane of the chamber was stained with a 50% methanol blue/ethanol solution overnight. The invasiveness of the cells was evaluated by counting the number of cells that had migrated through the membrane.
Flow cytometric analysis of cell cycle
The effect of BGN on cell cycle arrest in colon cancer cells was detected using a flow cytometer (FACSCalibur™; BD Biosciences). Briefly, the transfected HCT116 cells were collected and fixed in 70% ethanol at 4°C overnight. The cells were then stained with propidium iodide (PI)/RNase staining solution (BD Biosciences) for 30 min at room temperature in the dark. The cell cycle distribution was subsequently analysed using a flow cytometer based on the DNA content in the HCT116 cells.
Cell apoptosis assay
The percentage of apoptotic cells was assessed using the Annexin V-fluorescein isothiocyanate (FITC)/PI apoptosis detection kit (Nanjing KeyGen Biotech Co., Ltd.). Briefly, transfected HCT116 cells were collected, washed with ice-cold 0.01 M PBS (pH 7.2) and then resuspended in 500 µl of binding buffer containing 5 µl of Annexin V-FITC and 5 µl of PI. The cells were incubated in the dark for 15 min. Apoptotic cells were then quantified using a flow cytometer (FACSCalibur™; BD Biosciences).
Statistical analyses
All statistical analyses were performed using GraphPad Prism 9 (Graphpad Software Inc., San Diego, CA, USA). Unpaired Student's t-test was used to examine the statistical significance of comparisons between two groups. Survival curves were plotted and compared using the Kaplan–Meier method. A P-value <0.05 was considered statistically significant.
Results
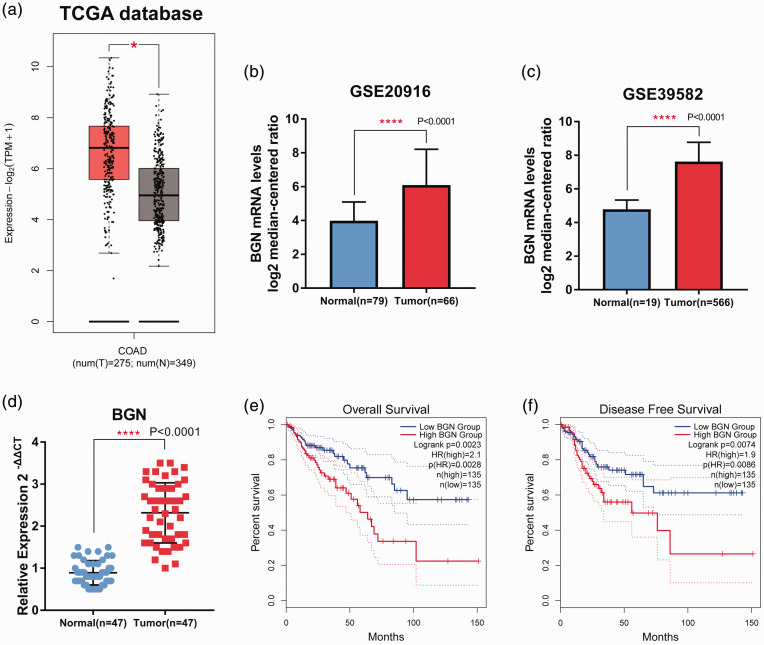
Biglycan expression data and corresponding clinical information were obtained from the TCGA database and GEO (GSE20916 and GSE39582). Analysis of BGN mRNA level profiles in TCGA and GEO revealed that BGN mRNA levels were higher in colon cancer tissues than in adjacent normal tissues (Figures 1a–1c). The results from the collected samples showed that BGN mRNA levels were higher in 47 fresh cancer tissues from patients with colon cancer compared with 47 adjacent normal tissues (Figure 1d). The relationship between BGN mRNA levels and both overall survival and disease-free survival were then analysed. The levels of BGN mRNA were significantly associated with both overall survival and disease-free survival times (P = 0.0023 and P = 0.0074, respectively); and high BGN mRNA levels indicated worse clinical outcomes (Figures 1e and 1f).
Figure 1.
Biglycan (BGN) mRNA levels were upregulated in colon cancer and correlated with prognosis. BGN mRNA levels in colon cancer tissues (T) and normal tissues (N) were analysed using data from The Cancer Genome Atlas (TCGA; tumour, n = 275; normal, n = 349) (a) (central black line is the mean; the extremities of the box are the 25th and 75th percentiles; error bars represent minimum and maximum outliers); and the Gene Expression Omnibus databases (b & c; GSE20916: tumour, n = 66; normal, n = 79; GSE39582: tumour, n = 19; normal, n = 566). (d) Levels of BGN mRNA in 47 tumour tissues and 47 adjacent normal tissues was examined using real-time polymerase chain reaction. (e & f) Overall survival and disease-free survival analyses using TCGA data. For b, c and d, the data are presented as mean ± SD; *P < 0.05, ****P < 0.0001. The colour version of this figure is available at: http://imr.sagepub.com. COAD, Colon Adenocarcinoma Collection.
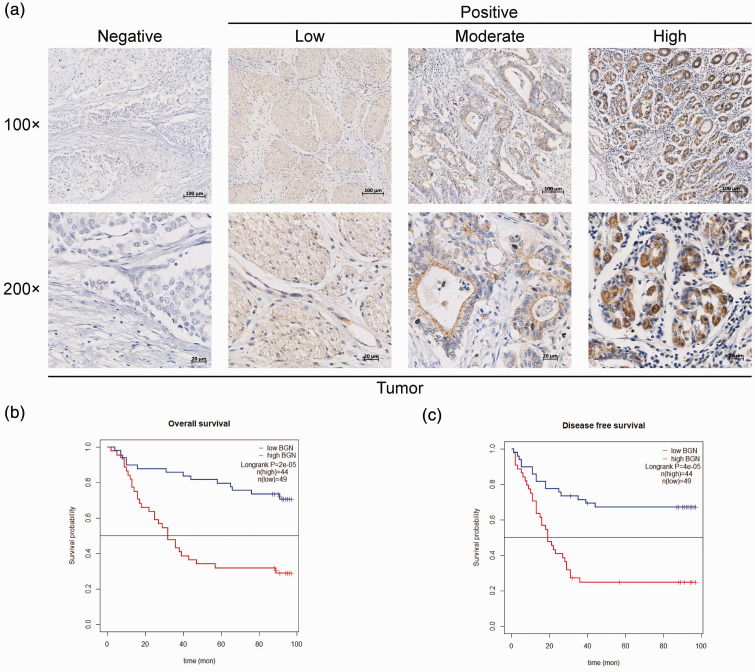
Biglycan protein levels in colon cancer and adjacent normal tissues were analysed using tissue array analysis (Figure 2a). BGN protein levels were significantly associated with both overall survival and disease-free survival times (P < 0.001 for both); and high levels indicated worse clinical outcomes (Figures 2b and 2c).
Figure 2.
High biglycan (BGN) protein levels indicate a poor prognosis for colon cancer patients. (a) Representative images of BGN immunostaining in tissue microarrays from 93 colon cancer patients. (b & c) Kaplan–Meier analyses of overall survival and disease-free survival based on low and high BGN protein levels in these 93 colon cancer patients. The colour version of this figure is available at: http://imr.sagepub.com.
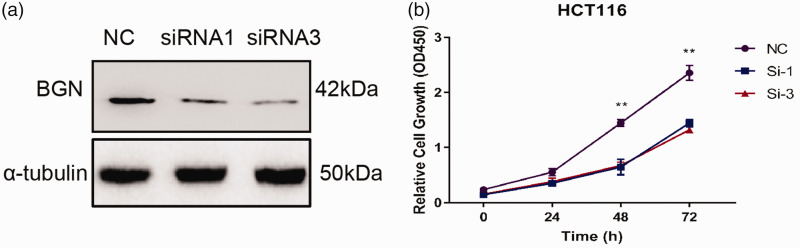
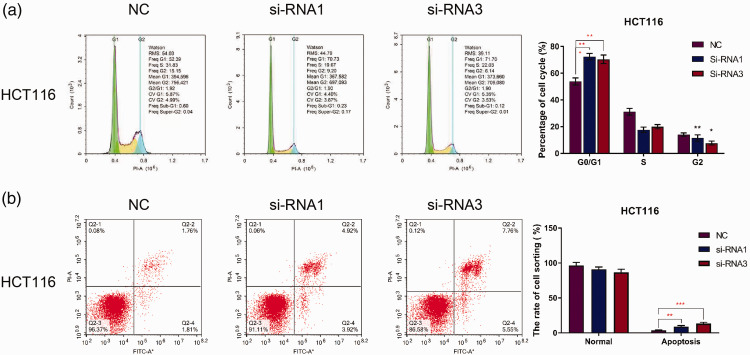
To investigate the role and molecular mechanisms of BGN in colon cancer, the HCT116 colon cancer cell line was transfected with siRNAs targeting BGN and control siRNA. BGN protein levels were measured using Western blot analysis. The results indicated that BGN protein levels were decreased in cells transfected with BGN-siRNA compared with the control group (Figure 3a). Cell viability was then assessed using a CCK-8 assay in BGN knockdown HCT116 cells. As shown in Figure 3b, cell viability was significantly inhibited 48 h after BGN knockdown compared with the control group (P < 0.01). In addition, the cell cycle in the BGN knockdown groups was arrested in the G0/G1 phase (Figure 4a). These results demonstrate that BGN knockdown inhibits the proliferation of colon cancer cells in vitro. Furthermore, BGN knockdown increased the percentage of apoptotic HCT116 cells (Figure 4b), indicating that BGN knockdown promotes apoptosis in colon cancer cells in vitro.
Figure 3.
Downregulation of biglycan (BGN) inhibits cell proliferation in HCT116 cells. (a) BGN protein levels after small interfering RNA (siRNA) transfection in HCT116 as measured by Western blot analysis. (b) Effect of BGN knockdown on cell viability detected by Cell Counting Kit-8 assays. n = 3. **P < 0.01. NC, normal control; kDa, kilodalton. The colour version of this figure is available at: http://imr.sagepub.com.
Figure 4.
Downregulation of biglycan (BGN) promotes apoptosis in HCT116 cells. Effect of BGN knockdown after small interfering RNA (siRNA) transfection on the cell cycle (a) and apoptosis (b) detected by flow cytometry. n = 3. *P < 0.05, **P < 0.01, ***P < 0.001. NC, normal control. The colour version of this figure is available at: http://imr.sagepub.com.
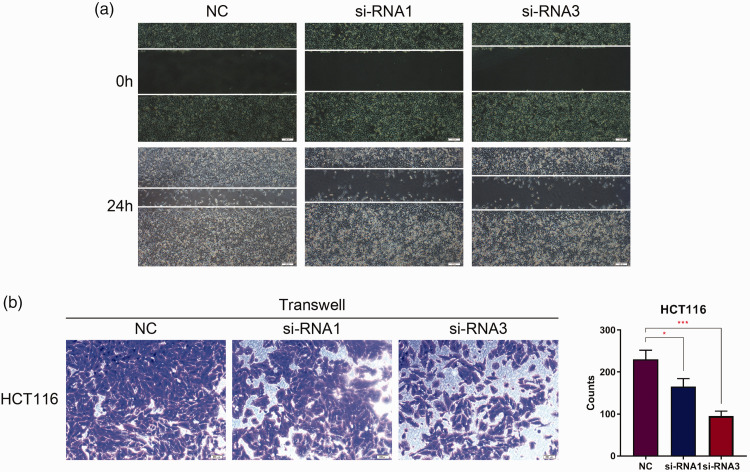
To further investigate the function of BGN in colon cancer, scratch wound healing and Matrigel® transwell® assays were undertaken to assess the role of BGN in cell motility. The results of the scratch wound healing assays indicated that cells transfected with BGN-siRNA closed the scratch wounds more slowly than control cells at 24 h in the HCT116 cell line (Figure 5a). In addition, the results of the Matrigel® transwell® assays demonstrated that the number of invasive HCT116 cells in the BGN-siRNA groups was lower than that in the control group (Figure 5b).
Figure 5.
Downregulation of biglycan (BGN) inhibits migration and invasion in HCT116 cells. (a) Effect of BGN knockdown after small interfering RNA (siRNA) transfection on wound healing (200× magnification). (b) Effect of BGN knockdown on cell invasion detected by Matrigel® transwell® assays. n = 3. *P < 0.05, ***P < 0.001. NC, normal control. The colour version of this figure is available at: http://imr.sagepub.com.
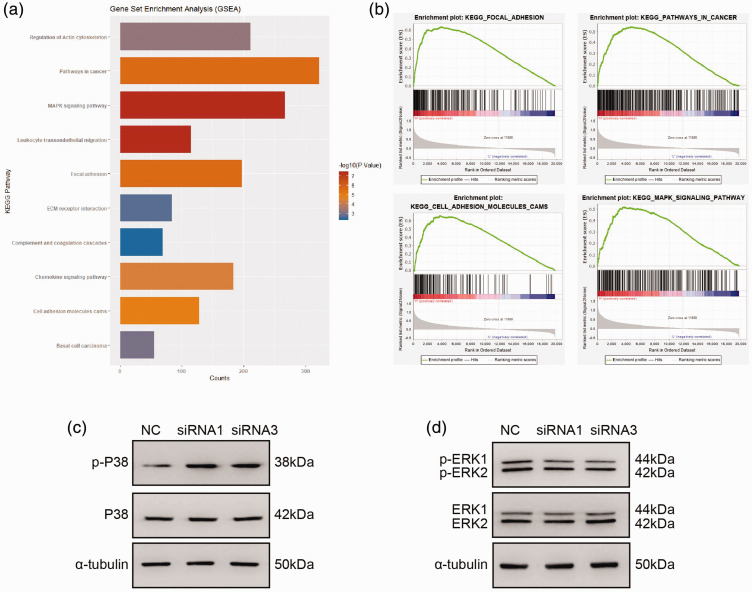
To further explore the mechanisms of BGN in colon cancer cells, GSEA was performed to identify gene sets and pathways associated with BGN, using data obtained from TCGA. This found significant correlations between BGN and gene sets in focal adhesion, cell adhesion molecules and the MAPK signalling pathway (Figures 6a and 6b). The levels of key components of the MAPK signalling pathway were then measured by Western blot analysis in BGN-knockdown HCT116 cells. The results showed that the levels of p-p38 were increased, while levels of p-ERK1/2 were decreased in the BGN-knockdown HCT116 cells compared with the control cells (Figures 6c and 6d).
Figure 6.
Biglycan (BGN) promotes colon cancer progression by regulating the p38 and ERK1/2 MAPK signalling pathways. (a & b) Gene Set Enrichment Analysis (GSEA) analysis showed that BGN correlates with gene sets involved in focal adhesion, cell adhesion molecules (CAMs), and the MAPK signalling pathway. (c & d) Western blot analysis was used to determine the protein levels of p38, p-P38, ERK1/2 and p-ERK1/2 after downregulating BGN in HCT116 cells. NC, normal control; siRNA, small interfering RNA; kDa, kilodalton. The colour version of this figure is available at: http://imr.sagepub.com.
Discussion
The current study found that BGN mRNA levels were higher in colon cancer tissues than in adjacent normal tissues. Elevated BGN mRNA levels were associated with worse clinical outcomes in patients with colon cancer, as determined through bioinformatics analysis of the TCGA and GEO databases. The tumour samples also showed upregulation of BGN, which indicated worse clinical outcomes in colon cancer patients. Moreover, BGN knockdown inhibited cell proliferation, migration, invasion; and induced cell cycle arrest in the G0/G1 phase and apoptosis in HCT116 cells. Therefore, BGN may play a significant role in the development of colon cancer.
Biglycan is a key SLRP that is ubiquitously distributed in the ECM. 18 The ECM, composed of non-cellular components, has unique properties that regulate cell behaviour. It also causes dynamic changes in the tumour microenvironment and plays an important role in tumour progression.9,19 Previous studies have linked the abnormal expression of BGN in tumours with cell proliferation, migration, metastasis and angiogenesis.15,20 Similarly, this current study demonstrated that BGN knockdown inhibited cell proliferation, migration and invasion; and promoted apoptosis in HCT116 cells. Analysis of cell cycle progression further revealed that reduced levels of BGN impacted cell cycle distribution, specifically causing cell cycle arrest in the G0/G1 phase. To further explore the mechanisms of BGN in colon cancer cells, GSEA was undertaken, which suggested that BGN might promote colon cancer progression through the MAPK signalling pathway. Moreover, downregulation of BGN increased p-p38 levels while decreasing p-ERK1/2.
The p38 MAPK and ERK1/2 signalling pathways are two important subgroups within the MAPK signalling pathway. The imbalance of p38 MAPK expression is closely related to tumour progression, influencing various cancers through biological processes such as proliferation, cell death, migration, invasion and differentiation. 21 In a previous study, long non-coding RNA ST8SIA6-AS1 was shown to promote proliferation, migration and invasion by inactivating the p38 MAPK signalling pathway in human breast cancer. 22 Similarly, these current results indicated that BGN knockdown activated the p38 MAPK signalling pathway. In addition, p38 can negatively regulate cell cycle progression by activating cyclin-dependent kinase inhibitors or downregulating the expression of cyclins.23,24 Interestingly, this current study found that BGN knockdown increased the levels of p-p38 and caused G0/G1 phase arrest in HCT116 cells. Previous studies have confirmed that the ERK1/2 signalling pathway plays a key role in the onset, invasion and drug resistance of various tumors.25,26 A previous study found that overexpression of miR-302a inhibited the proliferation and invasion of colorectal cancer cells by suppressing the MAPK/ERK and PI3K/Akt signalling pathways. 27 The current results also showed that levels of p-ERK1/2 decreased in BGN-knockdown HCT116 cells. Thus, the current findings indicate that BGN may promote colon cancer progression by regulating the p38 and ERK1/2 MAPK signalling pathways.
The current study had several limitations. First, this study may be limited by its sample size and population diversity, which could have potentially impacted the accuracy and applicability of the findings. BGN levels can vary across different colon cancer patients. Future research will analyse a larger cohort of colon cancer samples and collect more comprehensive patient information to further investigate the correlation between BGN levels and the various subtypes of colon cancer. Secondly, the current findings were preliminary. Future research will aim to utilize high-throughput sequencing, in vivo experiments and additional techniques to further explore the role of BGN and uncover the molecular mechanisms by which it influences colon cancer.
In conclusion, this current study found that BGN was upregulated in human colon cancer and that a high level of BGN was significantly associated with poor clinical outcomes in human patients with colon cancer. BGN promoted tumour proliferation and invasion in a colon cancer cell line. This current study suggests that BGN may serve as a potential therapeutic target for colon cancer.
Acknowledgements
We wish to acknowledge the support of the Tenth Affiliated Hospital, Southern Medical University.
Author contributions: Biyan Lu, Chuangyu Wen and Weibiao Ye made substantial contributions to the conception of this study and designed the experiments. Biyan Lu and Weibiao Ye performed most of the experiments and drafted the manuscript. Ying Zhu, Guangliu Li and Yongqiang Xu participated in sample collection and data analysis. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Funding: The study was supported by grants from the National Natural Science Foundation of China (no. 82103269), Guangdong Province Medical Scientific Research Foundation (no. C2023114) and Guangdong Basic and Applied Basic Research Foundation (no. 2022A1515140001).
ORCID iD: Weibiao Ye https://orcid.org/0000-0002-8881-9111
Data availability statement
The data analysed in the current study are available from the corresponding author upon reasonable request.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2.Zheng RS, Chen R, Han BF, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi 2024; 46: 221–231 [Article in Chinese, English abstract]. [DOI] [PubMed] [Google Scholar]
- 3.Ameye L, Aria D, Jepsen K, et al. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J 2002; 16: 673–680. [DOI] [PubMed] [Google Scholar]
- 4.Gesteira TF, Verma S, Coulson-Thomas VJ. Small leucine rich proteoglycans: Biology, function and their therapeutic potential in the ocular surface. Ocul Surf 2023; 29: 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechner BE, Lim JH, Mercado ML, et al. Developmental regulation of biglycan expression in muscle and tendon. Muscle Nerve 2006; 34: 347–355. [DOI] [PubMed] [Google Scholar]
- 6.Mercado ML, Amenta AR, Hagiwara H, et al. Biglycan regulates the expression and sarcolemmal localization of dystrobrevin, syntrophin, and nNOS. FASEB J 2006; 20: 1724–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miguez PA, Terajima M, Nagaoka H, et al. Recombinant biglycan promotes bone morphogenetic protein-induced osteogenesis. J Dent Res 2014; 93: 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreth K, Iozzo RV, Schaefer L. Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell Cycle 2012; 11: 2084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diehl V, Huber LS, Trebicka J, et al. The Role of Decorin and Biglycan Signaling in Tumorigenesis. Front Oncol 2021; 11: 801801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giatagana EM, Berdiaki A, Gaardlos M, et al. Biglycan Interacts with Type I Insulin-like Receptor (IGF-IR) Signaling Pathway to Regulate Osteosarcoma Cell Growth and Response to Chemotherapy. Cancers (Basel) 2022; 14: 1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber CK, Sommer G, Michl P, et al. Biglycan is overexpressed in pancreatic cancer and induces G1-arrest in pancreatic cancer cell lines. Gastroenterology 2001; 121: 657–667. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Wang R, Feng L, et al. LINC00460 Promotes Cell Proliferation, Migration, Invasion, and Epithelial-Mesenchymal Transition of Head and Neck Squamous Cell Carcinoma via miR-320a/BGN Axis. Onco Targets Ther 2021; 14: 2279–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furumido J, Maishi N, Yanagawa-Matsuda A, et al. Stroma biglycan expression can be a prognostic factor in prostate cancers. Int J Urol 2023; 30: 147–154. [DOI] [PubMed] [Google Scholar]
- 14.Pan S, Cheng L, White JT, et al. Quantitative proteomics analysis integrated with microarray data reveals that extracellular matrix proteins, catenins, and p53 binding protein 1 are important for chemotherapy response in ovarian cancers. OMICS 2009; 13: 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu H, Xiang Z, Huang G, et al. BGN/FAP/STAT3 positive feedback loop mediated mutual interaction between tumor cells and mesothelial cells contributes to peritoneal metastasis of gastric cancer. Int J Biol Sci 2023; 19: 465–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Wang F, Mi K, et al. Biglycan regulated colorectal cancer progress by modulating enteric neuron-derived IL-10 and abundance of Bacteroides thetaiotaomicron. iScience 2023; 26: 107515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto K, Ohga N, Hida Y, et al. Biglycan is a specific marker and an autocrine angiogenic factor of tumour endothelial cells. Br J Cancer 2012; 106: 1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nastase MV, Young MF, Schaefer L. Biglycan: a multivalent proteoglycan providing structure and signals. J Histochem Cytochem 2012; 60: 963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berdiaki A, Giatagana EM, Tzanakakis G, et al. The Landscape of Small Leucine-Rich Proteoglycan Impact on Cancer Pathogenesis with a Focus on Biglycan and Lumican. Cancers (Basel) 2023; 15: 3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Z, Lin J, Chen C, et al. Identification of BGN and THBS2 as metastasis-specific biomarkers and poor survival key regulators in human colon cancer by integrated analysis. Clin Transl Med 2022; 12: e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koul HK, Pal M, Koul S. Role of p38 MAP Kinase Signal Transduction in Solid Tumors. Genes Cancer 2013; 4: 342–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang K, Hu C, Zhang X, et al. LncRNA ST8SIA6-AS1 promotes proliferation, migration and invasion in breast cancer through the p38 MAPK signalling pathway. Carcinogenesis 2020; 41: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 23.Whitaker RH, Cook JG. Stress Relief Techniques: p38 MAPK Determines the Balance of Cell Cycle and Apoptosis Pathways. Biomolecules 2021; 11: 1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu S, Yue Z, Liu Q. Pectinose induces cell cycle arrest in luminal A and triple-negative breast cancer cells by promoting autophagy through activation of the p38 MAPK signaling pathway. BMC Cancer 2024; 24: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han HB, Gu J, Ji DB, et al. PBX3 promotes migration and invasion of colorectal cancer cells via activation of MAPK/ERK signaling pathway. World J Gastroenterol 2014; 20: 18260–18270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du J, Tong A, Wang F, et al. The Roles of PI3K/AKT/mTOR and MAPK/ERK Signaling Pathways in Human Pheochromocytomas. Int J Endocrinol 2016; 2016: 5286972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei ZJ, Tao ML, Zhang W, et al. Up-regulation of microRNA-302a inhibited the proliferation and invasion of colorectal cancer cells by regulation of the MAPK and PI3K/Akt signaling pathways. Int J Clin Exp Pathol 2015; 8: 4481–4491. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analysed in the current study are available from the corresponding author upon reasonable request.