Abstract
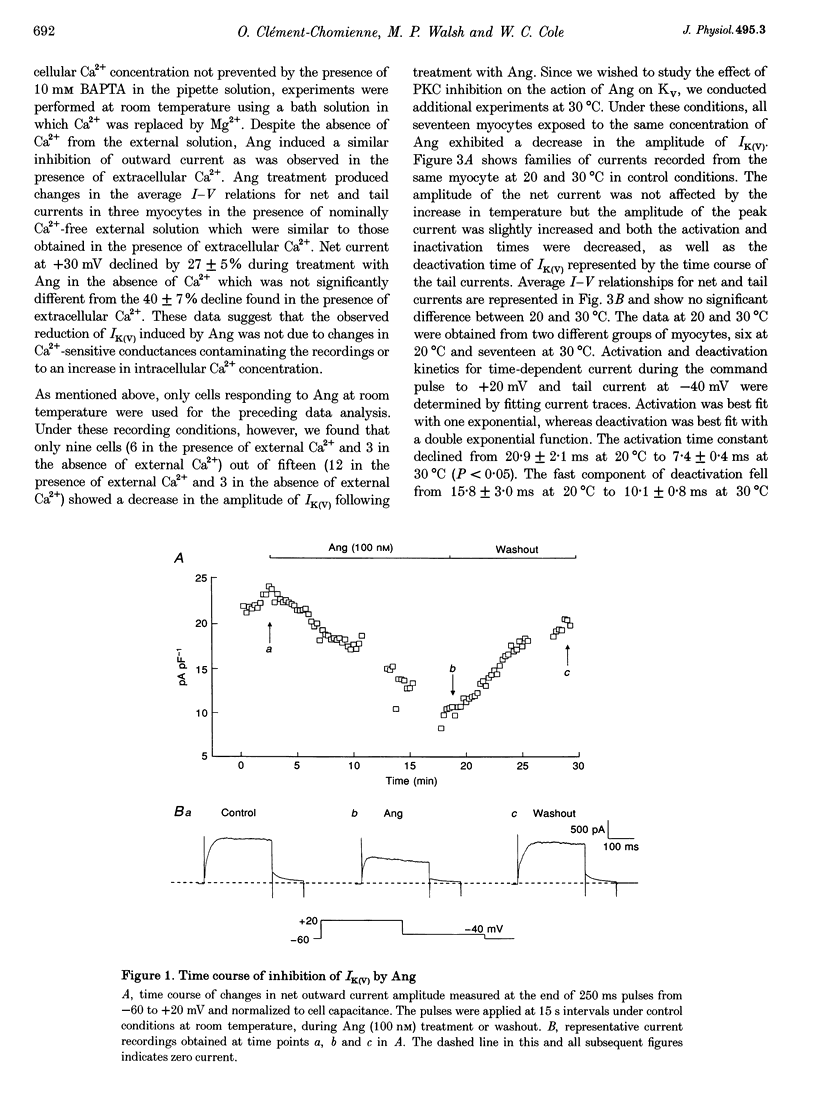
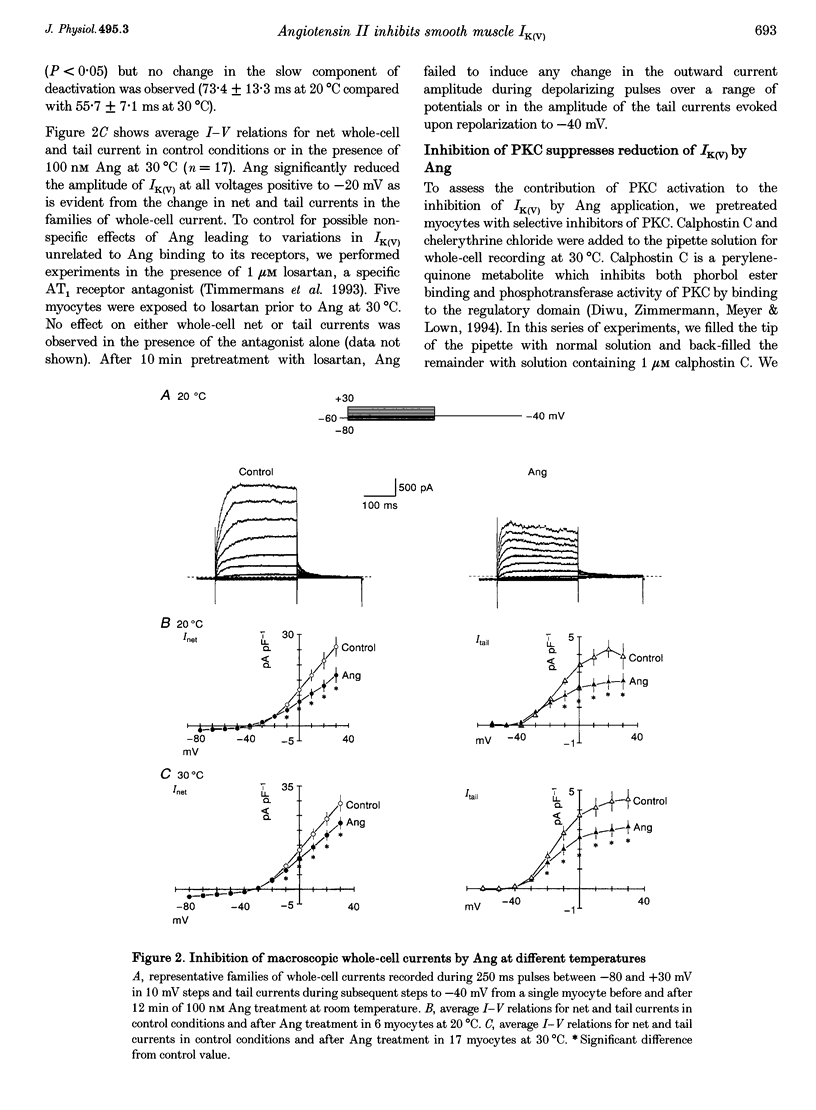
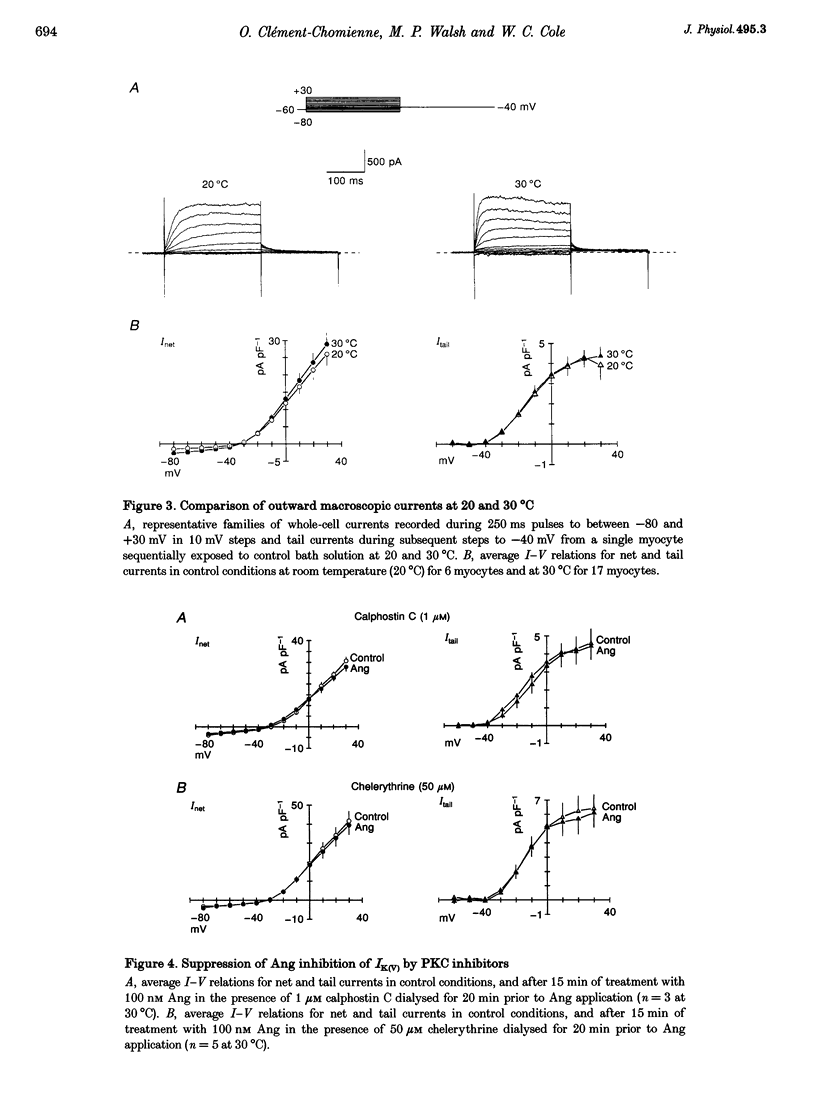
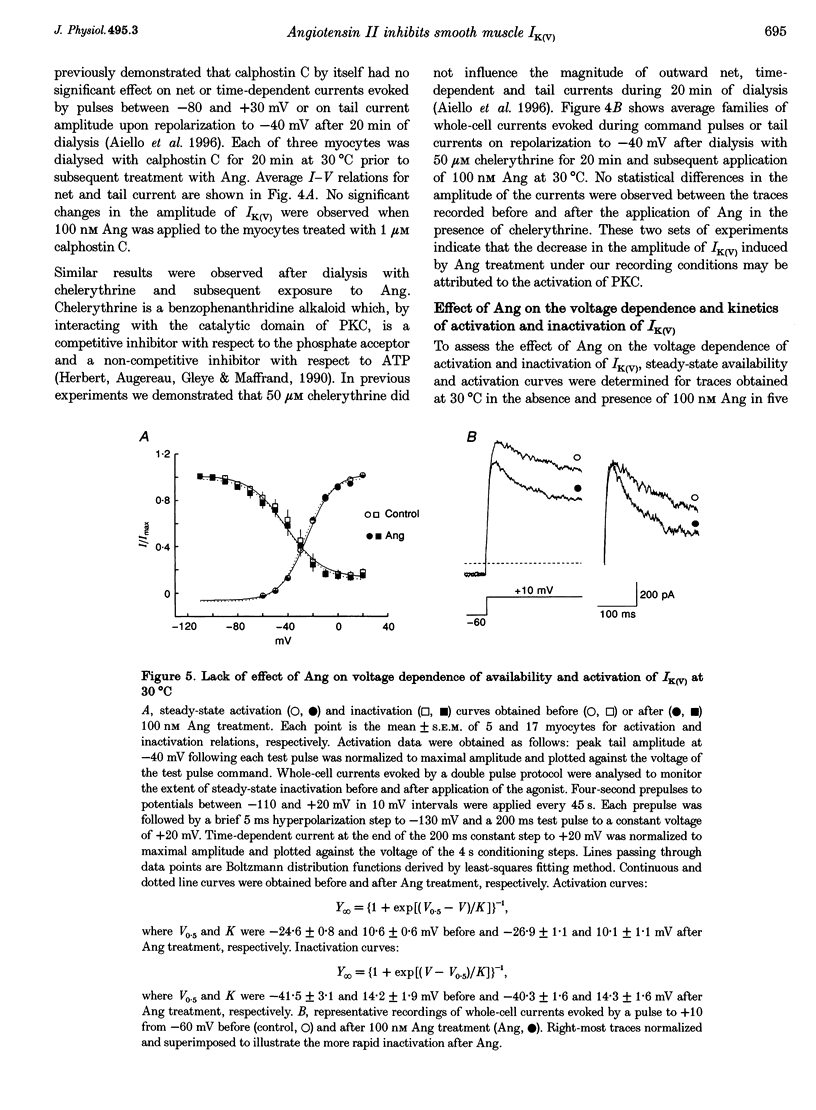
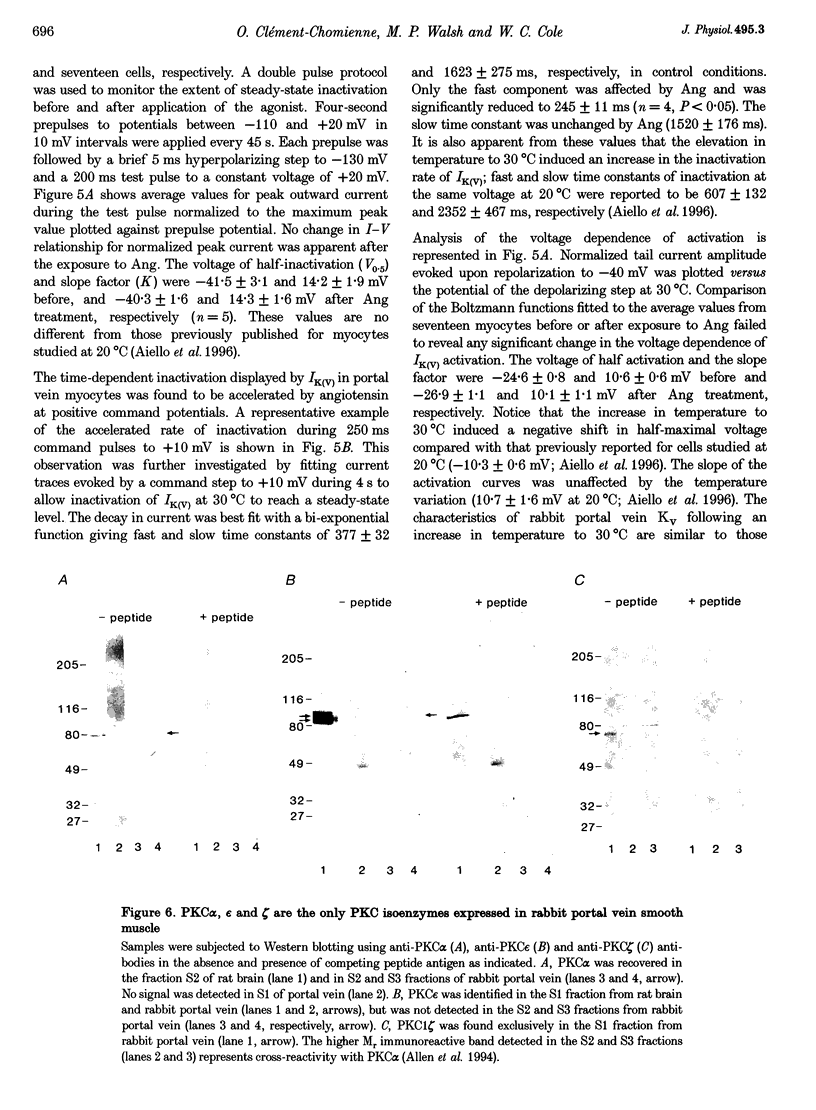
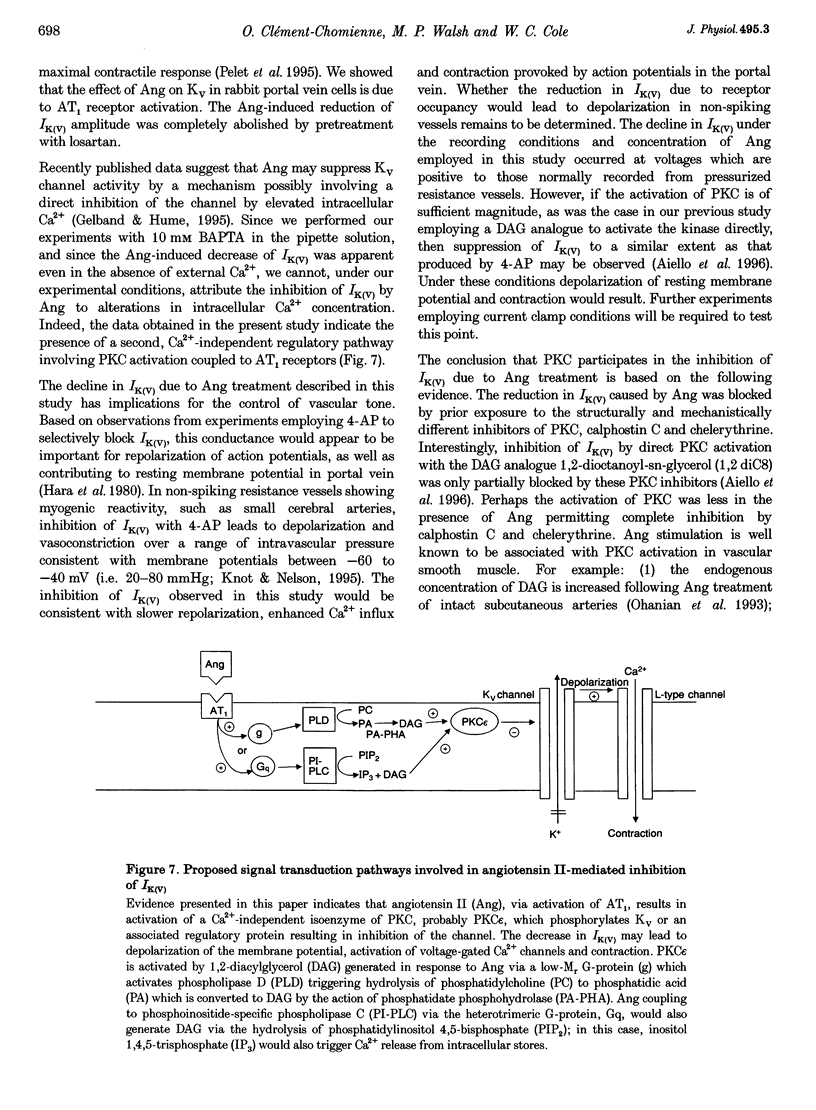
1. The effect of angiotension II (Ang) on delayed rectifier K+ current (IK(V)) was studied in isolated rabbit portal vein smooth muscle cells using standard whole-cell voltage clamp technique. The effect of 100 nM Ang on macroscopic, whole-cell IK(V) was assessed in myocytes dialysed with 10 mM BAPTA, 5 mM ATP and 1 mM GTP either at room temperature or at 30 degrees C. 2. Application of Ang caused a decline in IK(V) which was reversed upon washout of the drug. Tail current recorded after 250 ms pulses to +30 mV and repolarization to -40 mV was reduced from 3.9 +/- 0.7 to 2.5 +/- 0.5 pA pF-1 at 20 degrees C (n = 6) and from 4.5 +/- 0.5 to 3.13 +/- 0.4 pA pF-1 at 30 degrees C(n = 17). 3. Ang had no effect on outward current in the presence of an AT1 selective antagonist, losartan (1 microM), which alone had no direct effect on the amplitude of IK(V). Substitution of extracellular Ca2+ with Mg2+ in the presence of 10 microM intracellular BAPTA did not affect the suppression of IK(V) by Ang. 4. Ang induced a decrease in time constant for the rapid phase of inactivation of the macroscopic current (tau 1 reduced from 377 +/- 32 to 245 +/- 11 ms; tau 2 unchanged, n = 17). Neither the voltage dependence of activation nor inactivation were affected by Ang. 5. The inhibition of IK(V) by Ang was abolished by intracellular dialysis with the selective PKC inhibitors, calphostin C (1 microM) and chelerythrine (50 microM). These data provide strong evidence that the decline in IK(V) due to Ang treatment is due to PKC activation. 6. The pattern of expression of PKC isoforms was examined in rabbit portal vein using isoenzyme-specific antibodies: alpha, epsilon and zeta isoenzymes were detected, but beta, gamma, delta and eta isoenzymes were not. 7. The lack of requirement for Ca2+, as well as the sensitivity of the Ang response to chelerythrine, suggest the involvement of the Ca(2+)-independent PKC isoenzyme epsilon in the signal transduction pathway responsible for IK(V) inhibition by Ang.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiello E. A., Clément-Chomienne O., Sontag D. P., Walsh M. P., Cole W. C. Protein kinase C inhibits delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1996 Jul;271(1 Pt 2):H109–H119. doi: 10.1152/ajpheart.1996.271.1.H109. [DOI] [PubMed] [Google Scholar]
- Aiello E. A., Walsh M. P., Cole W. C. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995 Feb;268(2 Pt 2):H926–H934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- Allen B. G., Andrea J. E., Walsh M. P. Identification and characterization of protein kinase C zeta-immunoreactive proteins. J Biol Chem. 1994 Nov 18;269(46):29288–29298. [PubMed] [Google Scholar]
- Andrea J. E., Walsh M. P. Protein kinase C of smooth muscle. Hypertension. 1992 Nov;20(5):585–595. doi: 10.1161/01.hyp.20.5.585. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989 May;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretschneider E., Paintz M., Glusa E. Involvement of inositol 1,4,5-triphosphate and protein kinase C in thrombin-induced contraction of porcine pulmonary artery. Biochem Pharmacol. 1995 Jan 6;49(1):33–38. doi: 10.1016/0006-2952(94)00404-a. [DOI] [PubMed] [Google Scholar]
- Clément-Chomienne O., Walsh M. P. Identification of protein kinase C isoenzymes in smooth muscle: partial purification and characterization of chicken gizzard PKC zeta. Biochem Cell Biol. 1996;74(1):51–65. doi: 10.1139/o96-006. [DOI] [PubMed] [Google Scholar]
- Diwu Z., Zimmermann J., Meyer T., Lown J. W. Design, synthesis and investigation of mechanisms of action of novel protein kinase C inhibitors: perylenequinonoid pigments. Biochem Pharmacol. 1994 Jan 20;47(2):373–385. doi: 10.1016/0006-2952(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Dixon B. S., Sharma R. V., Dickerson T., Fortune J. Bradykinin and angiotensin II: activation of protein kinase C in arterial smooth muscle. Am J Physiol. 1994 May;266(5 Pt 1):C1406–C1420. doi: 10.1152/ajpcell.1994.266.5.C1406. [DOI] [PubMed] [Google Scholar]
- Farb A., Tang A. L., Burke A. P., Sessums L., Liang Y., Virmani R. Sudden coronary death. Frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation. 1995 Oct 1;92(7):1701–1709. doi: 10.1161/01.cir.92.7.1701. [DOI] [PubMed] [Google Scholar]
- Fleischmann B. K., Washabau R. J., Kotlikoff M. I. Control of resting membrane potential by delayed rectifier potassium currents in ferret airway smooth muscle cells. J Physiol. 1993 Sep;469:625–638. doi: 10.1113/jphysiol.1993.sp019834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E. J., Tallant E. A. Vascular smooth-muscle cells contain AT1 angiotensin receptors coupled to phospholipase D activation. Biochem J. 1994 Dec 1;304(Pt 2):543–548. doi: 10.1042/bj3040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelband C. H., Hume J. R. [Ca2+]i inhibition of K+ channels in canine renal artery. Novel mechanism for agonist-induced membrane depolarization. Circ Res. 1995 Jul;77(1):121–130. doi: 10.1161/01.res.77.1.121. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hara Y., Kitamura K., Kuriyama H. Actions of 4-aminopyridine on vascular smooth muscle tissues of the guinea-pig. Br J Pharmacol. 1980 Jan;68(1):99–106. doi: 10.1111/j.1476-5381.1980.tb10704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. J., Overturf K. E., Russell S. N., Carl A., Hume J. R., Sanders K. M., Horowitz B. Cloning and expression of a Kv1.2 class delayed rectifier K+ channel from canine colonic smooth muscle. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9659–9663. doi: 10.1073/pnas.90.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J. M., Augereau J. M., Gleye J., Maffrand J. P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990 Nov 15;172(3):993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hirakawa Y., Kuga T., Kobayashi S., Kanaide H., Takeshita A. Dual regulation of L-type Ca2+ channels by serotonin 2 receptor stimulation in vascular smooth muscle cells. Am J Physiol. 1995 Feb;268(2 Pt 2):H544–H549. doi: 10.1152/ajpheart.1995.268.2.H544. [DOI] [PubMed] [Google Scholar]
- Hughes A. D., Bolton T. B. Action of angiotensin II, 5-hydroxytryptamine and adenosine triphosphate on ionic currents in single ear artery cells of the rabbit. Br J Pharmacol. 1995 Oct;116(3):2148–2154. doi: 10.1111/j.1476-5381.1995.tb16424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Hume J. R., Keef K. D. Modulation of K+ and Ca2+ channels by histamine H1-receptor stimulation in rabbit coronary artery cells. J Physiol. 1993 Aug;468:379–400. doi: 10.1113/jphysiol.1993.sp019777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye A. D., Nossaman B. D., Ibrahim I. N., Feng C. J., Kadowitz P. J. Influence of protein kinase C inhibitors on vasoconstrictor responses in the pulmonary vascular bed of cat and rat. Am J Physiol. 1995 Mar;268(3 Pt 1):L532–L538. doi: 10.1152/ajplung.1995.268.3.L532. [DOI] [PubMed] [Google Scholar]
- Knot H. J., Nelson M. T. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol. 1995 Jul;269(1 Pt 2):H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- Koide H., Ogita K., Kikkawa U., Nishizuka Y. Isolation and characterization of the epsilon subspecies of protein kinase C from rat brain. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1149–1153. doi: 10.1073/pnas.89.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Morales E., Leblanc N. R., Cole W. C. Metabolic inhibition enhances Ca(2+)-activated K+ current in smooth muscle cells of rabbit portal vein. Am J Physiol. 1993 Dec;265(6 Pt 2):H2184–H2195. doi: 10.1152/ajpheart.1993.265.6.H2184. [DOI] [PubMed] [Google Scholar]
- Minami K., Hirata Y., Tokumura A., Nakaya Y., Fukuzawa K. Protein kinase C-independent inhibition of the Ca(2+)-activated K+ channel by angiotensin II and endothelin-1. Biochem Pharmacol. 1995 Apr 18;49(8):1051–1056. doi: 10.1016/0006-2952(95)98500-9. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Nilsson H., Flatman J. A. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J Physiol. 1982 Nov;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Quayle J. M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995 Apr;268(4 Pt 1):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Standen N. B., Brayden J. E., Worley J. F., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988 Nov 24;336(6197):382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- Ohanian J., Izzard A., Littlewood M., Heagerty A. Regulation of diacylglycerol metabolism by vasoconstrictor hormones in intact small arteries. Circ Res. 1993 Jun;72(6):1163–1171. doi: 10.1161/01.res.72.6.1163. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A. 1989 May;86(9):3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overturf K. E., Russell S. N., Carl A., Vogalis F., Hart P. J., Hume J. R., Sanders K. M., Horowitz B. Cloning and characterization of a Kv1.5 delayed rectifier K+ channel from vascular and visceral smooth muscles. Am J Physiol. 1994 Nov;267(5 Pt 1):C1231–C1238. doi: 10.1152/ajpcell.1994.267.5.C1231. [DOI] [PubMed] [Google Scholar]
- Pelet C., Mironneau C., Rakotoarisoa L., Neuilly G. Angiotensin II receptor subtypes and contractile responses in portal vein smooth muscle. Eur J Pharmacol. 1995 Jun 6;279(1):15–24. doi: 10.1016/0014-2999(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Huwiler A. A role for protein kinase C-epsilon in angiotensin II stimulation of phospholipase D in rat renal mesangial cells. FEBS Lett. 1993 Oct 4;331(3):267–271. doi: 10.1016/0014-5793(93)80350-4. [DOI] [PubMed] [Google Scholar]
- Timmermans P. B., Wong P. C., Chiu A. T., Herblin W. F., Benfield P., Carini D. J., Lee R. J., Wexler R. R., Saye J. A., Smith R. D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993 Jun;45(2):205–251. [PubMed] [Google Scholar]
- Ullian M. E., Walsh L. G., Wong K. C., Allan C. J. Potentiation of angiotensin II-stimulated vascular contraction by lithium. Am J Physiol. 1995 May;268(5 Pt 2):H2009–H2016. doi: 10.1152/ajpheart.1995.268.5.H2009. [DOI] [PubMed] [Google Scholar]
- Watanabe J., Keitoku M., Hangai K., Karibe A., Takishima T. alpha-Adrenergic augmentation of myogenic response in rat arterioles: role of protein kinase C. Am J Physiol. 1993 Feb;264(2 Pt 2):H547–H552. doi: 10.1152/ajpheart.1993.264.2.H547. [DOI] [PubMed] [Google Scholar]



