Abstract
Background:
Inflammatory bowel disease (IBD) affects 5%–10% of ankylosing spondylitis (AS) patients. Prior data suggest AS patients with IBD may have more severe disease and lower HLA-B27 prevalence. Yet, little is known about potential distinctions in AS with IBD compared to those without IBD.
Objective:
To investigate the clinical characteristics and radiographic differences between patients with (AS) with and without concurrent IBD.
Design:
This multicenter, observational, cross-sectional study included patients meeting European Spondyloarthropathy Study Group criteria from the Registry of Spondyloarthritis of Spanish Rheumatology (REGISPONSER) and Ibero-American Registry of Spondyloarthropathies (RESPONDIA) registries.
Methods:
Characteristics and disease burden were compared between patients with and without IBD. Multivariate logistic regression identified factors independently associated with IBD presence in patients with AS.
Results:
We included a total of 2766 patients with AS (1254 from REGISPONSER and 1512 from RESPONDIA), among whom 142 patients (5.13%) presented with concomitant IBD. AS patients with concurrent IBD were less frequently male, had a lower prevalence of HLA-B27 positivity, experienced a lower prolonged diagnostic delay, had a lower frequency of enthesitis, and received more commonly intensified treatment compared to those without IBD. In terms of structural damage, the Bath Ankylosing Spondylitis Radiology Index (BASRI) score for the sacroiliac joints (SIJs), cervical spine, and lumbar spine was lower in patients with AS and IBD than in those without IBD. In the multivariable analysis, the presence of IBD was significantly associated with a lower prevalence of HLA-B27 and enthesitis, with odds ratios (OR) of 0.32 (95% confidence interval (CI): 0.20–0.52) and 0.58 (95% CI: 0.33–0.97), respectively. Furthermore, structural damage in SIJs (BASRI) was significantly decreased in patients with IBD, with an OR of 0.79 (95% CI: 0.64–0.99).
Conclusion:
The presence of IBD in AS is associated with lower HLA-B27 positivity, less enthesitis, and less radiographic damage in this large population study.
Keywords: axial spondyloarthritis, HLA-B27, inflammatory bowel disease
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory condition primarily affecting the axial skeleton, which encompasses the spine and/or sacroiliac joints (SIJs). In addition to spinal inflammation and structural damage, peripheral symptoms (peripheral arthritis, enthesitis, dactylitis) and extra-musculoskeletal manifestations, such as inflammatory bowel disease (IBD), psoriasis, and anterior uveitis are common and contribute to the total burden of axSpA. 1
IBD, encompassing ulcerative colitis (UC) and Crohn’s disease (CD), is noted in 5%–10% of axSpA patients. 2 Prior research indicates that axSpA patients with concurrent IBD may require intensified treatment and exhibit higher disease activity.3,4 Notably, while the prevalence of HLA-B27 in radiographic axSpA or ankylosing spondylitis (AS) typically ranges from 85% to 90%, it is notably lower in AS associated with IBD, reported in up to 60%. 5
Despite all these data, little is known whether AS in patients with IBD differs from AS in patients without IBD, or whether the presence of IBD can influence the axSpA phenotype. Early radiographic studies suggest that axial disease in AS and SpA associated with IBD are morphologically similar. 6 However, no further studies explored this item.
This study explores the complex relationship between AS and IBD by comparing patients with AS with and without IBD. By examining two large cohorts of AS patients, we aim to identify key demographic, clinical characteristics, and radiographic differences between these two populations.
Methods
Study design and patients
This was an observational, cross-sectional, and multicenter study that included patients from the REGISPONSER (Registry of Spondyloarthritis of Spanish Rheumatology) and RESPONDIA (Ibero-American Registry of Spondyloarthropathies) registries. A detailed description of the two registries has been reported previously.7,8 Briefly, REGISPONSER is an observational, multicenter, national study that involved 31 centers from Spain. Patients who fulfilled the European Spondyloarthropathy Study Group (ESSG) 9 criteria for SpA between March 2004 and March 2007 were included consecutively. Thus, patients could have a diagnosis according to their rheumatologist of AS, psoriatic arthritis, IBD-associated SpA IBD, reactive arthritis, undifferentiated SpA, or juvenile SpA.
RESPONDIA has a similar design and shares the case report form and all the variables studied with REGISPONSER. It includes patients from 33 centers in eight Latin American countries and was conducted between 2006 and 2007. The inclusion criteria were the same as in REGISPONSER. Consecutive patients with SpA according to the criteria of the ESSG were included. Protocol design in each cohort predated the development of ASAS criteria, and hence the original nomenclature utilized in the different studies referring to AS rather than radiographic axSpA will be utilized in this report. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology statement. 10
The overall population included 4410 patients (2366 from REGISPONSER and 2044 from RESPONDIA). In this ancillary analysis, we analyzed 2766 patients, the total population of patients with AS included in REGISPONSER and RESPONDIA.
Clinical data
The variables recorded in the registries and selected for analysis in our study comprised demographic data: age, gender, body mass index (BMI), and smoking status. Disease characteristics analyzed were HLA-B27 status, family history of SpA, disease duration, inflammatory low back pain, alternating buttock pain, enthesitis, dactylitis, psoriasis, lower- and upper-limb arthritis ever, uveitis, and IBD. Treatment ever with conventional synthetic and biological disease-modifying antirheumatic drugs (csDMARDs and bDMARDs, respectively). Disease activity measurements included the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) 11 and Ankylosing Spondylitis Disease Activity Score (ASDAS). 12 Function was reported by the Bath Ankylosing Spondylitis Functional Index (BASFI). 13 Structural damage was evaluated by the local reader, who was in all cases a rheumatologist with an interest in SpA, using the Bath Ankylosing Spondylitis Radiology Index (BASRI). 14 Briefly, BASRI-spine is a combined score of the cervical spine, lumbar spine, and SIJs. BASRI uses the combination of AP and lateral views to score the lumbar spine and lateral views to score the cervical spine; then cervical and lumbar spine are scored separately on a scale from 0 to 4. SIJs are scored according to the modified New York criteria. The final BASRI-spine score results from the sum of the mean score for the right and left SIJs plus the scores for the lumbar and cervical spine.
Statistical analysis
Descriptive data were presented as proportions for qualitative variables and as means with standard deviation for continuous ones. Between-group differences were analyzed using the Pearson Chi-square or Fisher test for proportions and the Student’s t-test or the Mann–Whitney U test for continuous variables.
To assess the variables independently associated with the presence of IBD in AS patients, a comparative analysis between patients with IBD and without IBD was conducted using a multiple logistic regression analysis. In this model, we performed a backward stepwise model to select the relevant factors to be included in the analysis, variables known to be related to the presence of IBD, and others taken from the univariate analysis (p < 0.05).
The multivariate analysis evaluating factors associated with IBD between HLA-B27 positives and negatives included variables with a p < 0.20. This was decided because the univariable analysis showed very few significant associations, and we wanted to be less stringent and more inclusive in the multivariable analysis. Adjusted odds ratios (ORs) after controlling for all the variables in the model were presented with their 95% confidence intervals. Two-tailed p values < 0.05 were established to be statistically significant. All analyses were carried out using R Studio. 15
Results
We included a total of 2766 patients with AS (1254 from REGISPONSER and 1512 from RESPONDIA), among whom 142 patients (5.13%) presented with concomitant IBD. Among the 91 patients with available data, 53.8% (49/91) developed IBD after the onset of musculoskeletal symptoms, primarily axial symptoms (40/49), while 32.9% (30/91) had IBD prior to the onset of musculoskeletal symptoms, predominantly axial symptoms (28/30). The remaining 13.2% (12/91) developed IBD concurrently with musculoskeletal symptoms.
The clinical and sociodemographic characteristics of the two populations included in this study, REGISPONSER and RESPONDIA, are presented in Table 1. In summary, we found some differences concerning demographic and clinical features in the two registries, the studied populations were generally young and predominantly male. However, there were no significant differences in the prevalence of IBD between both registries, with rates of 4.6% in RESPONDIA compared to 5.6% in REGISPONSER (p = 0.270). When comparing patients with AS and IBD from the two cohorts, we found that RESPONDIA patients with AS and IBD had more arthritis, dactylitis, and enthesitis, and therefore were more often treated with csDMARDs. By contrast, REGISPONSER patients with AS and IBD were younger at diagnosis and were more frequently treated with bDMARDs (Supplemental Table S1).
Table 1.
The clinical and sociodemographic characteristics of the two populations included in this study, REGISPONSER and RESPONDIA, in patients with ankylosing spondylitis.
| RESPONDIA N = 1254 |
REGISPONSER N = 1512 |
p-Value | |
|---|---|---|---|
| Sex (male) | 916 (73.0%) | 1130 (74.7%) | 0.314 |
| Age | 44.3 (16.2) | 48.1 (12.8) | <0.001 |
| Age at diagnosis | 36.4 (15.6) | 34.8 (11.9) | 0.007 |
| Time since symptoms onset | 14.8 (11.6) | 21.0 (13.1) | <0.001 |
| Time since diagnosis | 7.3 (8.4) | 13.2 (10.4) | <0.001 |
| Diagnosis delay | 6.9 (8.9) | 7.7 (9.3) | 0.033 |
| BMI | 25.9 (4.5) | 26.7 (4.4) | <0.001 |
| HLA-B27 positive | 459/647 (70.9%) | 1114/1352 (82.4%) | <0.001 |
| Family history of SpA | 228/1228 (18.6%) | 285/1402 (20.3%) | 0.255 |
| Inflammatory back pain | 1196/1251 (95.6%) | 1474 (97.5%) | 0.008 |
| Alternating buttock pain | 693/1252 (55.4%) | 965/1492 (64.7%) | <0.001 |
| Arthritis ever | 696/1252 (55.6%) | 511/1508 (33.9%) | <0.001 |
| Enthesitis ever | 664/1244 (53.4%) | 461/1501 (30.7%) | <0.001 |
| Dactylitis ever | 155/1248 (12.4%) | 83/1506 (5.5%) | <0.001 |
| Cutaneous psoriasis | 138/1249 (11.0%) | 140/1408 (9.3%) | 0.126 |
| Nail disease | 83/1192 (7.0%) | 45/1507 (3.0%) | <0.001 |
| IBD | 58 (4.6%) | 84 (5.6%) | 0.270 |
| Uveitis | 274/1244 (22.0%) | 321/1502 (21.4%) | 0.678 |
| CRP | 9.7 (18.3) | 8.9 (13.5) | 0.236 |
| BASDAI | 4.4 (2.4) | 4.1 (2.3) | 0.002 |
| ASDAS-CRP | 2.6 (1.2) | 2.6 (1.1) | 0.227 |
| BASRI total | 7.9 (4.2) | 6.9 (4.1) | <0.001 |
| bDMARD ever | 113 (9.0%) | 304 (20.1%) | <0.001 |
| csDMARD ever | 621 (49.5%) | 441 (29.2%) | <0.001 |
Continuous variables are described by their mean and the standard deviation (between brackets), while absolute and percentages are shown for categorical variables.
ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASRI, Bath Ankylosing Spondylitis Radiology Index; bDMARDs, biological disease-modifying antirheumatic drugs; BMI, body mass index; CRP, C-reactive protein; csDMARD, conventional synthetic disease-modifying antirheumatic drugs; IBD, inflammatory bowel disease; SPA, spondyloarthritis.
Demographic and clinical characteristics of patients with AS and IBD compared to AS without IBD
Age and BMI were comparable between the two groups. Nevertheless, in AS patients with concurrent IBD, there was a lower frequency of males (62.0% vs 74.6% in IBD and non-IBD, respectively) and a reduced prevalence of HLA-B27 positivity (56.3% in IBD vs 79.9% in non-IBD). In addition, AS patients with IBD experienced a shorter diagnostic delay (5.3 years for AS with IBD vs 7.3 years for AS without IBD). However, the diagnostic delay varied depending on the onset of IBD. In patients where IBD onset occurred after musculoskeletal symptoms, the diagnostic delay was longer (8 years) compared to those where IBD onset preceded musculoskeletal symptoms (3 years). We further observed that AS patients with IBD were older at diagnosis (38.3 years in IBD vs 35.3 in non-IBD) and had a lower frequency of enthesitis (31.4% in IBD vs 41.5% in non-IBD). Pharmacological treatment also differed, with a higher frequency of bDMARDs (21.1% vs 14.7%) and csDMARDs (47.9% vs 37.9%) in AS patients with IBD compared to those without IBD. However, no statistically significant differences were observed between the groups in disease activity as evaluated by the BASDAI and ASDAS (Table 2).
Table 2.
Clinical characteristics of patients with AS and inflammatory bowel disease compared to AS without inflammatory bowel disease.
| Total N = 2766 |
IBD N = 142 |
No IBD N = 2624 |
p-Value | |
|---|---|---|---|---|
| Sex (male) | 2046 (74.0%) | 88 (62.0%) | 1958 (74.6%) | <0.001 |
| Age (years) | 47.2 (13.9) | 45.2 (16.0) | 47.3 (13.7) | 0.127 |
| Age at diagnosis (years) | 35.4 (13.5) | 38.3 (14.3) | 35.3 (13.4) | 0.013 |
| Time since symptoms onset | 19.5 (13.0) | 14.5 (11.2) | 19.8 (13.1) | <0.001 |
| Diagnosis delay | 7.4 (9.2) | 5.3 (8.0) | 7.5 (9.2) | 0.007 |
| BMI | 26.4 (4.5) | 26.0 (4.2) | 26.4 (4.5) | 0.278 |
| HLA-B27 positive | 1573/1999 (78.7%) | 58/103 (56.3%) | 1515/1896 (79.9%) | <0.001 |
| Inflammatory back pain | 2670/2763 (96.6%) | 134 (93.4%) | 2536/2621 (96.8%) | 0.144 |
| Alternating buttock pain | 1658/2744 (60.4%) | 90/141 (63.8%) | 1568/2603 (60.2%) | 0.396 |
| Arthritis ever | 1207/2760 (43.7%) | 61 (43.0%) | 1146/2618 (43.8%) | 0.849 |
| Enthesitis ever | 1125/2745 (41.0%) | 43/137 (31.4%) | 1082/2608 (41.5%) | 0.019 |
| Dactylitis ever | 238/2754 (8.6%) | 13/141 (9.2%) | 225/2613 (8.6%) | 0.802 |
| Psoriasis | 278/2757 (10.1%) | 12/140 (8.6%) | 266/2617 (10.2%) | 0.542 |
| Nail disease | 128/2699 (4.7%) | 5/136 (3.7%) | 123/2563 (4.8%) | 0.549 |
| Uveitis | 595/2746 (21.7%) | 32 (22.5%) | 563/2604 (21.6%) | 0.797 |
| bDMARD ever | 417 (15.1%) | 30 (21.1%) | 387 (14.7%) | 0.039 |
| csDMARD ever | 1062 (38.4%) | 68 (47.9%) | 994 (37.9%) | 0.017 |
| CRP | 9.3 (16.8) | 8.5 (11.5) | 9.3 (17.0) | 0.583 |
| ESR | 21.0 (18.3) | 23.0 (20.4) | 20.8 (18.2) | 0.190 |
| BASRI sacroiliac joint | 3.0 (1.0) | 2.6 (1.2) | 3.1 (1.0) | <0.001 |
| BASRI lumbar | 1.8 (1.4) | 1.2 (1.4) | 1.8 (1.4) | <0.001 |
| BASRI cervical | 1.5 (1.5) | 1.1 (1.4) | 1.5 (1.5) | <0.001 |
| BASRI spine | 6.4 (3.4) | 5.0 (3.6) | 6.4 (3.4) | <0.001 |
| VAS global | 4.7 (2.7) | 4.5 (2.9) | 4.7 (2.8) | 0.380 |
| BASDAI | 4.2 (2.4) | 4.0 (2.5) | 4.2 (2.4) | 0.274 |
| ASDAS-CRP | 2.6 (1.1) | 2.5 (1.1) | 2.6 (1.1) | 0.267 |
Continuous variables are described by their mean and the standard deviation (between brackets), while absolute and percentages are shown for categorical variables. Variables included in the multiple logistic regression analysis: sex, age at diagnosis (years), time since symptoms onset, HLA, enthesitis ever, bDMARD ever, csDMARD ever, BASRI hip, BASRI sacroiliac, BASRI lumbar, and BASRI cervical.
AS, ankylosing spondylitis; BMI, body mass index; IBD, inflammatory bowel disease; bDMARDs, biological disease-modifying antirheumatic drugs; csDMARD, conventional synthetic disease-modifying antirheumatic drugs; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; BASRI, Bath Ankylosing Spondylitis Radiology Index; VAS, visual analog scale; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; ASDAS, Ankylosing Spondylitis Disease Activity Score.
Regarding structural damage, there were also significant differences, with a lower BASRI score for SIJs (2.6 vs 3.1) and BASRI score of the spine (5 vs 6.4) in AS patients with IBD compared to those without IBD. These differences were even present when differentiated cervical and lumbar spine, with a lower BASRI score of the lumbar spine (1.2 vs 1.8) and BASRI score of the cervical spine (1.1 vs 1.5) in AS patients with IBD compared to those without IBD (Table 2).
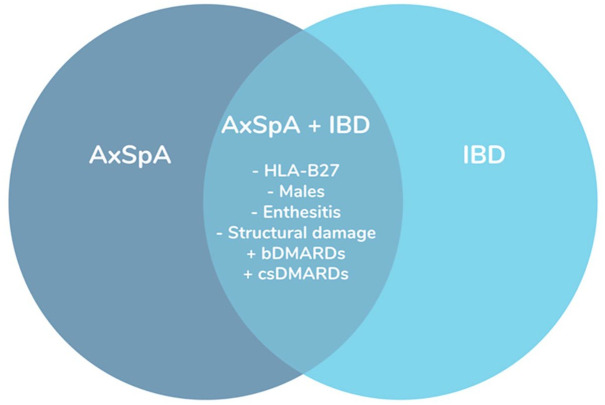
After adjusting for time since symptoms onset and bDMARDs, the multivariable analysis showed that the presence of IBD was significantly associated with a lower prevalence of HLA-B27 and enthesitis, with ORs of 0.32 (95% CI: 0.20–0.53) and 0.56 (95% CI: 0.32–0.94), respectively. Furthermore, structural damage in SIJs (BASRI) was significantly decreased in patients with IBD, with an OR of 0.78 (95% CI: 0.63–0.98) (Figure 1).
Figure 1.
Clinical picture of patients with axial spondyloarthritis and concomitant inflammatory bowel disease.
AxSpA, axial spondyloarthritis; bDMARDs, biological disease-modifying antirheumatic drugs; csDMARD, conventional synthetic disease-modifying antirheumatic drugs; IBD, inflammatory bowel disease.
When we analyzed the contribution of the HLA-B27 gene in patients with AS and concomitant IBD, we found that patients with HLA-B27 positive were younger at diagnosis (33.4 years in HLA-B27 positive vs 42.3 in HLA-B27 negative) and presented less nail disease than those with HLA-B27 negative (0% in HLA-B27 positive vs 8.9% in HLA-B27 negative). We did not observe any other notable differences in clinical characteristics, gender, disease activity, structural damage, or treatment concerning HLA-B27 status (Supplemental Table S2). After adjusting for time since symptoms onset, the multivariable analysis only showed that younger ager at diagnose was significantly associated with the positivity of HLA-B27, with ORs of 0.94 (95% CI: 0.90–0.97).
Discussion
Our study, which includes a substantial cohort of 2766 AS patients from two large registries (REGISPONSER and RESPONDIA), offers valuable insights into the clinical characteristics of AS patients with concomitant IBD. The prevalence of IBD among AS patients was 5.13%. Notably, we observed a lower prevalence of HLA-B27 and a lower proportion of male patients, which is consistent with existing literature.2,3,16 Moreover, our study provides new insights into the extent of structural damage in AS patients with concomitant IBD.
When comparing the clinical characteristics of AS patients with and without IBD, our study identified several important differences. Although age and BMI were comparable between the groups, those with both AS and IBD were less likely to be male and had a lower prevalence of HLA-B27 positivity. These findings align with a previous systematic review, 16 which demonstrated a positive association between IBD in AS patients and a higher proportion of women. In addition, previous studies have similarly reported a lower prevalence of HLA-B27 in AS patients with concurrent IBD (25%–78%) compared to patients without IBD (85%–90%).2,5,17–19
In our study, we further observed that these patients experienced a shorter diagnostic delay, were older at the time of diagnosis, and displayed a lower frequency of enthesitis. A previous study 17 reported a longer diagnostic delay in patients with SpA and IBD compared to those without IBD; notably, this study included all forms of SpA, including psoriatic arthritis. Another recent large retrospective study 20 reported that a longer diagnostic delay was associated with a higher probability of uveitis and IBD in AS patients. However, in that study, patients who presented with IBD before the SpA diagnosis were excluded. Although we do not have a clear explanation for this discrepancy, we found that the diagnostic delay varied depending on the onset of IBD. In patients where IBD onset occurred after musculoskeletal symptoms, the diagnostic delay was longer (8 years), as reported in previous research.16,18 Conversely, in patients where IBD onset preceded musculoskeletal symptoms, the diagnostic delay was shorter (3 years). In our data, a significant proportion of patients (32.95%) had IBD prior to the onset of musculoskeletal symptoms. This may explain the shorter diagnostic delay we observed in AS patients with IBD in our study and suggests that effective collaboration between gastroenterologists and rheumatologists is helping in the early recognition of SpA in patients presenting first with IBD.
Notably, as expected, and consistent with previous data, 3 pharmacological treatment patterns also differed, with a higher utilization of both bDMARDs and csDMARDs in those with IBD.
A prior study 3 reported higher disease activity in patients with axSpA with a history of IBD; however, the presence of current IBD did not show a significant association with disease activity or functional status. Interestingly, in our study, we did not find any statistically significant differences in disease activity assessed by BASDAI and ASDAS, nor in terms of functionality measured by BASFI between both groups. This suggests that while clinical presentation and treatment patterns may vary, the overall disease activity, at least as captured by these indices, remains comparable. Nevertheless, we must be cautious with these data because they come from a cross-sectional study.
Regarding structural damage, our results showed that patients with AS and IBD exhibited lower BASRI scores for both the SIJs and spine compared to those without IBD, even after adjusting for disease duration. This finding was consistent when examining specific regions of the spine, with lower BASRI scores for both the lumbar and cervical spine in patients with IBD. It is important to note that our study is the first to evaluate structural damage in patients with SpA with and without an association with IBD, providing new insights into structural damage in patients with AS and IBD.
The role of HLA-B27 in the phenotypic expression of axSpA is widely known; however, its role in patients with AS and concomitant IBD remains unknown. In our study, we found that the presence of HLA-B27 does not seem to contribute to a specific clinical differential profile between the two groups, except for its association with a younger age at diagnosis.
Our study has several limitations. First, this is an observational cross-sectional study including the collection of some data retrospectively, which inherently limits the ability to conclude causality or temporal relationships between variables. In addition, there is a high number of patients with AS, but the sample size for AS with IBD is modest, which may limit the ability to analyze certain associations. Furthermore, the radiographic assessment was evaluated using the BASRI score, not the modified Stoke Ankylosing Spondylitis Spine Score, which is the preferred method. 21 Another limitation is the lack of central reading of the radiographs, with multiple readers involved. However, it is important to note that the local readers participating in the REGISPONSER and RESPONDIA registries are rheumatologists with a special interest in SpA.
Conclusion
Our study provides comprehensive insights into the clinical characteristics and structural damage of patients with r-axSpA and concurrent IBD. The distinct features observed in this subgroup, such as reduced prevalence of HLA-B27, enthesitis, and structural damage, underscore the complexity of the relationship between these two conditions. Our findings contribute to the evolving understanding of the heterogeneity within the spectrum of axSpA and emphasize the importance of considering concomitant IBD as a relevant factor in the management of these patients.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X241303316 for Inflammatory bowel disease in axial spondyloarthritis patients. Is there any specific clinical picture? Data from the RESPONDIA and REGISPONSER registries by Maria Llop, Ignacio Gómez-García, Jordi Gratacós, Albert Villoria, Joan Calvet, Mireia Moreno, Marta Arévalo, Montserrat Cabanillas-Paredes, Eduardo Collantes-Estévez, Janitzia Vazquez-Mellado and Clementina López-Medina in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors would like to thank all the investigators from the REGISPONSER and RESPONDIA study groups. REGISPONSER: P. Zarco-Montejo, Hospital Fundación Alcorcón, Madrid; C. González, Hospital Gregorio Marañón, Madrid; J. Mulero-Mendoza, Hospital Puerta de Hierro, Madrid; J. L. Fernández Sueiro, Hospital Juan Canalejo, La Coruña; R. Almodóvar, Hospital Fundación Alcorcón, Madrid; J. Gratacós-Masmitjá, Hospital Parc Taulí, Barcelona; X. Juanola Roura, Hospital Bellvitge, Barcelona; C. Montilla, Hospital Virgen de la Vega, Salamanca; E. Moreno, Hospital San Rafael, Barcelona; A. Juan-Mas, Hospital Fundación Son Llatzer, Mallorca; P. Fernández-Dapica, Hospital 12 de Octubre, Madrid; M. C. Fernández-Espartero, Hospital de Móstoles, Madrid; V. Villaverde, Hospital de Móstoles, Madrid; M. E. Brito-Brito, Hospital Universitario Ramón y Cajal, Madrid; J. C. Torre-Alonso, Hospital Monte Naranco, Oviedo; E. Batlle-Gualda, Hospital General Universitario, Alicante; E. Cuende-Quintana, Hospital Universitario Príncipe de Asturias, Madrid; T. Clavaguera-Poch, Hospital de Palamós, Girona; M. Fernández-Prada, Hospital Universitario de Guadalajara, Guadalajara; and E. Judez Navarro, Hospital Virgen del Perpetuo Socorro, Albacete. RESPONDIA: A. Alvarellos, Hospital Privado de Córdoba, Córdoba, Argentina; C. Asnal, Hospital Alemán, Buenos Aires, Argentina; J. C. Barreira, Hospital Británico, Buenos Aires, Argentina; A. G. Bernard Medina, Hospital F. Antonio Alcalde, Guadalajara, México; M. B. Bertolo, Universidade Campinas, Brazil; W. A. Bianchi, Santa Casa do Rio de Janeiro, Brazil; R. Bonfiglioli, Pontifícia Universidade Católica de Campinas, Brazil; S. Carneiro, Universidade Federal do Rio de Janeiro, Brazil; H. M. S. Carvalho, Hospital de Base, Brasília, Brazil; G. C. Casado, Hospital Militar Central, Buenos Aires, Argentina; J. Casasola Vargas, Hospital General de México, México City, México; F. A. Castro da Rocha, Universidade Federal do Ceará, Fortaleza, Brazil; R. L. Chacón, Policlínica Méndez Gimón, Caracas, Venezuela; I. P. Costa, Universidade Federal do Mato Grosso do Sul, Campo Grande, Brazil; A. P. Duarte, Universidade Federal de Pernambuco, Recife, Brazil; J. Espinoza-Villalpando, Hospital Regional PEMEX, Reynosa, México; M. H. Esteva, Hospital Central San Cristóbal, San Cristóbal, Táchira, Venezuela; C. Fuentealba, Hospital San Borja Arriarán, Santiago, Chile; Y. Granados, Hospital Núñez Tovar, Maturín Monagas, Venezuela; G. Huerta-Sil, CLIDITER, México, México; M. Keiserman, Pontifícia Universidade Católica de Porto Alegre, Brazil; C. L. Kohem, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; N. H. Leite, Faculdade Souza Marques, Rio de Janeiro, Brazil; S. A. L. Lima, Hospital do Servidor Público Estadual de São Paulo, São Paulo, Brazil; J. A. Maldonado-Cocco, IREP, Buenos Aires, Argentina; E. S. Meirelles, Universidade de São Paulo, Brazil; R. Menin, Faculdade de Medicina de São José do Rio Preto, Brazil; O. Neira, Hospital del Salvador, Santiago, Chile; S. Paira, Hospital JM Cullen, Santa Fé, Argentina; F. Pimentel, Complexo Hospitalar Egas Moniz, Lisbon, Portugal; M. Pinheiro, Universidade Federal de São Paulo, Brazil; E. Polito, Santa Casa de Belo Horizonte, Brazil; G. Resende, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; S. L. E. Ribeiro, Universidade Federal do Amazonas, Manaus, Brazil; O. L. Rillo, Hospital Tornú, Buenos Aires, Argentina; M. B. Santiago, Escola de Saúde Pública da Bahia, Salvador, Brazil; H. Santos, Instituto Portugués de Reumatología, Lisboa, Portugal; H. Scherbarth, Mar del Plata, Argentina; M. F. L. C. Sauma, Universidade Federal do Pará, Belém, Brazil; T. L. Skare, Hospital Evangélico de Curitiba, Brazil; E. Sousa, Complexo Hospitalar Lisboa Norte, Lisboa, Portugal; E. Spangenberg, Instituto Nacional de Reumatología, Montevideo, Uruguay; V. Valin, Universidade Federal do Espírito Santo, Vitória, Brazil; C. Vera, Hospital Luis Vernaza, Guayaquil, Ecuador; U. Verdejo, Hospital Carlos van Buren, Valparaíso, Chile; W. P. Vieira, Hospital Geral de Fortaleza, Brazil; R. Wong, S. Plaza, Rosario, Argentina. The authors would like to thank all the investigators from the REGISPONSER and RESPONDIA study groups.
Appendix
Abbreviations
AS ankylosing spondylitis
ASDAS Ankylosing Spondylitis Disease Activity Score
AxSpA axial spondyloarthritis
BASDAI Bath Ankylosing Spondylitis Disease Activity Index
BASFI Bath Ankylosing Spondylitis Functional Index
BASRI Bath Ankylosing Spondylitis Radiology Index
bDMARDs biological disease-modifying antirheumatic drugs
BMI body mass index
CD Crohn’s disease
CRP C-reactive protein
csDMARDs conventional disease-modifying antirheumatic drugs
ESSG European Spondyloarthropathy Study Group
IBD inflammatory bowel disease
OR odds ratio
R-axSpA radiographic axial SpA
SD standard deviation
SIJ sacroiliac joints
UC ulcerative colitis
Footnotes
ORCID iDs: Maria Llop  https://orcid.org/0000-0001-5581-0965
https://orcid.org/0000-0001-5581-0965
Ignacio Gómez-García  https://orcid.org/0000-0002-4607-5644
https://orcid.org/0000-0002-4607-5644
Jordi Gratacós  https://orcid.org/0000-0003-4007-4103
https://orcid.org/0000-0003-4007-4103
Joan Calvet  https://orcid.org/0000-0002-0888-5152
https://orcid.org/0000-0002-0888-5152
Clementina López-Medina  https://orcid.org/0000-0002-2309-5837
https://orcid.org/0000-0002-2309-5837
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Maria Llop, Department of Rheumatology, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Universitat Autònoma de Barcelona, Sabadell, Barcelona, Spain.
Ignacio Gómez-García, Department of Rheumatology, Hospital Universitario Reina Sofía/IMIBIC/Universidad de Córdoba, Córdoba, Spain.
Jordi Gratacós, Department of Rheumatology, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Universitat Autònoma de Barcelona, Sabadell, Barcelona, Spain.
Albert Villoria, Department of Gastroenterology, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Universitat Autònoma de Barcelona, Sabadell, Barcelona, Spain.
Joan Calvet, Department of Rheumatology, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Universitat Autònoma de Barcelona, Sabadell, Barcelona, Spain.
Mireia Moreno, Department of Rheumatology, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Universitat Autònoma de Barcelona, Sabadell, Barcelona, Spain.
Marta Arévalo, Department of Rheumatology, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Universitat Autònoma de Barcelona, Sabadell, Barcelona, Spain.
Montserrat Cabanillas-Paredes, Department of Rheumatology, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí (I3PT-CERCA), Universitat Autònoma de Barcelona, Sabadell, Barcelona, Spain.
Eduardo Collantes-Estévez, Department of Medicine, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Córdoba, Spain.
Janitzia Vazquez-Mellado, Department of Rheumatology, Hospital General de Mexico, Mexico City, Mexico.
Clementina López-Medina, Department of Rheumatology, Hospital Universitario Reina Sofía/IMIBIC/Universidad de Córdoba, Córdoba, Spain.
Declarations
Ethics approval and consent to participate: This study was conducted according to the guidelines for Good Clinical Practice and was approved by the ethical committee from the Reina Sofia University Hospital (“Comisión de Ética e Investigación Sanitarias, REGISPON-2004y RESPONDIA-2006”). Written informed consent was obtained from all subjects before enrolment.
Consent for publication: Not applicable.
Author contributions: Maria Llop: Investigation; Methodology; Writing – original draft.
Ignacio Gómez-García: Conceptualization; Investigation; Methodology; Writing – review & editing.
Jordi Gratacós: Investigation; Supervision; Writing – review & editing.
Albert Villoria: Investigation; Supervision; Writing – review & editing.
Joan Calvet: Conceptualization; Investigation; Methodology; Supervision; Writing – review & editing.
Mireia Moreno: Data curation; Investigation; Writing – review & editing.
Marta Arévalo: Data curation; Investigation; Writing – review & editing.
Montserrat Cabanillas-Paredes: Data curation; Investigation; Writing – review & editing.
Eduardo Collantes-Estévez: Conceptualization; Investigation; Methodology; Supervision; Writing – review & editing.
Janitzia Vazquez-Mellado: Conceptualization; Data curation; Writing – review & editing.
Clementina López-Medina: Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017; 390(10089): 73–84. [DOI] [PubMed] [Google Scholar]
- 2. Schwartzman M, Ermann J, Kuhn KA, et al. Spondyloarthritis in inflammatory bowel disease cohorts: systematic literature review and critical appraisal of study designs. RMD Open 2022; 8(1): e001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Redeker I, Siegmund B, Ghoreschi K, et al. The impact of extra-musculoskeletal manifestations on disease activity, functional status, and treatment patterns in patients with axial spondyloarthritis: results from a nationwide population-based study. Ther Adv Musculoskelet Dis 2020; 12: 1759720X20972610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Essers I, Ramiro S, Stolwijk C, et al. Characteristics associated with the presence and development of extra-articular manifestations in ankylosing spondylitis: 12-year results from OASIS. Rheumatology (Oxford) 2015; 54(4): 633–640. [DOI] [PubMed] [Google Scholar]
- 5. Reveille JD. HLA-B27 and the seronegative spondyloarthropathies. Am J Med Sci 1998; 316(4): 239–249. [DOI] [PubMed] [Google Scholar]
- 6. McEwen C, DiTata D, Lingg C, et al. Ankylosing spondylitis and spondylitis accompanying ulcerative colitis, regional enteritis, psoriasis and Reiter’s disease. A comparative study. Arthritis Rheum 1971; 14(3): 291–318. [DOI] [PubMed] [Google Scholar]
- 7. Collantes E, Zarco P, Muñoz E, et al. Disease pattern of spondyloarthropathies in Spain: description of the first national registry (REGISPONSER) extended report. Rheumatology (Oxford) 2007; 46(8): 1309–1315. [DOI] [PubMed] [Google Scholar]
- 8. Vázquez-Mellado J, Ugalde PF, Gomáriz EM, et al. IberoAmerican Spondylarthritis Registry (RESPONDIA): what is, how came about, who we are and what we do. Reumatol Clin 2008; 4: S17–S22. [Google Scholar]
- 9. Dougados M, van der Linden S, Juhlin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991; 34(10): 1218–1227. [DOI] [PubMed] [Google Scholar]
- 10. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147(8): 573–577. [DOI] [PubMed] [Google Scholar]
- 11. Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994; 21(12): 2286–2291. [PubMed] [Google Scholar]
- 12. Lukas C, Landewé R, Sieper J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009; 68(1): 18–24. [DOI] [PubMed] [Google Scholar]
- 13. Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index.J Rheumatol 1994; 21(12): 2281–2285. [PubMed] [Google Scholar]
- 14. MacKay K, Mack C, Brophy S, et al. The Bath Ankylosing Spondylitis Radiology Index (BASRI): a new, validated approach to disease assessment. Arthritis Rheum 1998; 41(12): 2263–2270. [DOI] [PubMed] [Google Scholar]
- 15. R Core Team. R: A language and environment for statistical computing. Austria: R Foundation for Statistical Computing V, https://www.R-project.org/ (2019). [Google Scholar]
- 16. Stolwijk C, van Tubergen A, Castillo-Ortiz JD, et al. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74(1): 65–73. [DOI] [PubMed] [Google Scholar]
- 17. Ono K, Kishimoto M, Deshpande GA, et al. Clinical characteristics of patients with spondyloarthritis and inflammatory bowel disease versus inflammatory bowel disease-related arthritis. Rheumatol Int 2022; 42(10): 1751–1766. [DOI] [PubMed] [Google Scholar]
- 18. Rudwaleit M, Baeten D. Ankylosing spondylitis and bowel disease. Best Pract Res Clin Rheumatol 2006; 20(3): 451–471. [DOI] [PubMed] [Google Scholar]
- 19. Cozzi G, Scagnellato L, Lorenzin M, et al. Spondyloarthritis with inflammatory bowel disease: the latest on biologic and targeted therapies. Nat Rev Rheumatol 2023; 19(8): 503–518. [DOI] [PubMed] [Google Scholar]
- 20. Michelena X, Zhao SS, Marco-Pascual C, et al. Diagnostic delay is associated with uveitis and inflammatory bowel disease in AS: a study of extra-musculoskeletal manifestations in SpA. Rheumatology (Oxford) 2024; 63(2): 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Creemers MC, Franssen MJ, van’t Hof MA, et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005; 64(1): 127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X241303316 for Inflammatory bowel disease in axial spondyloarthritis patients. Is there any specific clinical picture? Data from the RESPONDIA and REGISPONSER registries by Maria Llop, Ignacio Gómez-García, Jordi Gratacós, Albert Villoria, Joan Calvet, Mireia Moreno, Marta Arévalo, Montserrat Cabanillas-Paredes, Eduardo Collantes-Estévez, Janitzia Vazquez-Mellado and Clementina López-Medina in Therapeutic Advances in Musculoskeletal Disease