Abstract
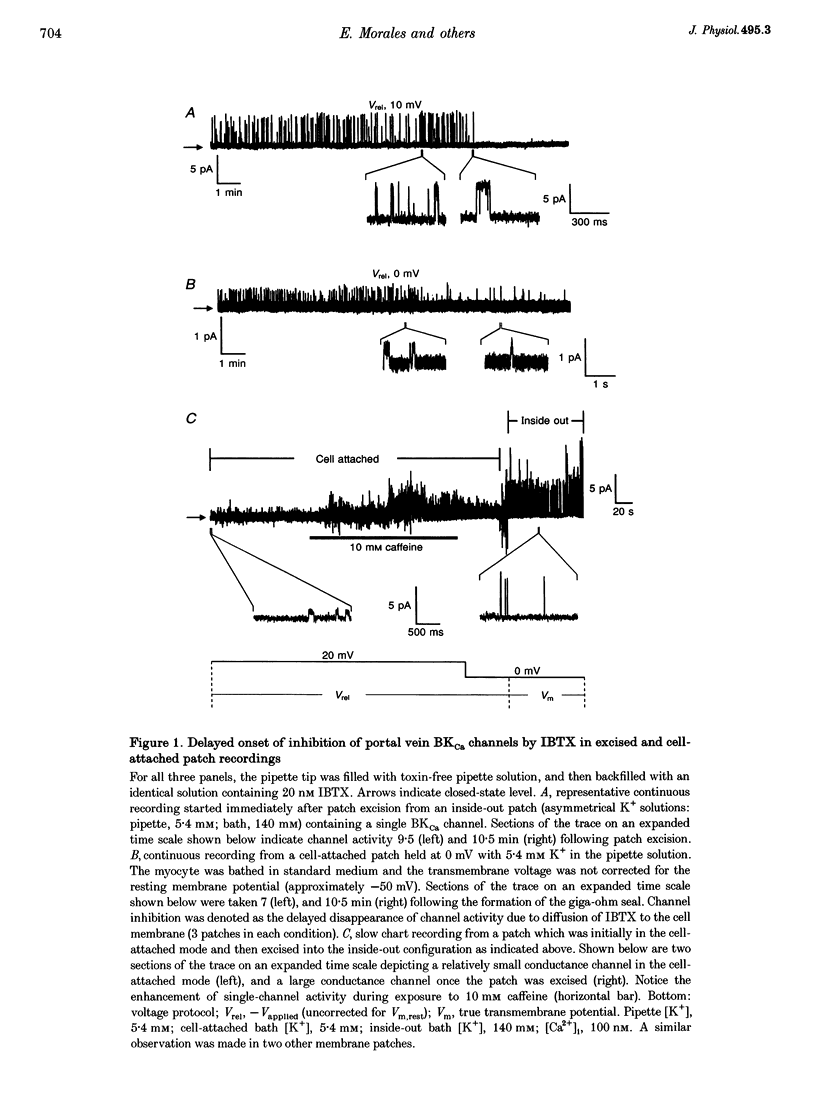
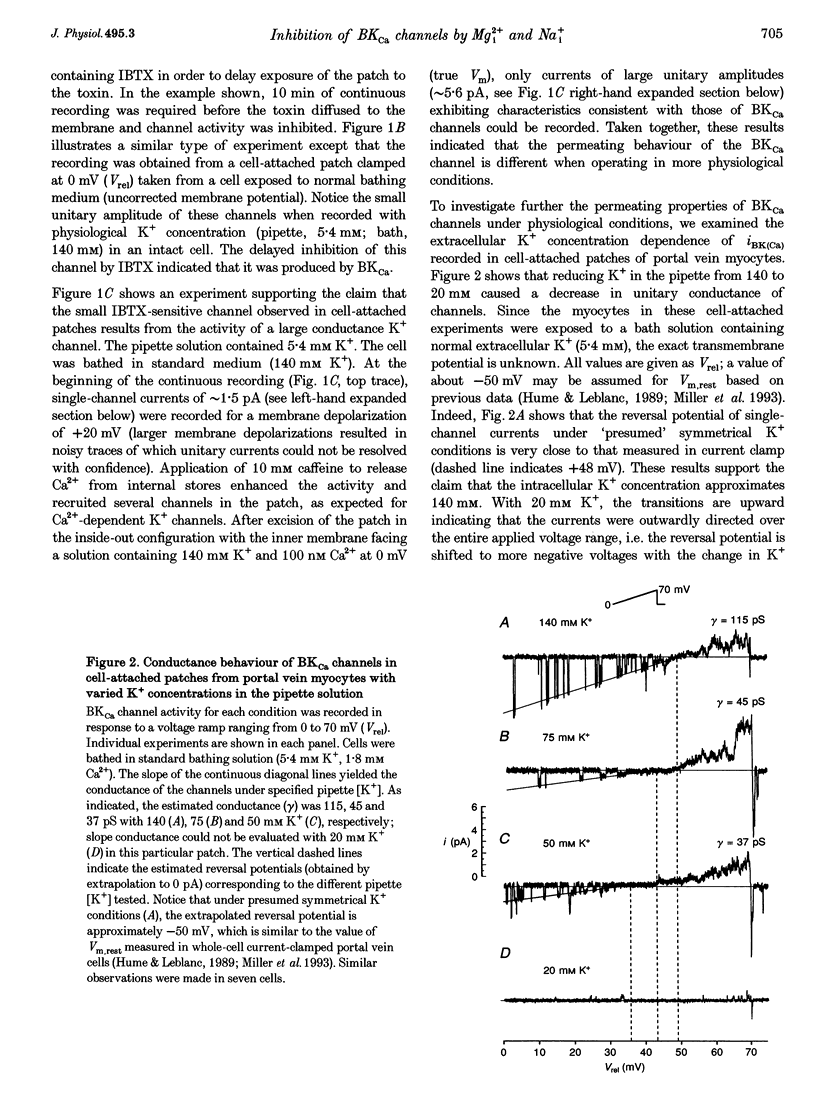
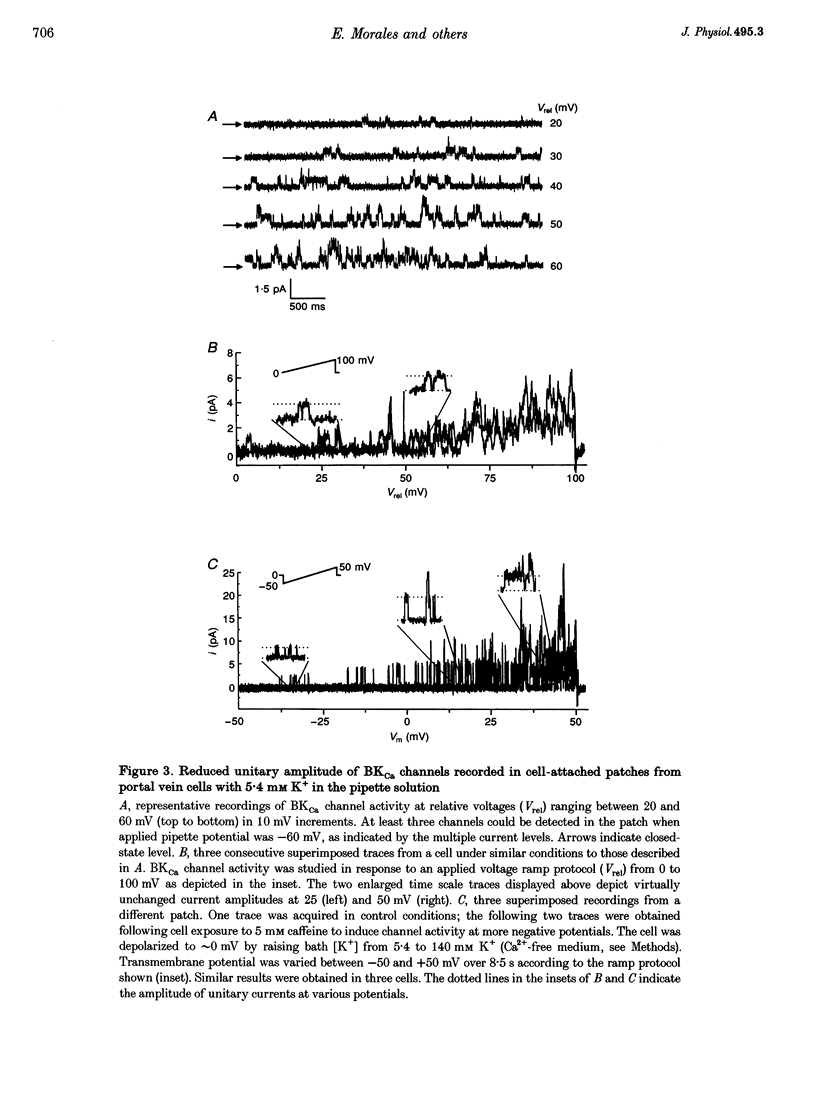
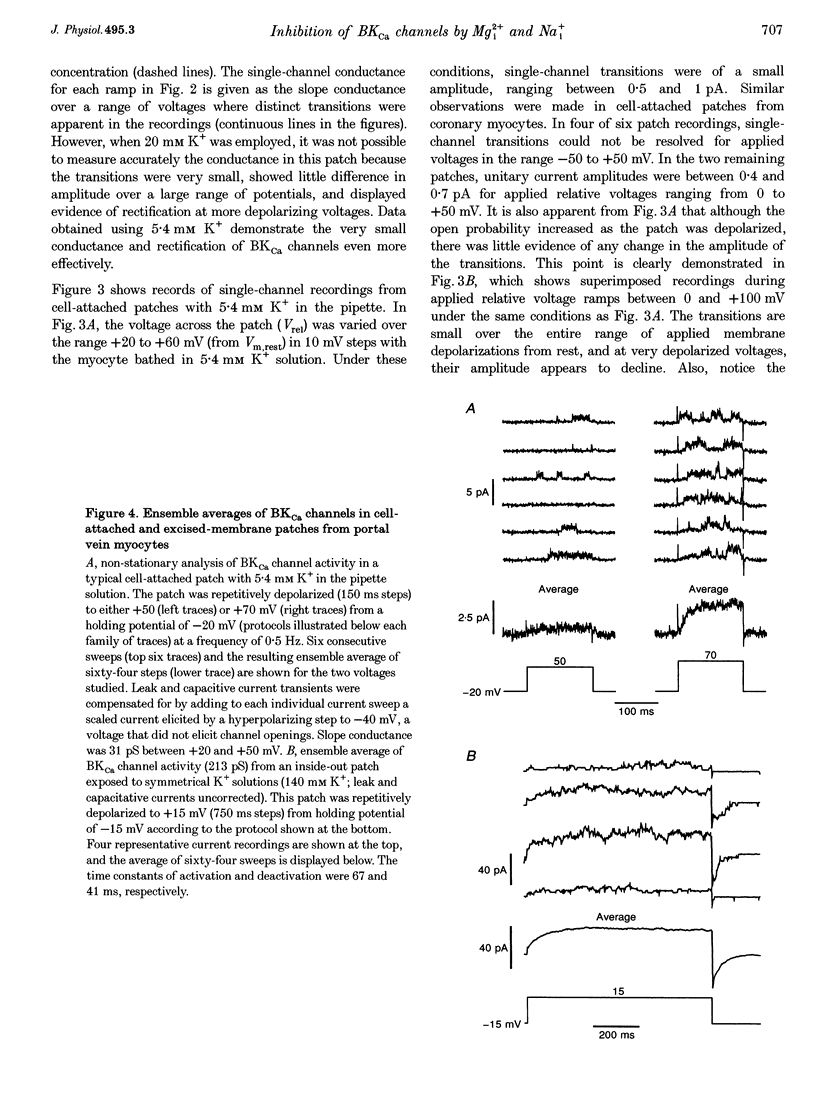
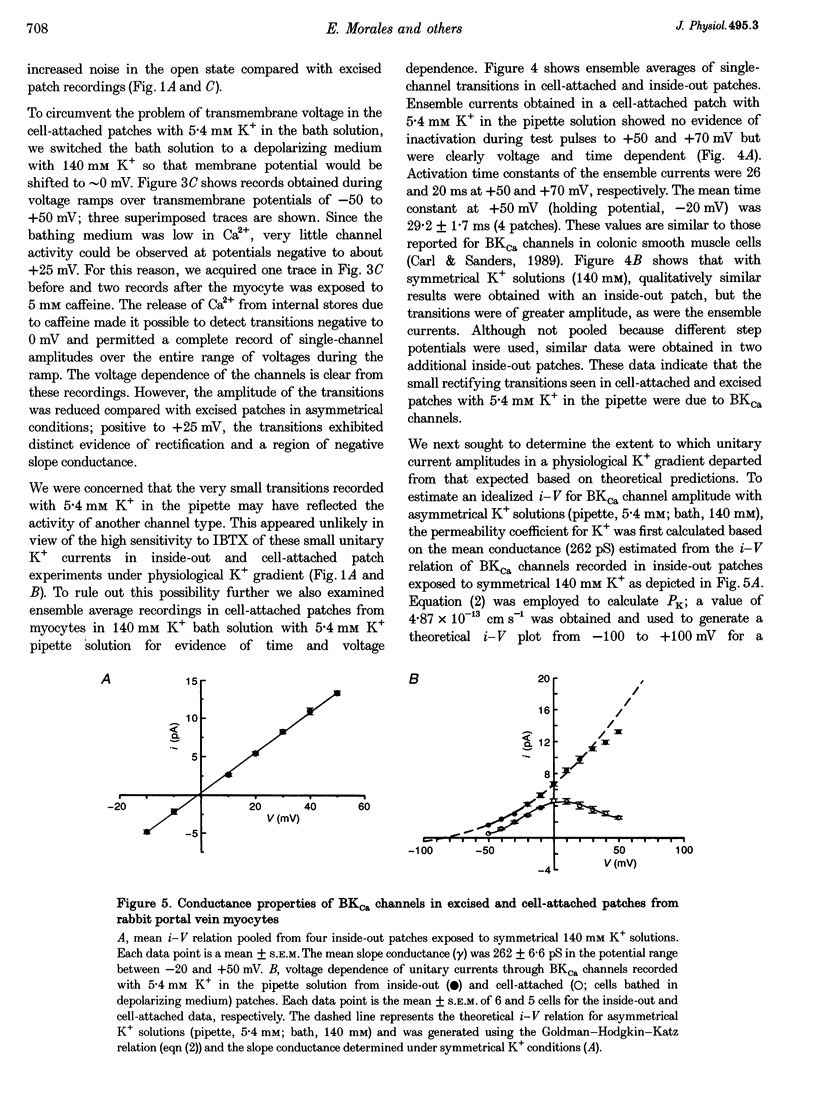
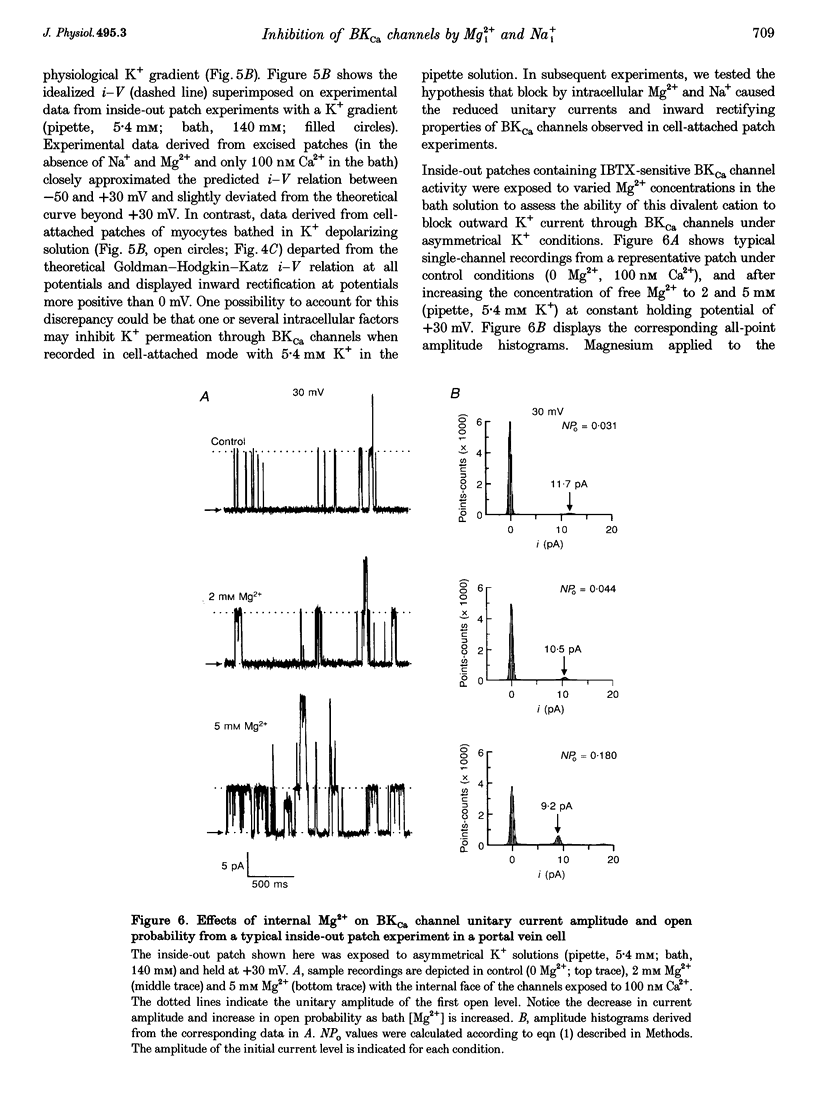
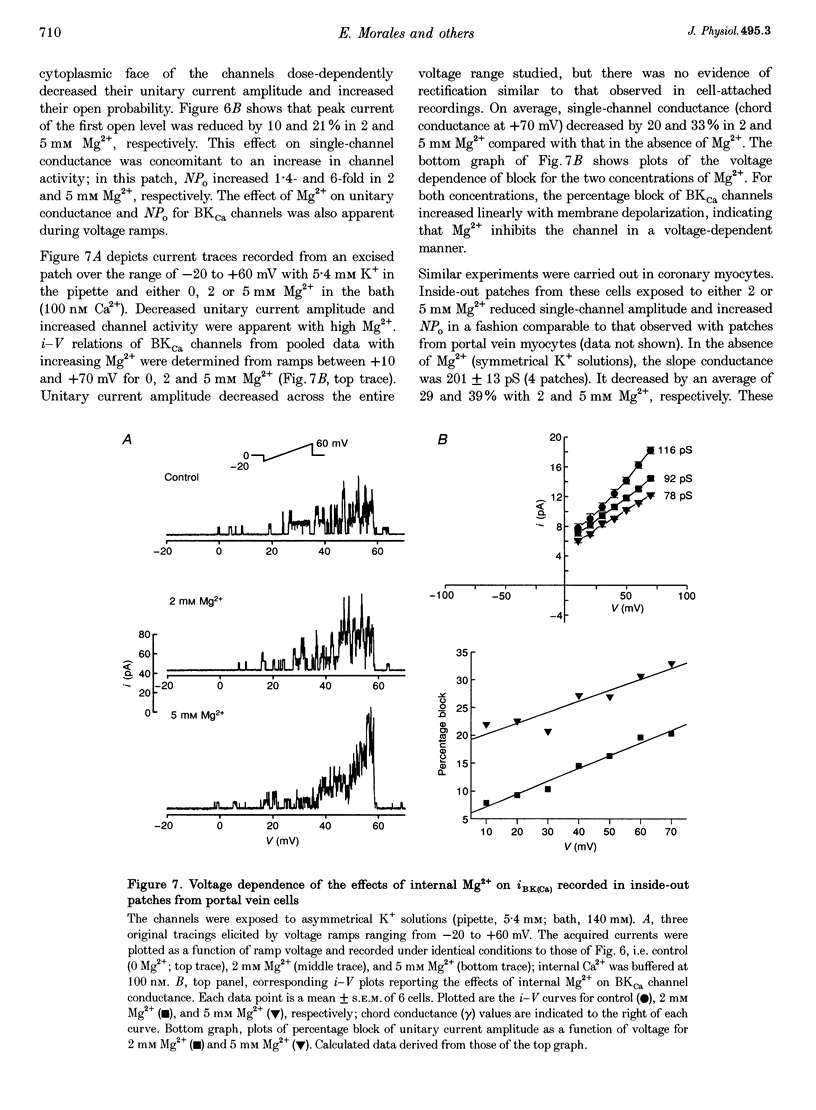
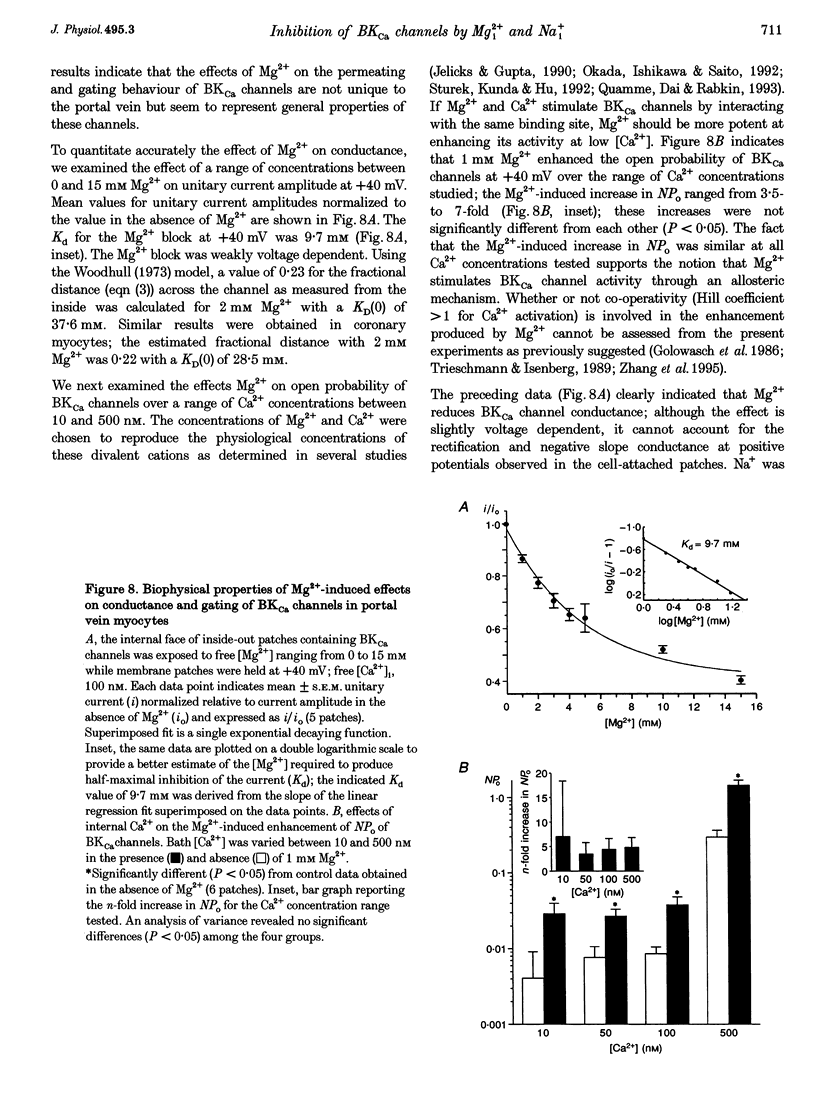
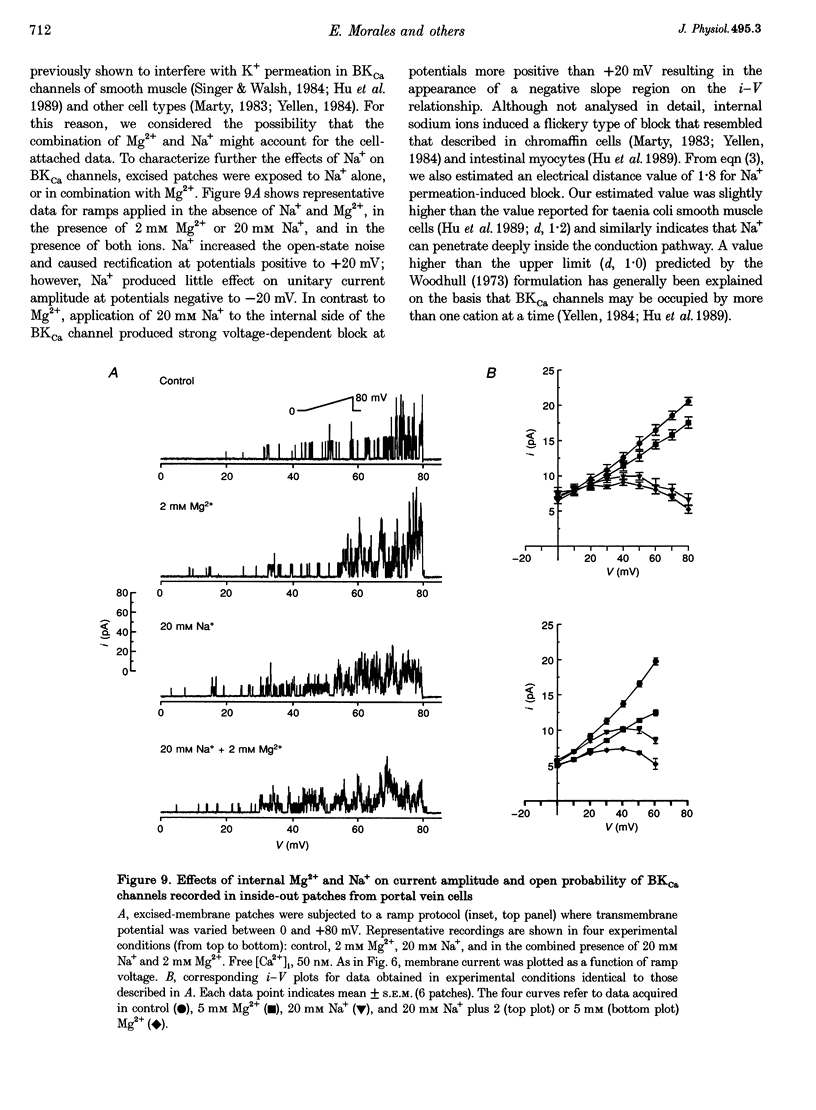
1. We studied the biophysical properties of single large conductance (> 200 pS in symmetrical K+ pipette and bath solutions) Ca(2+)-activated K+ (BKca) channels of rabbit portal vein and coronary arterial smooth muscle cells using the cell-attached and inside-out variants of the patch-clamp technique (at 22 degrees C). 2. The unitary conductance of BKca channels recorded in cell-attached patches with K+ concentrations in the range 5.4-140 mM was significantly lower than that predicted on the basis of the conductance measured in inside-out patches with symmetrical K+ pipette and bath solutions (140 mM) and the constant field equation. In cell-attached patches from cells bathed in depolarizing medium (140 mM) with 5.4 mM K+ in the pipette solution, BKca channels were difficult to detect on the physiological range of membrane potentials (approximately -50 mV). Unitary currents were smaller at all voltages in the range -50 to 0 mV and the i-V relationship exhibited strong inward rectification at potentials > 0 mV. These channels were unequivocally identified as BKca channels due to their sensitivity to caffeine (10 mM) and iberiotoxin (20 nM), and their non-stationary kinetic properties. 3. Exposure of the cytoplasmic side of excised patches to [Mg2+] in the range 0-15 mM produced two effects on BKca channel activity: the slope conductance and open probability were reduced and enhanced, respectively, in a concentration-dependent manner by this cation. The Mg(2+)-induced reduction in conductance exhibited weak voltage dependence. 4. Application of 20 mM Na+ to the internal face of BKca channels recorded in the inside-out configuration produced a flickery block at potentials > or = +20 mV resulting in reduced unitary current amplitudes and strong inward rectification of the i-V relationship. Exposure of inside-out patches to a combination of 20 mM Na+ and 2 mM Mg2+ further reduced unitary current amplitude to a level similar to the algebraic sum of the effect of each cation in isolation. 5. We conclude that Ca(2+)-dependent K+ channels of vascular smooth muscle cells display a lower unitary conductance when recorded under physiological conditions than that previously estimated on the basis of their behaviour in excised membrane patches. Our data indicate that the decreased permeation through BKca channels may be partly attributed to block by intracellular Mg2+ and Na+, which appear to interact with distinct binding sites along the inner side of the pore.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashida T., Blaustein M. P. Regulation of cell calcium and contractility in mammalian arterial smooth muscle: the role of sodium-calcium exchange. J Physiol. 1987 Nov;392:617–635. doi: 10.1113/jphysiol.1987.sp016800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Carl A., Sanders K. M. Ca2+-activated K channels of canine colonic myocytes. Am J Physiol. 1989 Sep;257(3 Pt 1):C470–C480. doi: 10.1152/ajpcell.1989.257.3.C470. [DOI] [PubMed] [Google Scholar]
- Elam T. R., Lansman J. B. The role of Mg2+ in the inactivation of inwardly rectifying K+ channels in aortic endothelial cells. J Gen Physiol. 1995 Apr;105(4):463–484. doi: 10.1085/jgp.105.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fakler B., Brändle U., Glowatzki E., Weidemann S., Zenner H. P., Ruppersberg J. P. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995 Jan 13;80(1):149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Ferguson W. B. Competitive Mg2+ block of a large-conductance, Ca(2+)-activated K+ channel in rat skeletal muscle. Ca2+, Sr2+, and Ni2+ also block. J Gen Physiol. 1991 Jul;98(1):163–181. doi: 10.1085/jgp.98.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Linsdell P., Stanfield P. R. Unitary A-currents of rat locus coeruleus neurones grown in cell culture: rectification caused by internal Mg2+ and Na+. J Physiol. 1992;451:553–583. doi: 10.1113/jphysiol.1992.sp019179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A., Gimenez-Gallego G., Reuben J. P., Roy-Contancin L., Feigenbaum P., Kaczorowski G. J., Garcia M. L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [PubMed] [Google Scholar]
- Gelband C. H., Hume J. R. Ionic currents in single smooth muscle cells of the canine renal artery. Circ Res. 1992 Oct;71(4):745–758. doi: 10.1161/01.res.71.4.745. [DOI] [PubMed] [Google Scholar]
- Gelband C. H., Ishikawa T., Post J. M., Keef K. D., Hume J. R. Intracellular divalent cations block smooth muscle K+ channels. Circ Res. 1993 Jul;73(1):24–34. doi: 10.1161/01.res.73.1.24. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J., Kirkwood A., Miller C. Allosteric effects of Mg2+ on the gating of Ca2+-activated K+ channels from mammalian skeletal muscle. J Exp Biol. 1986 Sep;124:5–13. doi: 10.1242/jeb.124.1.5. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horie M., Irisawa H., Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol. 1987 Jun;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. L., Yamamoto Y., Kao C. Y. Permeation, selectivity, and blockade of the Ca2+-activated potassium channel of the guinea pig taenia coli myocyte. J Gen Physiol. 1989 Nov;94(5):849–862. doi: 10.1085/jgp.94.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Leblanc N. Macroscopic K+ currents in single smooth muscle cells of the rabbit portal vein. J Physiol. 1989 Jun;413:49–73. doi: 10.1113/jphysiol.1989.sp017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Potassium channels and their evolving gates. Nature. 1994 Sep 8;371(6493):119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- Jelicks L. A., Gupta R. K. NMR measurement of cytosolic free calcium, free magnesium, and intracellular sodium in the aorta of the normal and spontaneously hypertensive rat. J Biol Chem. 1990 Jan 25;265(3):1394–1400. [PubMed] [Google Scholar]
- Johnson E. M., Theler J. M., Capponi A. M., Vallotton M. B. Characterization of oscillations in cytosolic free Ca2+ concentration and measurement of cytosolic Na+ concentration changes evoked by angiotensin II and vasopressin in individual rat aortic smooth muscle cells. Use of microfluorometry and digital imaging. J Biol Chem. 1991 Jul 5;266(19):12618–12626. [PubMed] [Google Scholar]
- Kajioka S., Kitamura K., Kuriyama H. Guanosine diphosphate activates an adenosine 5'-triphosphate-sensitive K+ channel in the rabbit portal vein. J Physiol. 1991 Dec;444:397–418. doi: 10.1113/jphysiol.1991.sp018885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc N., Wan X., Leung P. M. Physiological role of Ca(2+)-activated and voltage-dependent K+ currents in rabbit coronary myocytes. Am J Physiol. 1994 Jun;266(6 Pt 1):C1523–C1537. doi: 10.1152/ajpcell.1994.266.6.C1523. [DOI] [PubMed] [Google Scholar]
- Marty A. Blocking of large unitary calcium-dependent potassium currents by internal sodium ions. Pflugers Arch. 1983 Feb;396(2):179–181. doi: 10.1007/BF00615524. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Morales E., Leblanc N. R., Cole W. C. Metabolic inhibition enhances Ca(2+)-activated K+ current in smooth muscle cells of rabbit portal vein. Am J Physiol. 1993 Dec;265(6 Pt 2):H2184–H2195. doi: 10.1152/ajpheart.1993.265.6.H2184. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Quayle J. M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995 Apr;268(4 Pt 1):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Okada K., Ishikawa S., Saito T. Cellular mechanisms of vasopressin and endothelin to mobilize [Mg2+]i in vascular smooth muscle cells. Am J Physiol. 1992 Oct;263(4 Pt 1):C873–C878. doi: 10.1152/ajpcell.1992.263.4.C873. [DOI] [PubMed] [Google Scholar]
- Quamme G. A., Dai L. J., Rabkin S. W. Dynamics of intracellular free Mg2+ changes in a vascular smooth muscle cell line. Am J Physiol. 1993 Jul;265(1 Pt 2):H281–H288. doi: 10.1152/ajpheart.1993.265.1.H281. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., McCarron J. G., Brayden J. E., Nelson M. T. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol. 1993 Nov;265(5 Pt 1):C1363–C1370. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- Singer J. J., Walsh J. V. Large conductance ca-activated k channels in smooth muscle cell membrane: reduction in unitary currents due to internal na ions. Biophys J. 1984 Jan;45(1):68–70. doi: 10.1016/s0006-3495(84)84112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturek M., Kunda K., Hu Q. Sarcoplasmic reticulum buffering of myoplasmic calcium in bovine coronary artery smooth muscle. J Physiol. 1992;451:25–48. doi: 10.1113/jphysiol.1992.sp019152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepel M., Holthues J., Neusser M., Golinski P., Zhu Z., Mehring N., Zidek W. Reduced cytosolic free sodium concentration in vascular smooth muscle cells from spontaneously hypertensive rats. Clin Sci (Lond) 1994 Jun;86(6):741–747. doi: 10.1042/cs0860741. [DOI] [PubMed] [Google Scholar]
- Trieschmann U., Isenberg G. Ca2+-activated K+ channels contribute to the resting potential of vascular myocytes. Ca2+-sensitivity is increased by intracellular Mg2+-ions. Pflugers Arch. 1989;414 (Suppl 1):S183–S184. doi: 10.1007/BF00582296. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Puil E., Mathers D. A. Effects of intracellular Mg2+ on the properties of large-conductance, Ca(2+)-dependent K+ channels in rat cerebrovascular smooth muscle cells. J Cereb Blood Flow Metab. 1995 Nov;15(6):1066–1074. doi: 10.1038/jcbfm.1995.133. [DOI] [PubMed] [Google Scholar]


