Abstract
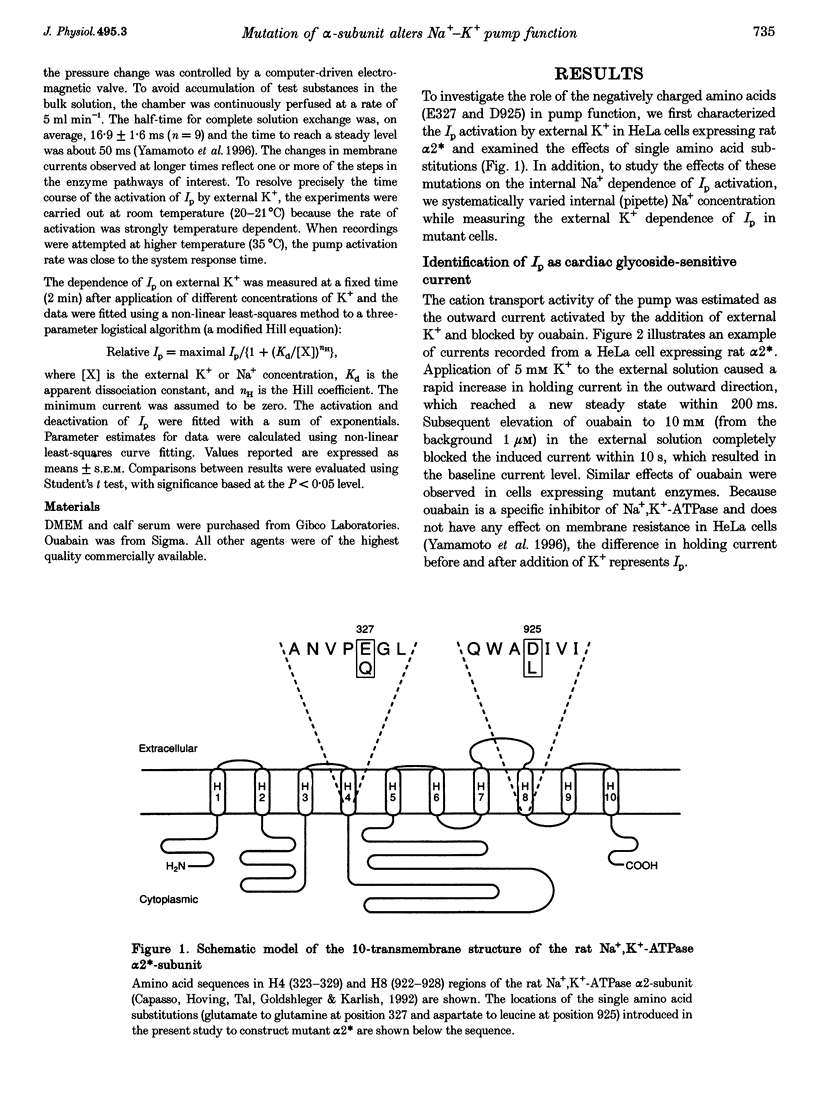
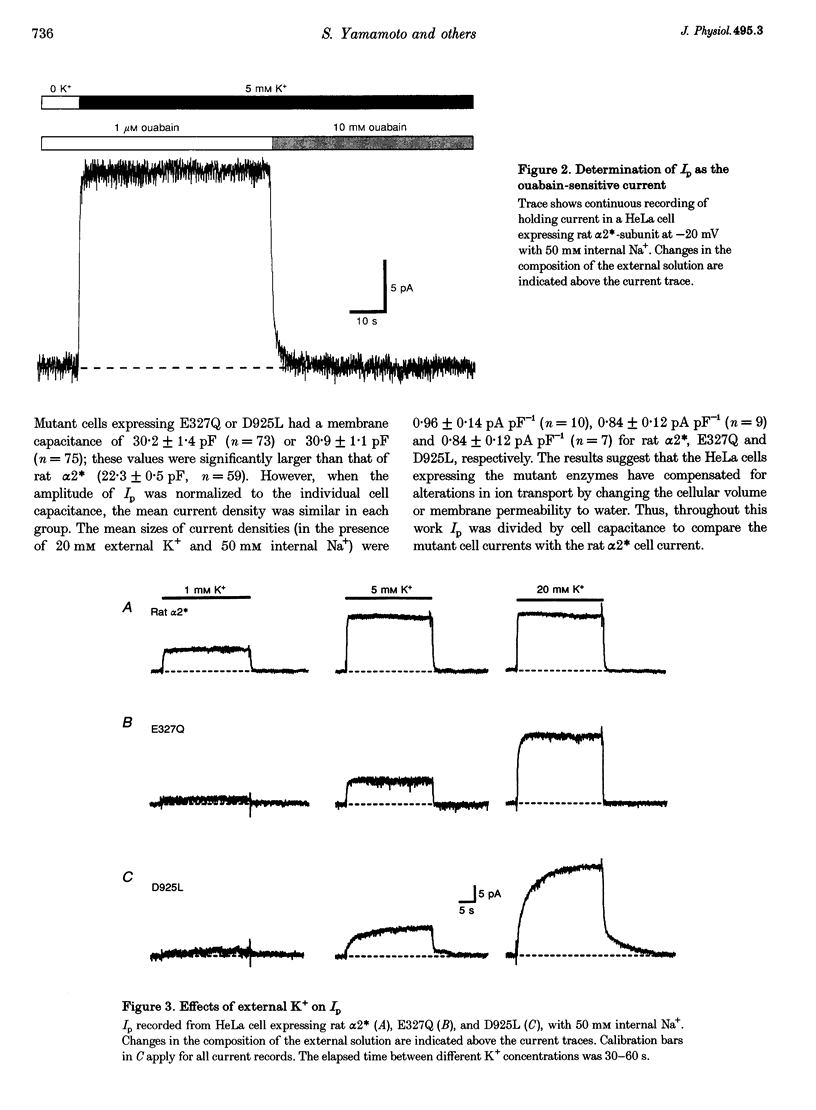
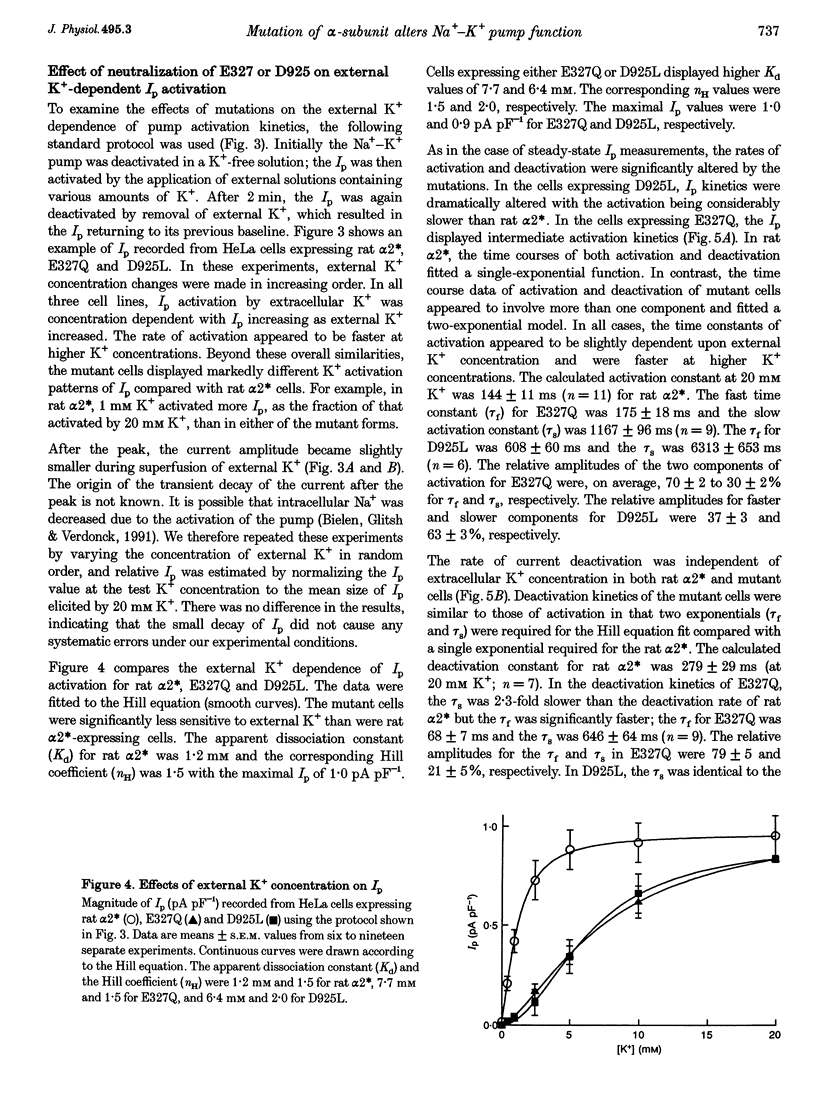
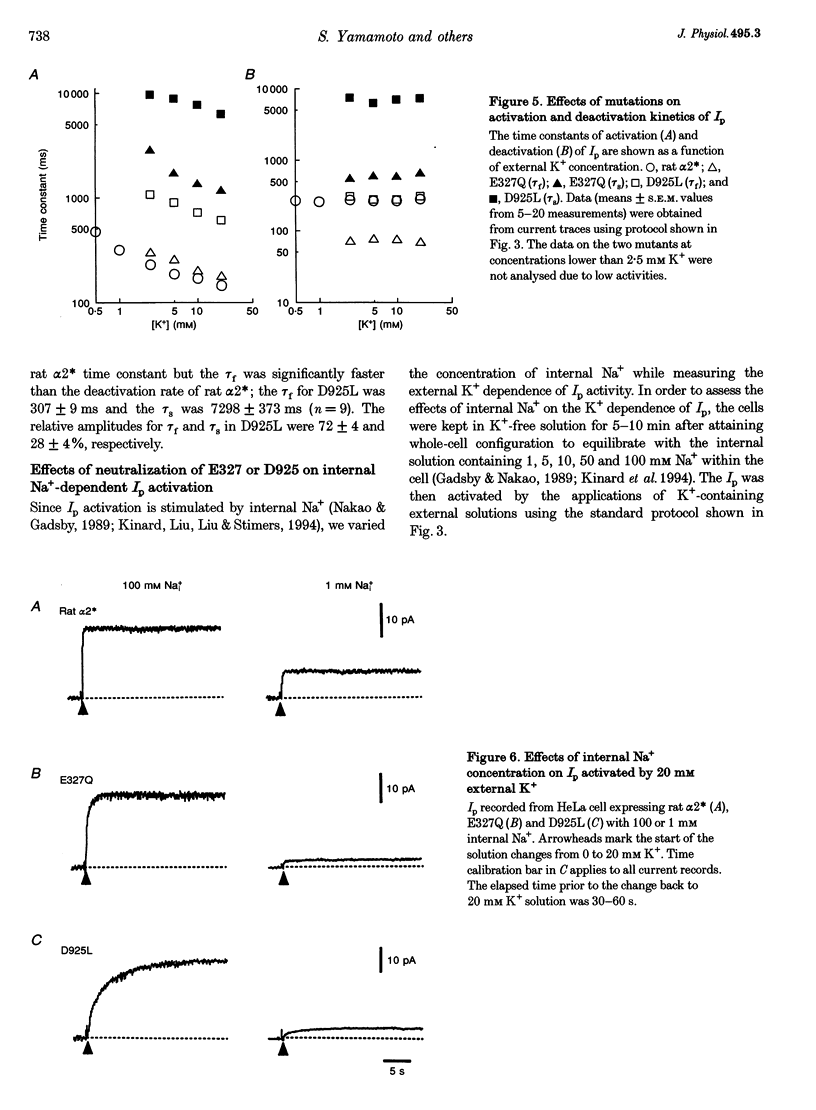
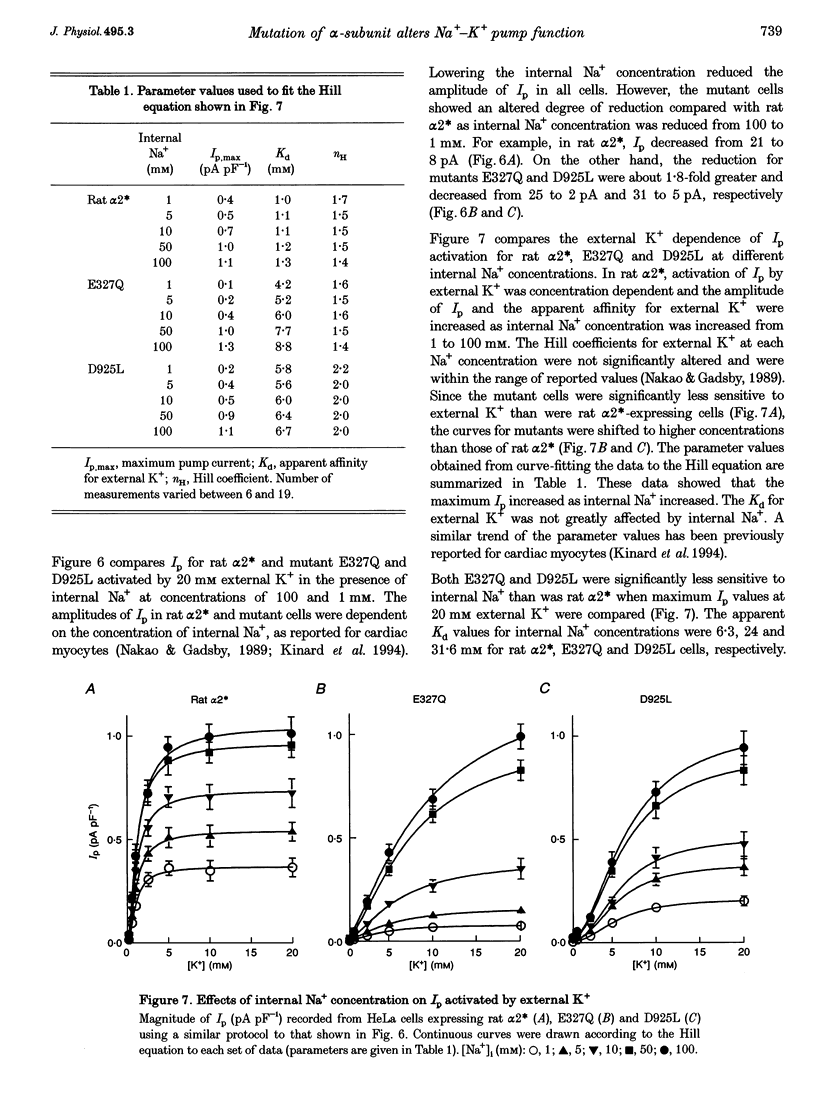
1. To study the functional role of negatively charged amino acids (E327 and D925) located in the transmembrane region of the rat alpha 2-isoform of the Na+, K(+)-ATPase (rat alpha 2*) in ion transport, the effects of mutations on external K+ dependence and internal Na+ dependence of pump currents were assessed by the patch-clamp technique in combination with a system for rapid solution changes. 2. Amino acid residues were replaced by glutamine (E327Q) or leucine (D925L) and were introduced into rat alpha 2* cDNA which encodes a ouabain-resistant isoform. These mutant enzymes were stably expressed in HeLa cells. The endogenous ouabain-sensitive HeLa cell Na+, K(+)-ATPase activity was selectively inhibited by 1 microM ouabain present in both the growing media and the assay solution. 3. External K(+)- and internal Na(+)-dependent pump activation was observed in all cells expressing rat alpha 2*, E327Q or D925L; however, the apparent affinities were significantly reduced by the mutations. 4. In E327Q, the activation of pump current was slightly slower than for rat alpha 2*, whereas the deactivation rate was faster. In contrast, D925L produced pump current having dramatically slower activation and deactivation kinetics. 5. These results indicate that these negatively charged amino acids (E327 and D925) are important in cation-induced conformational changes of the protein, which are intermediate steps in the pump mechanism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nakao M., Gadsby D. C. Potassium translocation by the Na+/K+ pump is voltage insensitive. Proc Natl Acad Sci U S A. 1988 May;85(10):3412–3416. doi: 10.1073/pnas.85.10.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielen F. V., Glitsch H. G., Verdonck F. Changes of the subsarcolemmal Na+ concentration in internally perfused cardiac cells. Biochim Biophys Acta. 1991 Jun 18;1065(2):269–271. doi: 10.1016/0005-2736(91)90239-5. [DOI] [PubMed] [Google Scholar]
- Canessa C. M., Horisberger J. D., Louvard D., Rossier B. C. Mutation of a cysteine in the first transmembrane segment of Na,K-ATPase alpha subunit confers ouabain resistance. EMBO J. 1992 May;11(5):1681–1687. doi: 10.1002/j.1460-2075.1992.tb05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso J. M., Hoving S., Tal D. M., Goldshleger R., Karlish S. J. Extensive digestion of Na+,K(+)-ATPase by specific and nonspecific proteases with preservation of cation occlusion sites. J Biol Chem. 1992 Jan 15;267(2):1150–1158. [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. S., Datyner N. B., Gintant G. A., Mulrine N. K., Pennefather P. Properties of an electrogenic sodium-potassium pump in isolated canine Purkinje myocytes. J Physiol. 1987 Feb;383:251–267. doi: 10.1113/jphysiol.1987.sp016407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbush B., 3rd Rapid 86Rb release from an occluded state of the Na,K-pump reflects the rate of dephosphorylation or dearsenylation. J Biol Chem. 1988 Jun 15;263(17):7961–7969. [PubMed] [Google Scholar]
- Gadsby D. C., Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. Occluded cations in active transport. Annu Rev Biochem. 1990;59:171–205. doi: 10.1146/annurev.bi.59.070190.001131. [DOI] [PubMed] [Google Scholar]
- Heyse S., Wuddel I., Apell H. J., Stürmer W. Partial reactions of the Na,K-ATPase: determination of rate constants. J Gen Physiol. 1994 Aug;104(2):197–240. doi: 10.1085/jgp.104.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell-Motz E. A., Lingrel J. B. Site-directed mutagenesis of the Na,K-ATPase: consequences of substitutions of negatively-charged amino acids localized in the transmembrane domains. Biochemistry. 1993 Dec 14;32(49):13523–13530. doi: 10.1021/bi00212a018. [DOI] [PubMed] [Google Scholar]
- Jewell E. A., Lingrel J. B. Comparison of the substrate dependence properties of the rat Na,K-ATPase alpha 1, alpha 2, and alpha 3 isoforms expressed in HeLa cells. J Biol Chem. 1991 Sep 5;266(25):16925–16930. [PubMed] [Google Scholar]
- Kinard T. A., Liu X. Y., Liu S., Stimers J. R. Effect of Napip on K0 activation of the Na-K pump in adult rat cardiac myocytes. Am J Physiol. 1994 Jan;266(1 Pt 1):C37–C41. doi: 10.1152/ajpcell.1994.266.1.C37. [DOI] [PubMed] [Google Scholar]
- Kuntzweiler T. A., Wallick E. T., Johnson C. L., Lingrel J. B. Glutamic acid 327 in the sheep alpha 1 isoform of Na+,K(+)-ATPase stabilizes a K(+)-induced conformational change. J Biol Chem. 1995 Feb 17;270(7):2993–3000. doi: 10.1074/jbc.270.7.2993. [DOI] [PubMed] [Google Scholar]
- Lingrel J. B., Kuntzweiler T. Na+,K(+)-ATPase. J Biol Chem. 1994 Aug 5;269(31):19659–19662. [PubMed] [Google Scholar]
- Lingrel J. B., Orlowski J., Shull M. M., Price E. M. Molecular genetics of Na,K-ATPase. Prog Nucleic Acid Res Mol Biol. 1990;38:37–89. doi: 10.1016/s0079-6603(08)60708-4. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Shirasaki T., Wakamori M., Fukuda A., Akaike N. Excitatory amino acid response in isolated nucleus tractus solitarii neurons of the rat. Neurosci Res. 1990 Jun;8(2):114–123. doi: 10.1016/0168-0102(90)90063-k. [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):539–565. doi: 10.1085/jgp.94.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte C. H., Kaplan J. H. Chemical modification as an approach to elucidation of sodium pump structure-function relations. Am J Physiol. 1990 Jan;258(1 Pt 1):C1–23. doi: 10.1152/ajpcell.1990.258.1.C1. [DOI] [PubMed] [Google Scholar]
- Rakowski R. F., Gadsby D. C., De Weer P. Stoichiometry and voltage dependence of the sodium pump in voltage-clamped, internally dialyzed squid giant axon. J Gen Physiol. 1989 May;93(5):903–941. doi: 10.1085/jgp.93.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilets L. A., Schwarz W. Structure-function relationships of cation binding in the Na+/K(+)-ATPase. Biochim Biophys Acta. 1993 Oct 29;1154(2):201–222. doi: 10.1016/0304-4157(93)90012-d. [DOI] [PubMed] [Google Scholar]
- Vilsen B. A Glu329-->Gln variant of the alpha-subunit of the rat kidney Na+,K(+)-ATPase can sustain active transport of Na+ and K+ and Na+,K(+)-activated ATP hydrolysis with normal turnover number. FEBS Lett. 1993 Oct 25;333(1-2):44–50. doi: 10.1016/0014-5793(93)80372-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Askew G. R., Heiny J., Masaki H., Yatani A. Modulation of pump function by mutations in the first transmembrane region of Na(+)-K(+)-ATPase alpha 1-subunit. Am J Physiol. 1996 Feb;270(2 Pt 1):C457–C464. doi: 10.1152/ajpcell.1996.270.2.C457. [DOI] [PubMed] [Google Scholar]
- Yatani A., Okabe K., Birnbaumer L., Brown A. M. Detergents, dimeric G beta gamma, and eicosanoid pathways to muscarinic atrial K+ channels. Am J Physiol. 1990 May;258(5 Pt 2):H1507–H1514. doi: 10.1152/ajpheart.1990.258.5.H1507. [DOI] [PubMed] [Google Scholar]
- Yatani A., Wakamori M., Mikala G., Bahinski A. Block of transient outward-type cloned cardiac K+ channel currents by quinidine. Circ Res. 1993 Aug;73(2):351–359. doi: 10.1161/01.res.73.2.351. [DOI] [PubMed] [Google Scholar]


