Abstract
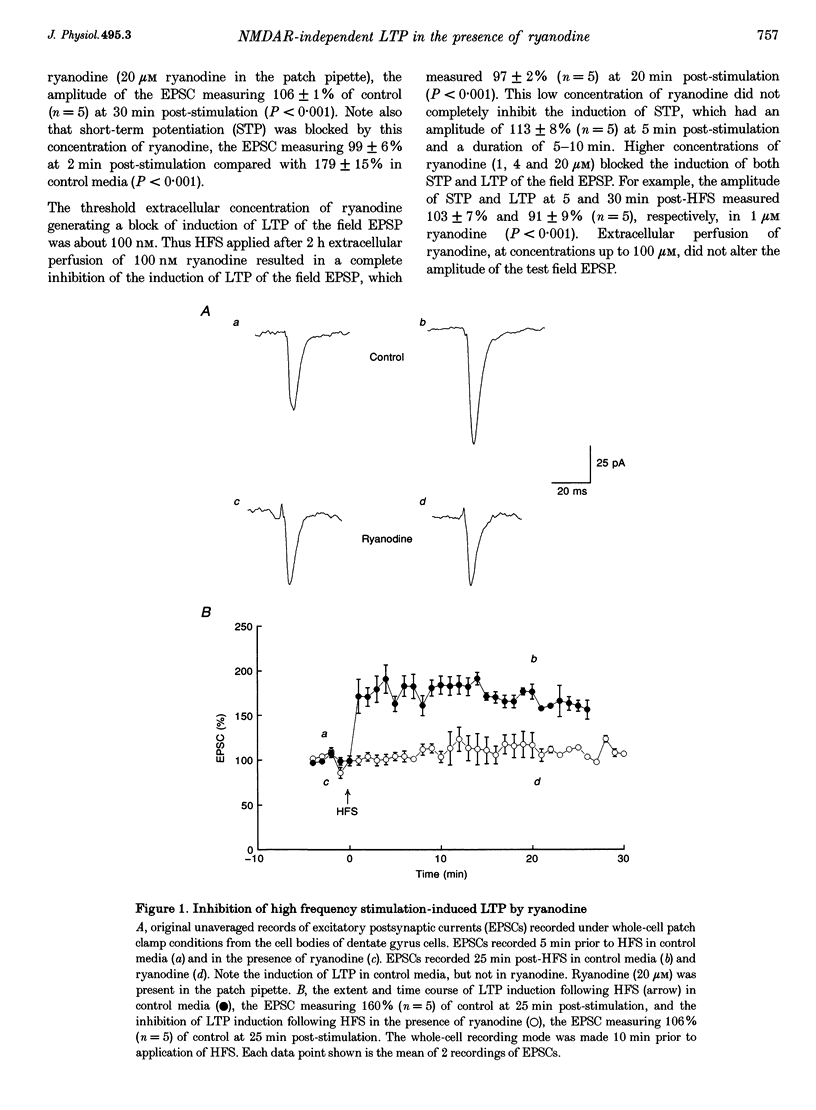
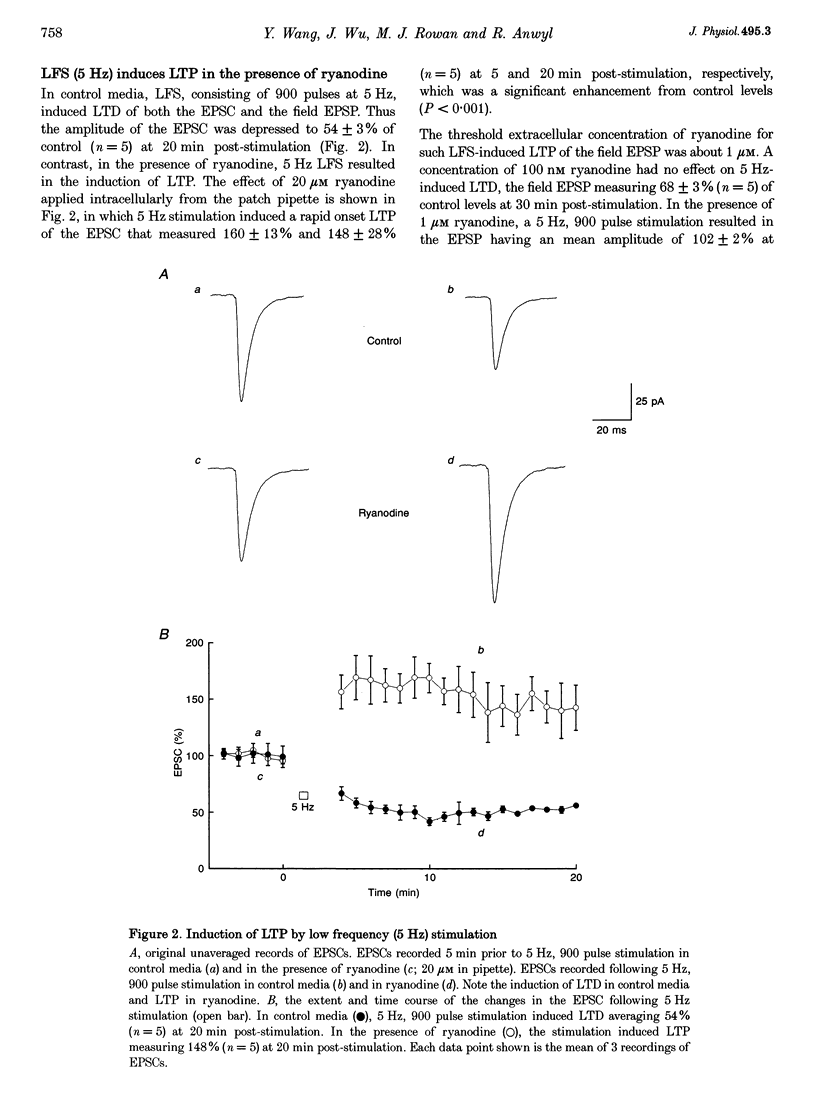
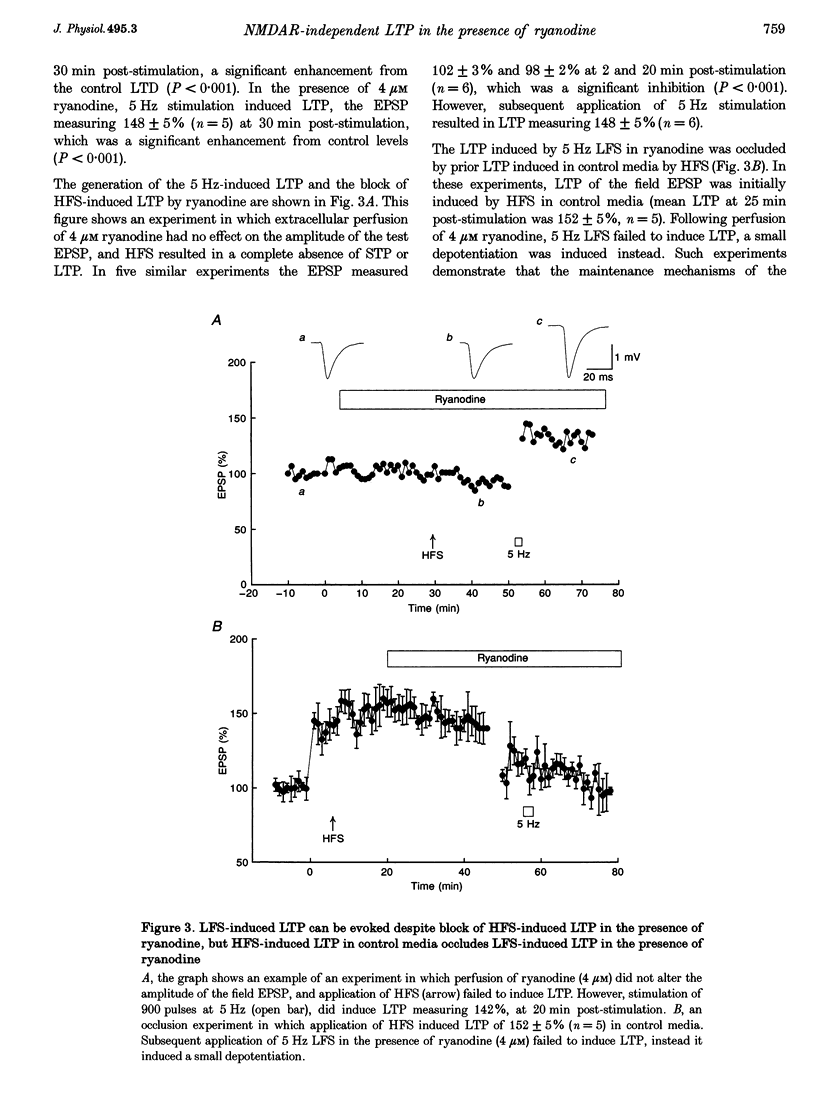
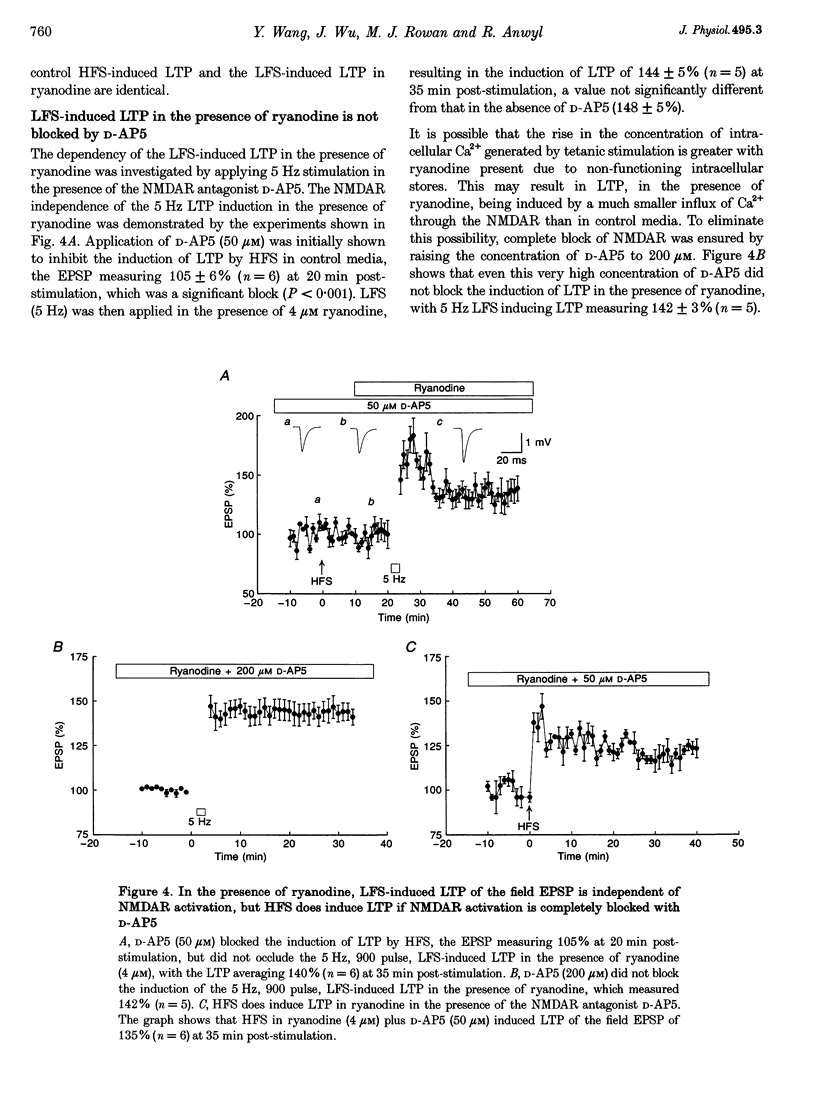
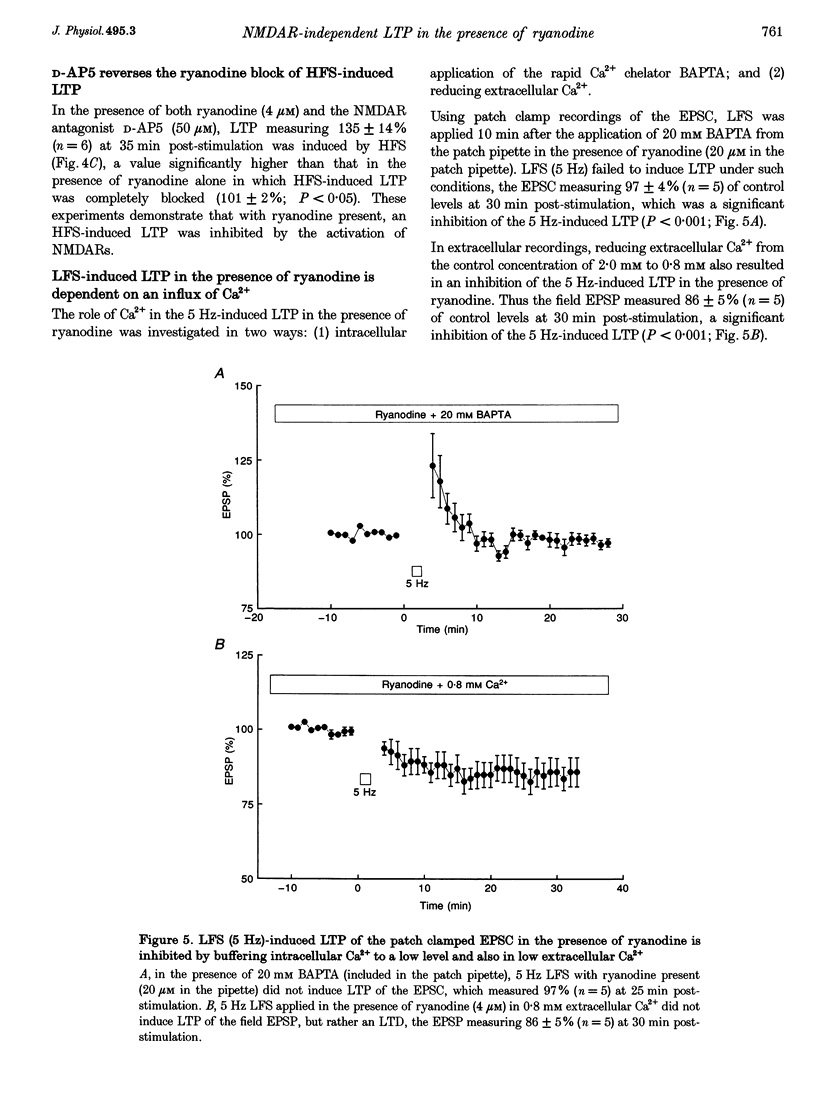
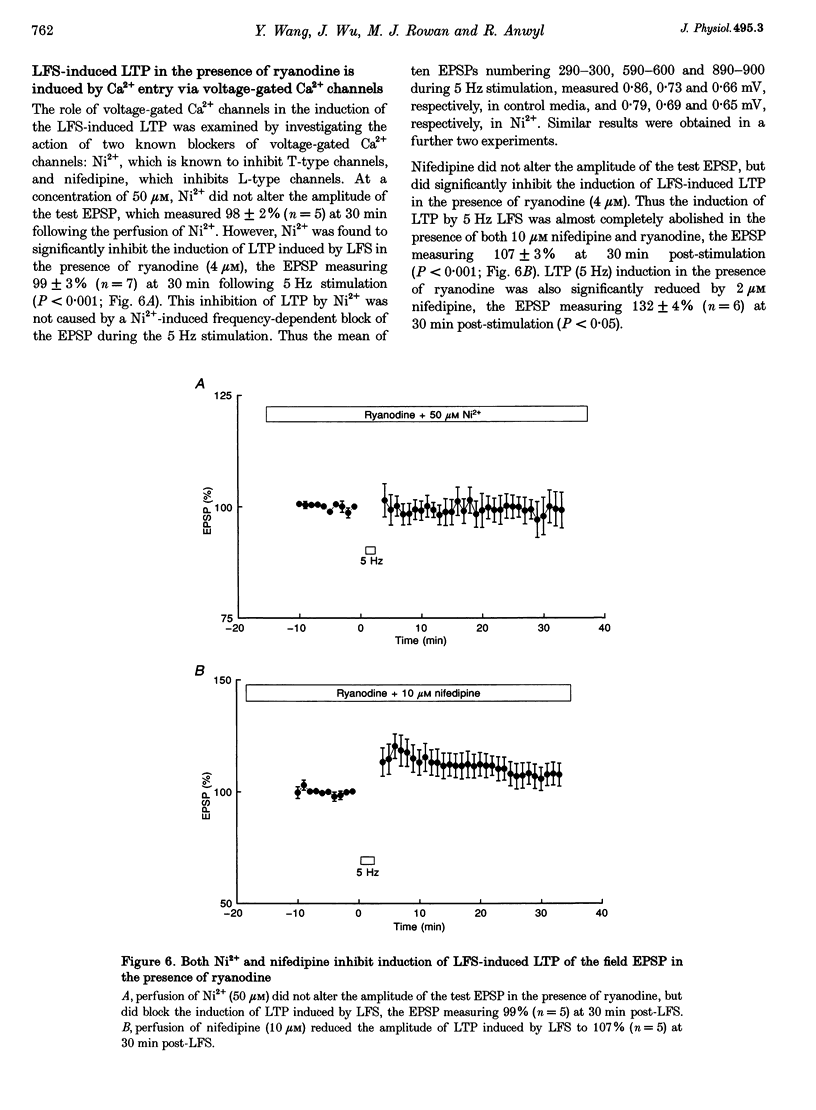
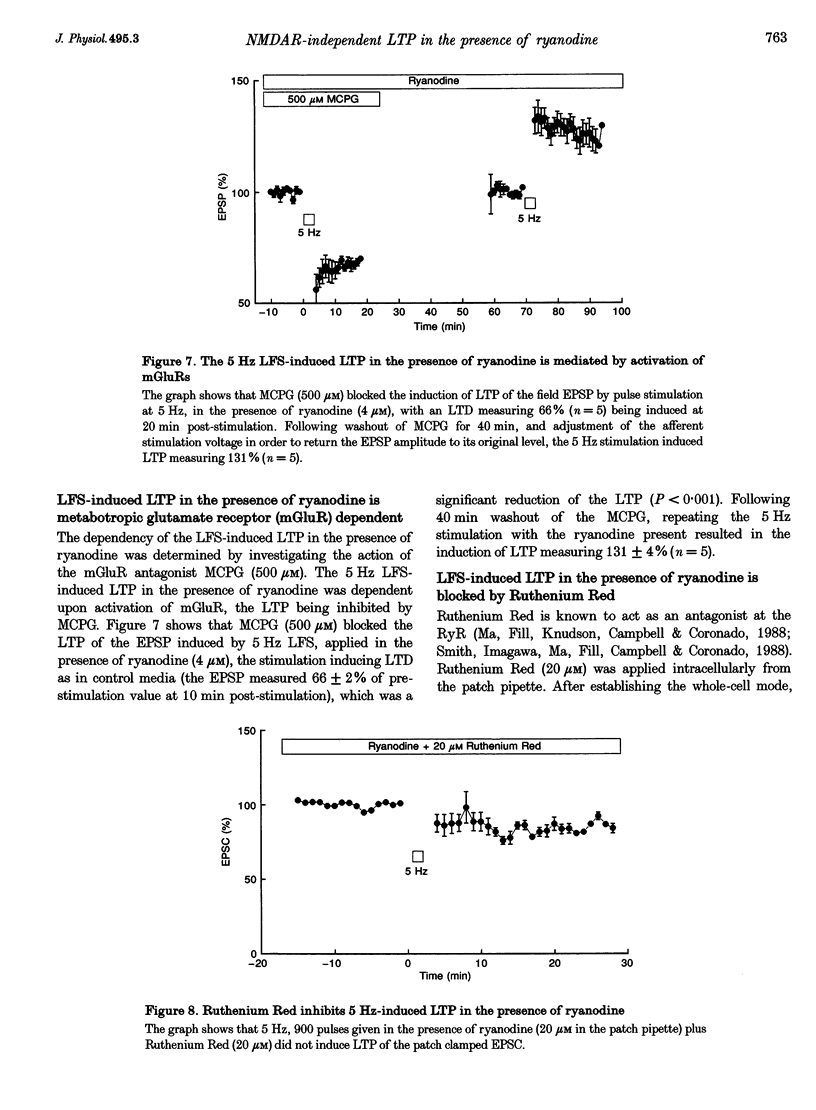
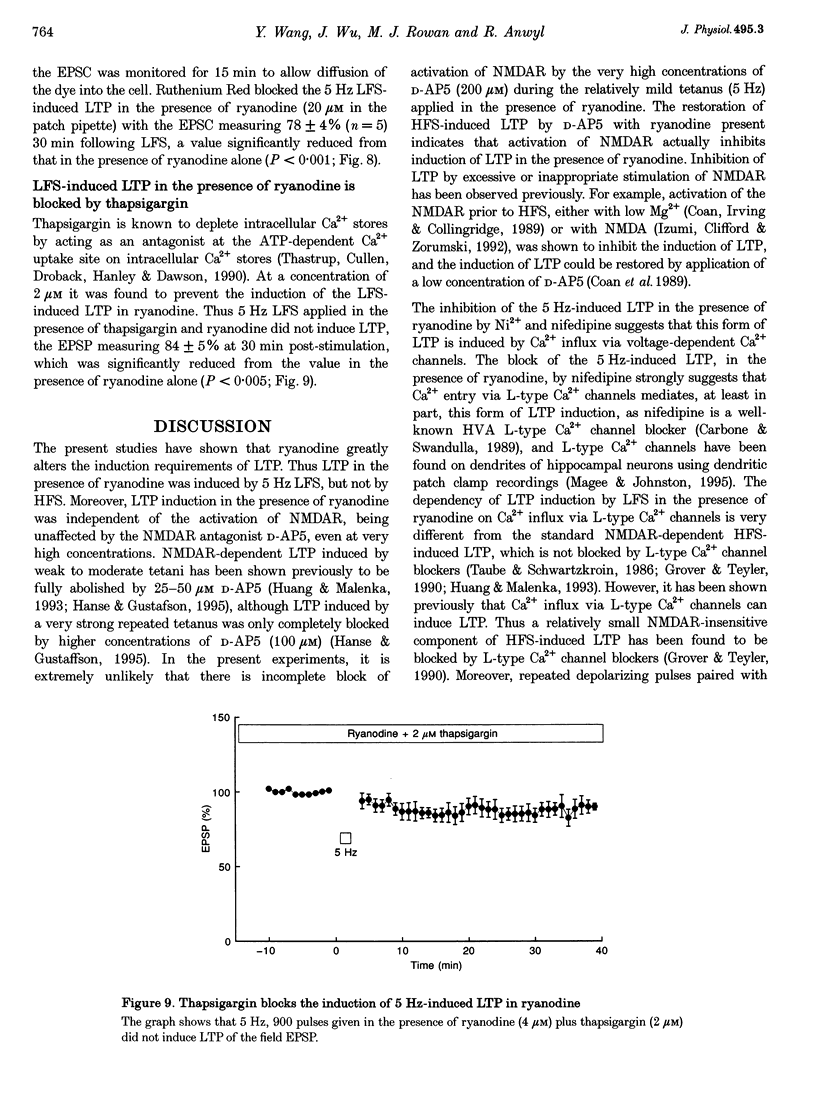
1. The induction of long-term potentiation (LTP) was investigated in the rat dentate gyrus in the presence of ryanodine, an agent which is known to selectively bind to the ryanodine receptor (RyR) Ca2+ channels which regulate Ca2+ release from intracellular Ca2+ stores. 2. In control media, high frequency stimulation (HFS) induced LTP, and prolonged low frequency stimulation (LFS) induced long-term depression (LTD), of field excitatory postsynaptic potentials (EPSPs) and patch clamped excitatory postsynaptic currents (EPSCs). 3. In the presence of ryanodine, at a threshold concentration of about 1 microM, HFS-induced LTP was inhibited, whereas LFS (5 Hz, 900 pulses) now induced LTP. 4. The N-methyl-D-aspartate receptor (NMDAR) antagonist D-2-amino-phosphonopentanoate (D-AP5), at both 50 and 200 microM, did not prevent the induction of LTP by 5 Hz LFS in the presence of ryanodine. This demonstrates the NMDAR independence of LTP induction in the presence of ryanodine. Furthermore, D AP5 reversed the block of HFS-induced LTP by ryanodine. 5. The induction of LTP by 5 Hz LFS in the presence of ryanodine was blocked by lowering extracellular Ca2+, or by rapidly buffering intracellular Ca2+ to very low levels with BAPTA. 6. The induction of LTP by 5 Hz LFS was inhibited by the L-type voltage-gated Ca2+ channel blocker nifedipine, and also by Ni2+ a commonly used T type voltage-gated Ca2+ channel blocker. 7. The 5 Hz LFS-induced LTP in the presence of ryanodine was inhibited by the metabotropic glutamate receptor (mGluR) antagonist (+)-alpha-methyl 4-carboxyphenylglycine (MCPG). 8. The 5 Hz LFS-induced LTP in the presence of ryanodine was blocked by Ruthenium Red, an agent known to block RyR channel opening, and also by thapsigargin, an agent known to block-ATP-dependent Ca2+ uptake into endoplasmic reticulum. 9. The results of the present studies emphasize the importance of intracellular Ca2+ stores in the induction of LTP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford S., Frenguelli B. G., Schofield J. G., Collingridge G. L. Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. J Physiol. 1993 Sep;469:693–716. doi: 10.1113/jphysiol.1993.sp019838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir Z. I., Bortolotto Z. A., Davies C. H., Berretta N., Irving A. J., Seal A. J., Henley J. M., Jane D. E., Watkins J. C., Collingridge G. L. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993 May 27;363(6427):347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bortolotto Z. A., Collingridge G. L. Characterisation of LTP induced by the activation of glutamate metabotropic receptors in area CA1 of the hippocampus. Neuropharmacology. 1993 Jan;32(1):1–9. doi: 10.1016/0028-3908(93)90123-k. [DOI] [PubMed] [Google Scholar]
- Carbone E., Swandulla D. Neuronal calcium channels: kinetics, blockade and modulation. Prog Biophys Mol Biol. 1989;54(1):31–58. doi: 10.1016/0079-6107(89)90008-4. [DOI] [PubMed] [Google Scholar]
- Coan E. J., Irving A. J., Collingridge G. L. Low-frequency activation of the NMDA receptor system can prevent the induction of LTP. Neurosci Lett. 1989 Oct 23;105(1-2):205–210. doi: 10.1016/0304-3940(89)90038-4. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J. E., Hainaut K. Inhibition of the intracellular release of calcium by Dantrolene in barnacle giant muscle fibres. J Physiol. 1977 Feb;265(2):565–585. doi: 10.1113/jphysiol.1977.sp011731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot L. S., Johnston D. Multiple components of calcium current in acutely dissociated dentate gyrus granule neurons. J Neurophysiol. 1994 Aug;72(2):762–777. doi: 10.1152/jn.1994.72.2.762. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992 May;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover L. M., Teyler T. J. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990 Oct 4;347(6292):477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- Hanse E., Gustafsson B. Long-term potentiation in the hippocampal CA1 region in the presence of N-methyl-D-aspartate receptor antagonists. Neuroscience. 1995 Aug;67(3):531–539. doi: 10.1016/0306-4522(95)00090-6. [DOI] [PubMed] [Google Scholar]
- Harvey J., Collingridge G. L. Thapsigargin blocks the induction of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1992 May 25;139(2):197–200. doi: 10.1016/0304-3940(92)90551-h. [DOI] [PubMed] [Google Scholar]
- Huang Y. Y., Malenka R. C. Examination of TEA-induced synaptic enhancement in area CA1 of the hippocampus: the role of voltage-dependent Ca2+ channels in the induction of LTP. J Neurosci. 1993 Feb;13(2):568–576. doi: 10.1523/JNEUROSCI.13-02-00568.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Miura M., Furuse H., Zhixiong C., Kato H., Yasutomi D., Inoue T., Mikoshiba K., Kimura T., Sakakibara S. Voltage-gated Ca2+ channel blockers, omega-AgaIVA and Ni2+, suppress the induction of theta-burst induced long-term potentiation in guinea-pig hippocampal CA1 neurons. Neurosci Lett. 1995 Jan 2;183(1-2):112–115. doi: 10.1016/0304-3940(94)11127-5. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Clifford D. B., Zorumski C. F. 2-Amino-3-phosphonopropionate blocks the induction and maintenance of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1991 Jan 28;122(2):187–190. doi: 10.1016/0304-3940(91)90854-m. [DOI] [PubMed] [Google Scholar]
- Kano M., Garaschuk O., Verkhratsky A., Konnerth A. Ryanodine receptor-mediated intracellular calcium release in rat cerebellar Purkinje neurones. J Physiol. 1995 Aug 15;487(1):1–16. doi: 10.1113/jphysiol.1995.sp020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann D. M., Perkel D. J., Manabe T., Nicoll R. A. Ca2+ entry via postsynaptic voltage-sensitive Ca2+ channels can transiently potentiate excitatory synaptic transmission in the hippocampus. Neuron. 1992 Dec;9(6):1175–1183. doi: 10.1016/0896-6273(92)90075-o. [DOI] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- Ma J., Fill M., Knudson C. M., Campbell K. P., Coronado R. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science. 1988 Oct 7;242(4875):99–102. doi: 10.1126/science.2459777. [DOI] [PubMed] [Google Scholar]
- Magee J. C., Christofi G., Miyakawa H., Christie B., Lasser-Ross N., Johnston D. Subthreshold synaptic activation of voltage-gated Ca2+ channels mediates a localized Ca2+ influx into the dendrites of hippocampal pyramidal neurons. J Neurophysiol. 1995 Sep;74(3):1335–1342. doi: 10.1152/jn.1995.74.3.1335. [DOI] [PubMed] [Google Scholar]
- Magee J. C., Johnston D. Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J Physiol. 1995 Aug 15;487(1):67–90. doi: 10.1113/jphysiol.1995.sp020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness N., Anwyl R., Rowan M. Trans-ACPD enhances long-term potentiation in the hippocampus. Eur J Pharmacol. 1991 May 17;197(2-3):231–232. doi: 10.1016/0014-2999(91)90529-y. [DOI] [PubMed] [Google Scholar]
- McPherson P. S., Kim Y. K., Valdivia H., Knudson C. M., Takekura H., Franzini-Armstrong C., Coronado R., Campbell K. P. The brain ryanodine receptor: a caffeine-sensitive calcium release channel. Neuron. 1991 Jul;7(1):17–25. doi: 10.1016/0896-6273(91)90070-g. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Miller R. J. The control of neuronal Ca2+ homeostasis. Prog Neurobiol. 1991;37(3):255–285. doi: 10.1016/0301-0082(91)90028-y. [DOI] [PubMed] [Google Scholar]
- Mogul D. J., Fox A. P. Evidence for multiple types of Ca2+ channels in acutely isolated hippocampal CA3 neurones of the guinea-pig. J Physiol. 1991 Feb;433:259–281. doi: 10.1113/jphysiol.1991.sp018425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkeen D., Anwyl R., Rowan M. The effects of external calcium on long-term potentiation in the rat hippocampal slice. Brain Res. 1988 May 3;447(2):234–238. doi: 10.1016/0006-8993(88)91124-9. [DOI] [PubMed] [Google Scholar]
- Nakai J., Dirksen R. T., Nguyen H. T., Pessah I. N., Beam K. G., Allen P. D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996 Mar 7;380(6569):72–75. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- O'Connor J. J., Rowan M. J., Anwyl R. Tetanically induced LTP involves a similar increase in the AMPA and NMDA receptor components of the excitatory postsynaptic current: investigations of the involvement of mGlu receptors. J Neurosci. 1995 Mar;15(3 Pt 1):2013–2020. doi: 10.1523/JNEUROSCI.15-03-02013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mara S. M., Rowan M. J., Anwyl R. Dantrolene inhibits long-term depression and depotentiation of synaptic transmission in the rat dentate gyrus. Neuroscience. 1995 Oct;68(3):621–624. doi: 10.1016/0306-4522(95)00233-9. [DOI] [PubMed] [Google Scholar]
- Obenaus A., Mody I., Baimbridge K. G. Dantrolene-Na (Dantrium) blocks induction of long-term potentiation in hippocampal slices. Neurosci Lett. 1989 Mar 27;98(2):172–178. doi: 10.1016/0304-3940(89)90505-3. [DOI] [PubMed] [Google Scholar]
- Regan L. J. Voltage-dependent calcium currents in Purkinje cells from rat cerebellar vermis. J Neurosci. 1991 Jul;11(7):2259–2269. doi: 10.1523/JNEUROSCI.11-07-02259.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham J. S., Cleemann L., Morad M. Functional coupling of Ca2+ channels and ryanodine receptors in cardiac myocytes. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):121–125. doi: 10.1073/pnas.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A. H., McPherson P. S., Dawson T. M., Aoki C., Campbell K. P., Snyder S. H. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J Neurosci. 1993 Jul;13(7):3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. B., Challiss R. A., Nahorski S. R. Involvement of intracellular stores in the Ca2+ responses to N-Methyl-D-aspartate and depolarization in cerebellar granule cells. J Neurochem. 1993 Aug;61(2):760–763. doi: 10.1111/j.1471-4159.1993.tb02184.x. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J. S., Schwartzkroin P. A. Ineffectiveness of organic calcium channel blockers in antagonizing long-term potentiation. Brain Res. 1986 Aug 6;379(2):275–285. doi: 10.1016/0006-8993(86)90781-x. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Hirning L. D., Miller R. J. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol. 1988 Nov;34(5):664–673. [PubMed] [Google Scholar]
- Valdivia H. H., Coronado R. Inhibition of dihydropyridine-sensitive calcium channels by the plant alkaloid ryanodine. FEBS Lett. 1989 Feb 27;244(2):333–337. doi: 10.1016/0014-5793(89)80557-5. [DOI] [PubMed] [Google Scholar]
- Wang Y., Rowan M. J., Anwyl R. (RS)-alpha-Methyl-4-carboxyphenylglycine inhibits long-term potentiation only following the application of low frequency stimulation in the rat dentate gyrus in vitro. Neurosci Lett. 1995 Sep 15;197(3):207–210. doi: 10.1016/0304-3940(95)11937-r. [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Randall A. D., Ellinor P. T., Horne W. A., Sather W. A., Tanabe T., Schwarz T. L., Tsien R. W. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993 Nov;32(11):1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]


