Abstract
Background
Tubular anastomosis is commonly used in proximal gastrectomy; however, its use in stage II esophagogastric conjugate cancer is currently unclear. In this study, we investigated the short- and long-term clinical outcomes of Siewert II/III adenocarcinoma of the esophagogastric junction after modified proximal gastrectomy with tubular esophagogastric anastomosis compared with total gastrectomy with Roux-en-Y reconstruction.
Methods
We collected the clinical data of patients who underwent proximal gastrectomy tubular esophagogastric anastomosis (PG-TEA) and total gastrectomy Roux-en-Y reconstruction (TG-RY) from October 2015 to October 2018. The clinical characteristics, postoperative quality of life, nutritional status, and long-term survival outcomes of the two groups were compared.
Results
There were 43 patients in the PG-TEA group and 80 patients in the TG-RY group, and there was no significant difference between the baseline data of the groups. The operation time of the PG-TEA group was shorter, there was less intraoperative bleeding, and the feeding time was earlier, which was conducive to postoperative recovery. Reflux esophagitis was more evident in the PG-TEA group than in the TG-RY group, and there was no significant difference in the incidence of anastomotic ulcers or other complications. Three months after surgery, the nutritional status of the PG-TEA group was better than the TG-RY group. By the 6th postoperative month, there was no significant difference between the two groups. Regarding quality of life, the PG-TEA group was superior to the TG-RY group in terms of diarrhea and dumping syndrome. In addition, the PG-TEA group had higher satisfaction with daily life and higher-quality meals. There was no significant difference in overall survival between the two groups.
Conclusions
Proximal gastrectomy tubular gastroesophageal anastomosis is a surgical procedure for stage II Siewert type II and III AEG. It achieves similar clinical outcomes to those after total gastrectomy and can be further applied in the clinic.
Keywords: Adenocarcinoma of the esophagogastric junction, Total gastrectomy Roux-en-Y reconstruction, Proximal gastrectomy tube anastomosis, Comparative quality of life
Introduction
Esophagogastric junction cancer (EGC) is defined as adenocarcinoma, in which the center of the tumor is located within 5 cm above or below the esophagogastric junction (EGJ) and invades the dentate line [1]. The prevalence of adenocarcinoma of the esophagogastric junction (AEG) has continuously increased in recent years due to the high prevalence of gastroesophageal reflux disease (GERD) and obesity [2, 3].
Surgical resection is the main method of treating AEG and can be divided into total and partial gastrectomy [4]. Proximal gastrectomy, as a function-preserving surgery, is beneficial to the long-term prognosis of patients [5, 6]. However, reflux esophagitis is unavoidable. Various anti-reflux surgeries have been developed to prevent postoperative reflux, including esophageal tube-gastric anastomosis, double-channel anastomosis, side-overlap (SOFY) anastomosis, bimuscular valvuloplasty (Kamikawa anastomosis) and jejunal interposition anastomosis [7, 8]. Each operation has advantages and disadvantages, and finding a method that combines postoperative gastric function preservation and eases the operation’s side effects is difficult.
Tube-gastroesophageal anastomosis involves cutting the remnant stomach into a tube for anti-reflux effects. It is a simple operation with low surgical difficulty and short operation time [9]. Compared with traditional gastroesophageal anastomosis, tube gastroesophageal anastomosis preserves the normal structure of the digestive tract. Hence, this procedure has a precise anti-reflux effect and better quality of life and nutritional status [10, 11].
Few studies exist on the efficacy of tube esophagogastric anastomosis, especially in patients with progressive gastric cancer. This study compared the immediate and long-term clinical outcomes and prognoses of patients with stage II Siewert II/III AEG after undergoing proximal gastrectomy with tube esophagogastric anastomosis versus total gastrectomy.
Methods and materials
Study design and patients
We retrospectively evaluated patients who were diagnosed with AEG (Siewert type II or Siewert type III AEG) from October 2015 to October 2018 at Shaanxi Provincial People’s Hospital and were pathologically confirmed as having stage IIA or IIB disease, who underwent radical proximal gastrectomy with tubular esophagogastric anastomosis (PG-TEA) or total gastrectomy with Roux-en-Y reconstruction (TG-RY). Patients who met the following criteria were excluded: 1) had a combination of malignant tumors from other sites; 2) had a history of gastrectomy; 3) had distant metastases; 4) were unable to understand and answer the questionnaire correctly; and 5) had missing clinicopathological and follow-up data.
Surgical methods
For the choice of surgical approach, we preoperatively clarified the tumor size and clinical T-stage by performing High Resolution CT (HRCT), fiberoptic gastroscopy, etc., and after initially determining the surgical plan, the final surgical plan was determined after multidisciplinary treatment through thoracic surgery, gastroenterology, gastrointestinal surgery, and imaging. All surgeries were D2/R0 resections [4], and the extent of lymph node dissection included No. 1, No. 2, No. 3a, No.3b, No.4sa, No.4sb, No.4d, No. 5, No. 6, No. 7, No. 8a, No. 9, No. 11p, No. 11d, and No. 12a; If the esophagus was involved, we increased the clearance of lymph nodes in groups No.19, No.20 and No.110. To ensure R0 resection, surgical margins were routinely sent intraoperatively for rapid frozen section examination to ensure negative margins [12].
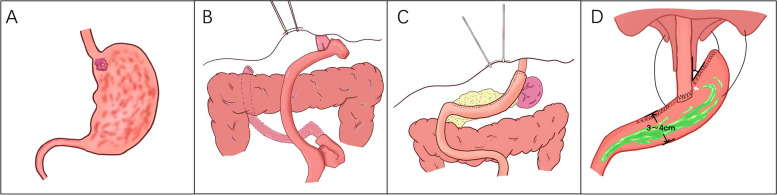
The PG-TEA procedure was performed as follows: we isolated approximately 8 cm of the esophagus, removed the mediastinal lymph nodes around the esophagus, and cut the esophagus 3 cm above the tumor. We preserved the greater curvature of the stomach and dissected the gastric tissue 5 cm from the tumor. The stomach was further made into a 4–5 cm wide tube, and the anterior wall of the stomach was then anastomosed to the esophagus 4 cm from the tubular stomach stump. Finally, the fundus was reconstructed by suspending the severed end of the tubular stomach over the diaphragm with three needles. The anti-reflux effect was achieved by creating a structure similar to the fundus and His horn (Fig. 1).
Fig. 1.
Surgical schematic. A Tumor location; B TG-RY anastomosis approach; C PG-TEA anastomotic approach; D Suspension of the tube stomach at the foot of the diaphragm after PG-TEA
The choice of GI reconstruction after total gastrectomy for AEG is currently the more commonly used esophagojejunal Roux-en-Y anastomosis: it is a simple, safe, and reliable procedure that significantly reduces the incidence of reflux esophagitis and improves patients’ quality of life [13].
Intraoperative pathology of the proximal esophageal margin was routinely performed on rapidly frozen sections, and all patients achieved a margin free of tumor tissue. All these patients were subjected to chemotherapy for six months, and the chemotherapy regimens used were SOX, XELOX, or FOLFOX6 [14], which were adjusted according to the side effects and recurrence of the patients.
Observation indicators
We collected the following clinical data:
Surgical indicators: surgical procedure, duration of surgery, intraoperative bleeding, number of dissected lymph nodes, and whether the anastomosis was reinforced. Postoperative indicators: postoperative time of defecation, bowel movement, and food intake; postoperative time of gastric tube removal; time of abdominal drain removal; and postoperative hospital stay.
Postoperative nutritional indicators: white blood cell count, hemoglobin count, and serum albumin count. Postoperative distant complications: reflux esophagitis was assessed via gastroscopy findings at three and six months postoperatively, and the severity of reflux esophagitis was evaluated via the Los Angeles grading scale [4]. Distant complications were diagnosed and graded via the Clavien–Dindo grading criteria [15]. Quality of life: The quality of life of patients one year after surgery was assessed via the Post Gastrostomy Syndrome Assessment Scale (PGSAS-45) [16]. The scale consists mainly of a symptom domain, a life status domain, and a quality-of-life domain. The results of the above indicators were obtained by reviewing the electronic medical records.
Statistical analysis
All the statistical analyses were performed via SPSS software (SPSS, version 27.0; Chicago, USA). Descriptive statistics with a normal distribution are presented as the means ± standard deviations, and continuous variables without a normal distribution are presented as medians (interquartile ranges). The t-test and Mann–Whitney U tests were used to compare continuous variables with and without a normal distribution. Categorical variables are presented as numbers (percentages) and were compared via the chi-square test or Fisher’s exact test. Survival curves were plotted via the Kaplan‒Meier method, and survival analyses were performed via the log-rank test. Statistical significance was set at P < 0.05.
Results
Baseline characteristics
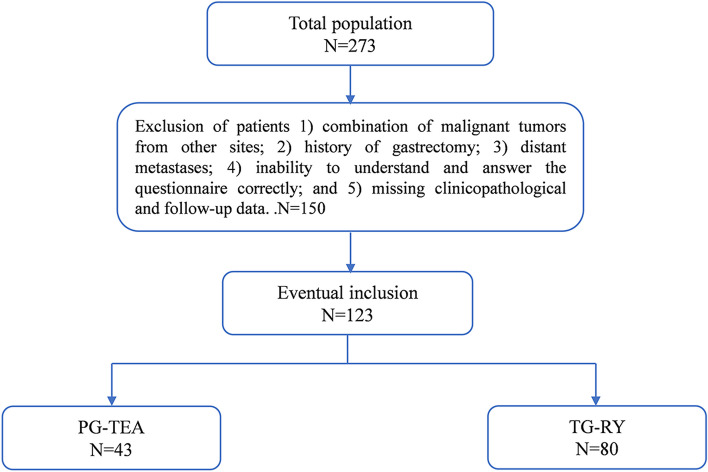
One hundred twenty-three patients were enrolled in this study: 43 underwent proximal gastrectomy with tube gastroesophageal anastomosis, and 80 underwent total gastrectomy with Roux-en-Y reconstruction (Fig. 2).
Fig. 2.
Patient intake flowchart
All included patients had Siewert type II/III adenocarcinoma of the esophagogastric junction, and there was no difference in staging between the two groups. There were no statistically significant differences in sex, age, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, or preoperative serological indicators between the two groups. There were no differences in pathological TNM stage, tumor nature, vascular invasion, or lymphatic invasion between the two groups (Table 1).
Table 1.
Comparison of general data between the PG-TEA and TG-RY groups
| Factors | PG-TEA (n = 43) | TG-RY (n = 80) | t/X2 | P |
|---|---|---|---|---|
| Gender | 0.116 | 0.733 | ||
| Male | 36 | 65 | ||
| Female | 7 | 15 | ||
| Age (years, x ± s) | 61 ± 10 | 61 ± 9 | 0.082 | 0.935 |
| BMI (kg/m2, x ± s) | 22.54 ± 1.56 | 22.71 ± 1.40 | 0.642 | 0.522 |
| Siewert type | 0.218 | 0.641 | ||
| II | 18 | 37 | ||
| III | 25 | 43 | ||
| ASA Score | 1.846 | 0.379 | ||
| I | 11 | 14 | ||
| II | 31 | 61 | ||
| III | 1 | 5 | ||
| WBC before surgery (× 109/L, x ± s) | 5.5 ± 1.4 | 5.2 ± 1.3 | -0.980 | 0.332 |
| Hb before surgery (g/L, x ± s) | 110 ± 17 | 113 ± 18 | -1.120 | 0.265 |
| ALB before surgery (g/L, x ± s) | 36.4 ± 3.1 | 36.7 ± 3.9 | 0.445 | 0.675 |
| Tumor diameter(cm, x ± s) | 2.3 ± 0.6 | 2.5 ± 0.6 | 1.968 | 0.051 |
| Degree of differentiation | 1.514 | 0.469 | ||
| Well | 14 | 26 | ||
| Moderate | 28 | 47 | ||
| Worse | 1 | 6 | ||
| Pathologic T classification | 5.246 | 0.144 | ||
| 1 | 15 | 39 | ||
| 2 | 23 | 26 | ||
| 3 | 4 | 13 | ||
| 4 | 1 | 2 | ||
| Pathologic N classification | 4.043 | 0.242 | ||
| 0 | 2 | 2 | ||
| 1 | 22 | 29 | ||
| 2 | 14 | 31 | ||
| 3 | 5 | 18 | ||
| Pathological stage | 3.066 | 0.091 | ||
| IIA | 28 | 38 | ||
| IIB | 15 | 42 | ||
| Presence of vascular thrombus invasion | 0.158 | 0.691 | ||
| Yes | 13 | 27 | ||
| No | 30 | 53 | ||
| Neurological violation | 3.607 | 0.072 | ||
| Yes | 14 | 14 | ||
| No | 29 | 66 |
PG-TEA proximal gastrectomy tubular esophagogastric anastomosis, TG-RY total gastrectomy Roux-en-Y reconstruction, BMI body mass index, WBC white blood cell, Hb hemoglobin, ALB albumin
Surgical outcomes and complications
The operative time in the TG-RY group (235 min) was significantly longer than that in the PG-TEA group (188 min); moreover, the PG-TEA group had statistically significantly less intraoperative bleeding and fewer lymph nodes dissected. There were no statistically significant differences between the two groups regarding time to first postoperative deflation, gastric tube removal, first meal, or drain removal. In terms of postoperative hospitalization days, the PG-TEA group had fewer postoperative hospitalization days than the TG-RY group, but the difference between the groups was not statistically significant (Table 2).
Table 2.
Comparison of short-term outcomes between the PG-TEA and TG-RY groups
| Factor | PG-TEA(N = 43) | TG-RY(N = 80) | t/X2 | P |
|---|---|---|---|---|
| Operation time(min,x ± s) | 188 ± 18 | 235 ± 22 | 12.142 | 0.010 |
| Blood loss(mL,x ± s) | 35 ± 15 | 40 ± 13 | 2.014 | 0.046 |
| Lymph node number(x ± s) | 21 ± 3 | 30 ± 4 | 0/171 | 0.001 |
| First exhaust time(days) | 3.4 ± 0.9 | 3.5 ± 0.9 | 0.383 | 0.703 |
| First fluid taken time(days) | 3.6 ± 0.7 | 3.6 ± 0.7 | -0.032 | 0.975 |
| First defecation time(days) | 4.1 ± 0.8 | 4.2 ± 0.8 | 0.067 | 0.946 |
| Gastric tube removal time(days) | 2.6 ± 0.7 | 2.4 ± 0.6 | -1.404 | 0.163 |
| Drainage tube removal time(days) | 7.0 ± 1.1 | 6.9 ± 1.2 | -0.343 | 0.732 |
| Anastomotic fistula | 0.058 | 0.809 | ||
| Yes | 2 | 3 | ||
| No | 42 | 77 | ||
| Anastomotic ulcer | 2.536 | 0.149 | ||
| Yes | 8 | 7 | ||
| No | 35 | 73 | ||
| Anastomotic stenosis | 2.789 | 0.182 | ||
| Yes | 4 | 2 | ||
| No | 39 | 78 | ||
| Anastomosis reinforcement | 3.086 | 0.079 | ||
| No | 10 | 9 | ||
| Yes | 33 | 71 | ||
| Clavien‒Dindo degree | 4.025 | 0.232 | ||
| I | 6 | 18 | ||
| II | 26 | 52 | ||
| IIIA | 9 | 8 | ||
| IIIB | 2 | 2 | ||
| Postoperative hospitalization days(days) | 12.3 ± 2.0 | 11.9 ± 1.8 | -1.289 | 0.200 |
| Postoperative adjuvant therapy | 3.419 | 0.078 | ||
| Yes | 35 | 74 | ||
| No | 8 | 6 |
PG-TEA proximal gastrectomy tubular esophagogastric anastomosis, TG-RY total gastrectomy Roux-en-Y reconstruction
Postoperative complications affect the number of days in the hospital and the quality of life of patients. There were no statistically significant differences between the two groups in terms of anastomotic ulceration, anastomotic fistula, anastomotic stenosis, anastomotic reinforcement, Clavien-Dindo grading, first gas evacuation, first bowel movement, anastomotic fistula appearance, or length of hospital stay. Within three months after surgery, two patients in the PG-TEA group developed anastomotic ulcers, which led to gastrointestinal bleeding. They were cured after rehospitalization with nutritional support and symptomatic treatment (P > 0.05).
Nutritional status
Nutritional status was compared between groups by assessing BMI and hemoglobin and albumin levels. There was no statistically significant difference in preoperative nutritional indicators between the groups. At one month post-surgery, the nutritional indices of both groups were significantly lower than before surgery, and there was no statistically significant difference between the two groups. At three months postsurgery, the nutritional status of the PG-TEA group was significantly better than the TG-RY group, but at six months postsurgery, the hemoglobin count of the PG-TEA group was greater than the TG-RY group, and there was no significant difference in body weight change or albumin count (Table 3).
Table 3.
Comparison of nutrition conditions between the PG-TEA and TG-RY groups
| Factor | PG-TEA (N = 43) | TG-RY (N = 80) | t/X2 | P |
|---|---|---|---|---|
| 1 month after surgery | ||||
| BMI | 20.15 ± 1.07 | 20.47 ± 1.17 | 1.501 | 0.136 |
| Hb (g/L,x ± s) | 98.2 ± 8.1 | 95.6 ± 8.3 | -1.691 | 0.093 |
| ALB (g/L,x ± s) | 35.0 ± 3.6 | 33.9 ± 2.8 | -1.933 | 0.056 |
| 3 months after surgery | ||||
| BMI | 22.25 ± 1.22 | 21.72 ± 1.67 | -2.429 | 0.017 |
| Hb (g/L,x ± s) | 114.3 ± 13.0 | 103.3 ± 14.1 | -4.264 | 0.001 |
| ALB (g/L,x ± s) | 36.2 ± 4.5 | 32.8 ± 3.0 | -4.966 | 0.001 |
| 6 months after surgery | ||||
| BMI | 22.56 ± 1.58 | 22.71 ± 1.17 | 0.642 | 0.522 |
| Hb (g/L,x ± s) | 122.1 ± 12.2 | 107.7 ± 15 | -5.413 | 0.001 |
| ALB (g/L,x ± s) | 35.4 ± 4.6 | 34.2 ± 3.7 | -1.544 | 0.125 |
PG-TEA proximal gastrectomy tubular esophagogastric anastomosis, TG-RY total gastrectomy Roux-en-Y reconstruction, WBC white blood cell, Hb hemoglobin, ALB albumin
Reflux esophagitis
We collected gastroscopic findings at three and six months postoperatively and counted the severity of reflux esophagitis according to the LA classification. Gastroscopy revealed that reflux esophagitis occurred more in the PG-TEA group at three months post-surgery than in the TG-RY group. In addition, two patients with anastomotic ulcers in the PG-TEA group had severe reflux esophagitis. At six months postoperatively, there was no significant difference in the incidence of reflux esophagitis between the two groups. All patients with combined mild reflux esophagitis improved their symptoms after active treatment (Tables 4 and 5).
Table 4.
Comparison of gastroscopy findings between PG-TEA and TG-RY groups based on Los Angeles classification at 3 months postoperatively
| Factor | PG-TEA | TG-RY | P |
|---|---|---|---|
| Cases | 42 | 75 | |
| LA grade | 0.001 | ||
| A | 20(47.6%) | 62(82.7%) | |
| B | 20(47.6%) | 13(17.3%) | |
| C/D | 2(4.8%) | 0(0%) |
Based on the degree of endoscopic damage to the esophageal mucosa, reflux esophagitis is classified as grades A, B, C, or D, with severity increasing in each grade
PG-TEA proximal gastrectomy tubular esophagogastric anastomosis, TG-RY total gastrectomy Roux-en-Y reconstruction
Table 5.
Comparison of gastroscopy findings between PG-TEA and TG-RY groups based on Los Angeles classification at 6 months postoperatively
| Factor | PG-TEA | TG-RY | P |
|---|---|---|---|
| Cases | 37 | 64 | |
| LA grade | 0.234 | ||
| A | 29(78.4%) | 57(89%) | |
| B | 8(21.6%) | 7(10.9%) | |
| C/D | 0(0%) | 0(0%) |
Based on the degree of endoscopic damage to the esophageal mucosa, reflux esophagitis is classified as grades A, B, C, or D, with severity increasing in each grade
PG-TEA proximal gastrectomy tubular esophagogastric anastomosis, TG-RY total gastrectomy Roux-en-Y reconstruction
Comparative quality of life (PGSAS-45)
We used the PGSAS-45 scale to compare patients’ quality of life one year after surgery. There were no differences in the esophageal reflux subscale in the domain of somatic symptoms. However, the PG-TEA group was more likely to have constipation, whereas the TG-RY group was more likely to have diarrhea, with the same findings reported by Nobuhiro et al. [16]. In addition, the TG-TEA group was more likely to have dumping syndrome, which affected quality of life.
In contrast, the PG-TEA group had better overall physical symptom scores than the TG-RY group. For the daily life dissatisfaction subscale, the TG-RY group scored higher than those that underwent proximal gastrectomy and esophagogastric anastomosis. The TG-RY group scored higher than the PG-TEA group (3.26 ± 0.65; 2.9 ± 0.65). For the meal quality subscale, the PG-TEA group scored higher than the TG-RY group (3.28 ± 0.3; 3.41 ± 0.30). In terms of quality of life, there was no significant difference between the two groups, which indicated that at one year postoperatively, the PG-TEA group had a better overall condition than the TG-RY group (Table 6).
Table 6.
Comparison of quality of life between the PG-TEA and TG-RY Groups 1 year post-surgery
| Factor | PG-TEA (N = 43) | TG-RY (N = 80) | t/X2 | P | |
|---|---|---|---|---|---|
| Physical symptom | Esophageal reflux table | 2.5 ± 0.4 | 2.4 ± 0.5 | 1.893 | 0.061 |
| Abdominal Pain Scale | 1.5 ± 0.3 | 1.6 ± 0.3 | -1.301 | 0.196 | |
| Eating-Related Distress Scale | 2.5 ± 0.4 | 2.5 ± 0.4 | -0.236 | 0.813 | |
| Dyspepsia Scale | 2.2 ± 0.3 | 2.3 ± 0.3 | -1.110 | 0.269 | |
| Diarrhea Scale | 2.1 ± 0.4 | 2.5 ± 0.3 | -5.110 | 0.001 | |
| Constipation Scale | 2.9 ± 0.6 | 2.7 ± 0.4 | 3.476 | 0.001 | |
| Dumping scale | 1.5 ± 0.6 | 1.8 ± 0.7 | -2.330 | 0.021 | |
| Total Symptoms Scale | 2.1 ± 0.1 | 2.2 ± 0.2 | -0.747 | 0.457 | |
| Condition of life | Weight loss (%)* | -14 ± 1.75 | -18 ± 1.19 | 0.132 | 0.895 |
| Food Consumption per Meal* | 5.5 ± 0.7 | 5.4 ± 0.6 | 0.801 | 0.425 | |
| The need for extra meals | 2.9 ± 0.65 | 3.26 ± 0.65 | -2.893 | 0.005 | |
| Meal Quality Scale* | 3.28 ± 0.3 | 3.41 ± 0.30 | -2.192 | 0.030 | |
| Working ability | 2.07 ± 0.77 | 2.29 ± 0.64 | -1.675 | 0.097 | |
| Quality of life | Unsatisfied with symptoms | 2.28 ± 0.93 | 2.51 ± 0.84 | -1.411 | 0.161 |
| Dissatisfied with the meal | 2.6 ± 0.5 | 2.7 ± 0.6 | -6.33 | 0.528 | |
| Dissatisfied with work | 2.0 ± 0.8 | 2.3 ± 0.8 | -1.81 | 0.073 | |
| Dissatisfaction with Daily Life Scale | 2.3 ± 0.5 | 2.5 ± 0.4 | -2.434 | 0.016 | |
| General measure of physical health* | 71.6 ± 6.1 | 69.9 ± 4.4 | 1.75 | 0.083 | |
| General Measures of Mental Health* | 76.5 ± 5.3 | 75.8 ± 4.5 | 0.776 | 0.439 |
Higher scores on items or subscales with * indicate better conditions. For items or subscales without *, higher scores indicate worse conditions
PG-TEA proximal gastrectomy tubular esophagogastric anastomosis, TG-RY total gastrectomy Roux-en-Y reconstruction
Long-term prognosis
We also compared the long-term prognoses of patients in the two groups. The 5-year overall survival (OS) rates of patients in the PG-TEA group and the TG-RY group were 72.1% and 73.7%, respectively, with no significant difference between the two groups (χ2 = 0.517, P = 0.472) (Fig. 3). The 5-year OS rates of the Siewert II and Siewert III types were 72.7% and 73.5%, respectively. There was no statistically significant difference between the two groups (χ2 = 0.517, P = 0.472) (Fig. 4).
Fig. 3.
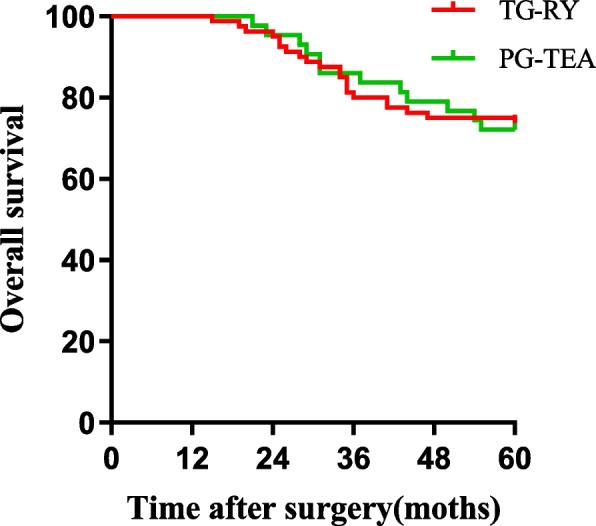
Comparison of overall survival between TG-RY and PG-TEA
Fig. 4.
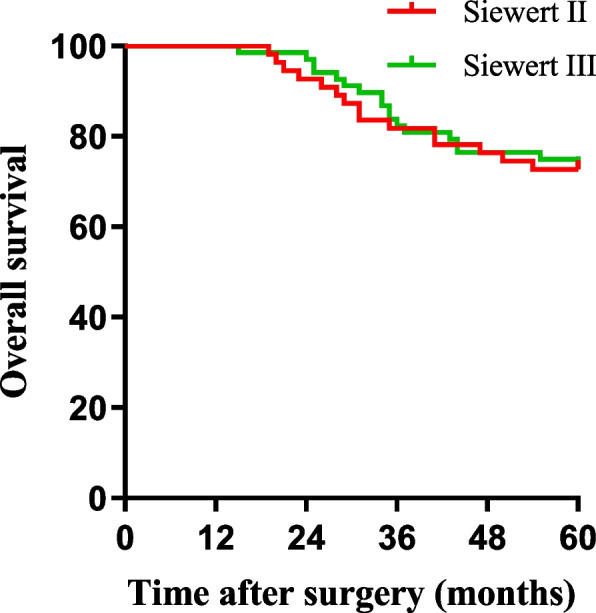
Comparison of Siewert II/III overall survival
There was no significant difference in 5-year disease-free survival (DFS) between patients in the PG-TEA and TG-RY groups (62.8% and 67.5%, χ2 = 0.049, P = 0.824) (Fig. 5). Similarly, there was no significant difference in DFS between Siewert type II patients and Siewert type III patients (74.5% and 58.8%, χ2 = 0.727, P = 0.394) (Fig. 6).
Fig. 5.
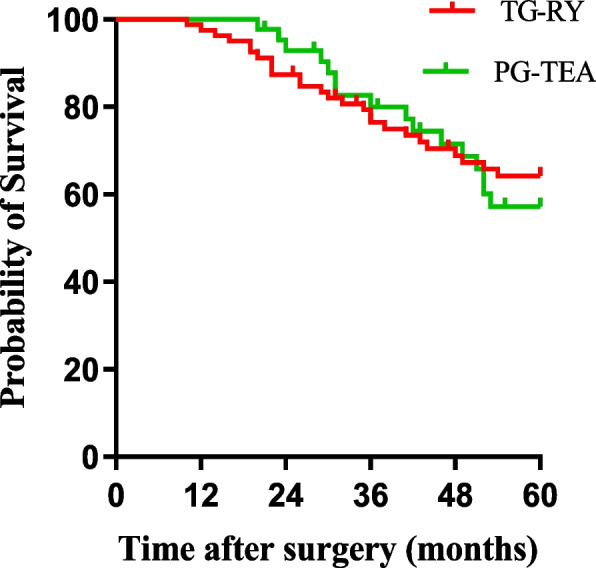
Comparison of disease-free survival between TG-RY and PG-TEA
Fig. 6.
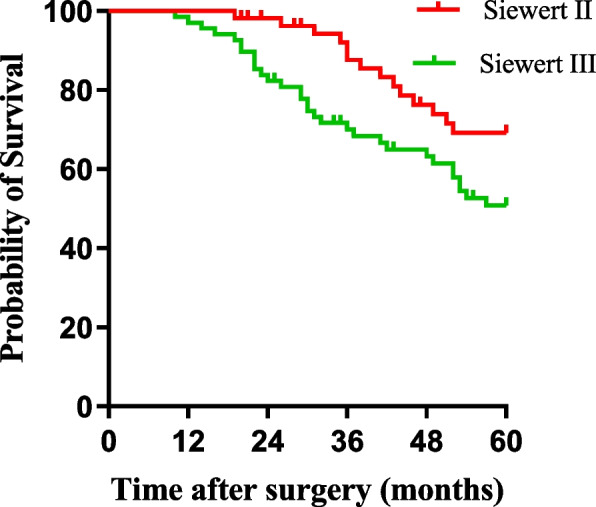
Comparison of Siewert II/III disease-free survival
Discussion
The incidence of AEG has been increasing annually [6]. The standard surgical approaches for Siewert type II and type III AEG are proximal gastrectomy (PG) and TG-RY. Chen et al. [17] reported that PG followed by tube gastroesophageal anastomosis for the treatment of upper 1/3 gastric cancer is a safe surgical technique with few early postoperative complications and good clinical outcomes. Studies have shown that patients who undergo proximal gastrectomy have a longer 5-year OS than those who undergo total gastrectomy [18]. However, the occurrence of impaired gastric emptying and reflux esophagitis after proximal gastrectomy affects patients’ postoperative quality of life and survival rate to different degrees.
After PG-TEA, lesions in the lower esophageal sphincter and gastric fundus structure are destroyed, and their anti-reflux effect is lost, increasing the incidence of gastroesophageal reflux [19]. In this study, the fundus structure was artificially reconstructed by anastomosing the esophagus to the anterior wall of the stomach 4 cm from the gastric stump and suspending the gastric stump under the pedicle of the diaphragm, which provided some anti-reflux effects. The results revealed that patients in the PG-TEA group had significantly more reflux esophagitis than those in the TG-RY Group 3 months after surgery. Nevertheless, there was no difference between the two groups at six months. The symptoms of reflux were less severe, suggesting that reconstruction of the gastric fundus structure and suspension of the gastric fundus under the foot of the diaphragm could have an anti-reflux effect. In addition, we preserved the right gastroepiploic artery and the right gastroepiploic vein, which provided more blood flow to the remnant stomach, thus reducing the incidence of anastomotic fistula. Although two patients in the PG-TEA group developed severe anastomotic ulcers and gastrointestinal bleeding within three months after surgery, which required endoscopic hemostasis or reoperation, the sample size was limited. The two cases of severe complications in this study were not representative, and larger sample numbers are needed to confirm the data.
Restoration of nutritional status contributes to the quality of life of patients and influences long-term prognosis. When preoperative BMI was used as the baseline, the postoperative weight of patients in both groups decreased. However, the weight loss in the TG-RY group was greater than in the PG-TEA group. In addition, the nutritional status of both groups was poorer in the first month after surgery, and the nutritional status of the PG-TEA group was better than that of the TG-RY group in the third month after surgery, which was more evident in the sixth month after surgery. Some studies have shown that the difference in the nutritional status of patients at 12 months post-surgery is more evident [20].
Nevertheless, because data from patients’ reviews during long-term follow-ups were missing, only serological indices at six months postoperatively were included in the present study. We also found that the hemoglobin count of the PG-TEA group was significantly higher than that of the TG-RY group in the 6th postoperative month. This result was similar to that of the study by Nobuhiro et al. [16]. The possible reasons for this result are as follows: first, the fundic glands retain the region of the fundic glands that secrete gastric acid and intracardiac factor, and vitamin B12 deficiency rarely occurs in patients undergoing fundoplication [21]; thus, hemoglobin counts in the PG-TEA group were significantly greater than those in the total gastrectomy group at the 6th postoperative month. Second, the duodenal channel, which plays an important role in the absorption of iron from food during food intake, was also preserved in the PG-TEA. Finally, the distal stomach and pylorus are preserved during surgery, which is beneficial for digestion and absorption [6, 21].
In accordance with Lin et al. [16], who reported that quality of life after gastrectomy decreased significantly in the postoperative months but stabilized substantially at approximately six months postoperatively, we chose to score patients’ quality of life at 12 months postoperatively. We assessed the patients’ somatic symptoms via the PGSAS-45 scale, and there was no significant difference between the PG-TEA and TG-RY groups in terms of the mean scores on the esophageal reflux subscale. On the dumping assessment subscale, patients in the PG-TEA group had milder symptoms and lower scores, and most of the patients in this study presented with early dumping syndrome in the form of malaise, cold sweats, bloating, and diarrhea, and late dumping syndrome in the form of hunger, malaise, and cold sweats. In addition, the PG-TEA group was superior to the PG-TEA group in terms of the incidence of diarrhea, necessity of meal refilling, and daily dissatisfaction subscale scores. In conclusion, patients in the PG-TEA group had superior long-term quality of life compared to those in the TG-RY group.
Overall survival is the most important indicator for assessing the clinical efficacy of treatment [22]. Currently, the optimal extent of resection and surgical approach for progressive (TNM stages II and III) Siewert type II and type III AEG are controversial [23, 24]. Some studies have shown that the transabdominal approach has significant advantages over the epigastric right thoracic approach in terms of postoperative long-term survival, number of lymph nodes cleared, and in terms of postoperative complications and overall quality of life than the transthoracic or combined thoraco-abdominal approach [25]. However, when the tumor invades the lower esophagus, the transabdominal approach often fails to completely resect the tumor resulting in residual cancerous tissue and, is not conducive to intrathoracic lymph node dissection [26]. The study by Xing et al. suggests that patients with Siewert type II AEG may benefit more from a transthoracic approach than a transabdominal approach, which may lead to higher survival and better R0 resection, affecting long-term quality of life, and they suggest that the main factor affecting long-term survival is the timing of postoperative chemotherapy initiation. [27]. Studies have shown that the thoracoabdominal approach allows for better mediastinal lymph node clearance and does not increase the incidence of postoperative complications compared to the transabdominal approach [28]. The results of this study showed no significant difference in OS between stage II Siewert type II and Siewert type III AEG patients who underwent TG-RY and PG-TEA. The main reason for this is that this may be due to the relatively small sample size of this study which affected the validity of the study. The cases included in this study were all operated by transabdominal approach; therefore, comparing the effects of different surgical approaches on the long-term survival of Siewert type II patients still needs to be further analyzed and explored in large-data, prospective multicenter studies.
This was a retrospective, single-center study involving a small sample size. Therefore, the results are unlikely to fully reflect the advantages, disadvantages, and long-term prognoses of the surgical approaches in both groups. This study did not statistically analyze the size of the residual stomach, mainly because these data were not entirely recorded in the medical records, resulting in missing data. However, the size of the remnant stomach is undoubtedly an important factor affecting patients’ quality of life, and previous studies have shown that the larger the remnant stomach is, the better the quality of life [29]. Therefore, these conclusions need to be validated by further studies, including a multicenter prospective randomized controlled trial with a larger sample size and a more extended follow-up period.
Conclusion
This study revealed that TG-RY and PG-TEA were associated with the exact incidence of complications and survival outcomes. Nevertheless, the quality of life of patients after tube gastroesophageal anastomosis was greater than that of patients in the total gastrectomy group, and the long-term postoperative nutritional status was also better than that of patients in the total gastrectomy group. Therefore, the authors concluded that proximal gastrectomy tube gastroesophageal anastomosis can be used as a surgical procedure for stage II Siewert II/III AEG can achieve similar clinical outcomes after total gastrectomy and can be further applied in the clinic.
Acknowledgements
This study was completed in the general department of Shaanxi Provincial People's Hospital. We would like to thank Dr. Zhijun Mao, Department of General Surgery, Shaanxi Provincial People's Hospital, for his help in the preliminary data collection. We also thank all the staff again for their help.
Declaration of conflicts of interest
The authors declare that they have no potential conflicts of interest.
Authors’ contributions
ZX and QG wrote the main manuscript text. ZX, TT and YX collected the clinical data. FX, YS and YH analyzed the data. All authors read and approved the final manuscript.
Funding
This study was supported by the Youth Fund of the National Natural Science Foundation of China (No. 82200563); Shaanxi Province Natural Science Basic Researches (No. 2022JQ-763) and the Project Fund of the Key Research and Development Programme of Shaanxi Province (No. 2023-YBSF-532); General Project of Shaanxi Provincial Science and Technology Department (No. 2024JC-YBMS-715) and Xi’an Science and Technology Program (No.2022JH-YBYJ-0465);Shaanxi Province Natural Science Basic Research Program(No.2023-JC-YB-639).
Data availability
The datasets used and/or analyzed in this study are available upon request from the corresponding author.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of Shaanxi Provincial People’s Hospital. Informed consent was obtained from all study participants before surgery (2024-R164).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qingguo Du, Email: dr_duqingguo@163.com.
Yunhua Wu, Email: wuyunhua199011@163.com.
References
- 1.Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiong HC, Wu N, Chen JF, Zhang LJ, Ji JF, Yang Y. [A clinical study of thoracic-abdominal double-incision and two-field lymphadenectomy in treatment of esophagogastric junction cancer]. Zhonghua Yi Xue Za Zhi. 2007;87(21):1478–81. [PubMed]
- 3.Jung MK, Schmidt T, Chon SH, et al. Current surgical treatment standards for esophageal and esophagogastric junction cancer. Ann N Y Acad Sci. 2020;1482(1):77–84. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T, Yoshikawa T. Optimal surgery for esophagogastric junctional cancer. Langenbecks Arch Surg. 2022;407(4):1399–407. [DOI] [PubMed] [Google Scholar]
- 5.Sun K-K, Yong-You Wu. Current status of laparoscopic proximal gastrectomy in proximal gastric cancer: technical details and oncologic outcomes. Asian J Surg. 2021;44(1):54–8. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Gong S, Tingting Lu, et al. Proximal gastrectomy versus total gastrectomy for Siewert II/III adenocarcinoma of the gastroesophageal junction: a systematic review and meta-analysis. J Gastrointest Surg. 2022;26(6):1321–35. [DOI] [PubMed] [Google Scholar]
- 7.Li Li, Cai X, Liu Z, et al. Digestive tract reconstruction after laparoscopic proximal gastrectomy for gastric cancer: a systematic review. J Cancer. 2023;14(16):3139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Lin S, Wang H, et al. Reconstruction methods after radical proximal gastrectomy. Medicine. 2018;97(11):e0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu BY, Wu S, Xu Y. Clinical efficacy and safety of double-channel anastomosis and tubular gastroesophageal anastomosis in gastrectomy. World J Gastrointest Surg. 2024;16(7):2012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Li, Zheng-Hui L, Xu-Fan C, et al. Cardia function-preserving surgery and anti-reflux anastomotic method after proximal gastrectomy for gastric cancer: current status and future perspectives. Front Oncol. 2022;12:1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Ma Y, Liu G, et al. Proximal gastrectomy with gastric tube reconstruction or jejunal interposition reconstruction in upper-third gastric cancer: which offers better short-term surgical outcomes? BMC Surg. 2021;1:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K, Zhu YF, Yang YS, et al. Interpretation of Chinese expert consensus on the surgical treatment for adenocarcinoma of esophagogastric junction(2024 edition). Zhonghua Wei Chang Wai Ke Za Zhi. 2024;27(2):127–31. [DOI] [PubMed] [Google Scholar]
- 13.Tono C, Terashima M, Takagane A, Abe K. Ideal reconstruction after total gastrectomy by the interposition of a jejunal pouch considered by emptying time. World J Surg. 2003;27(10):1113–8. [DOI] [PubMed]
- 14.Jain VK, Cunningham D, Chau I. Preoperative and postoperative chemotherapy for gastric cancer. Surg Oncol Clin N Am. 2012;21(1):99–112. [DOI] [PubMed] [Google Scholar]
- 15.Bolliger M, Kroehnert J-A, Molineus F, et al. Experiences with the standardized classification of surgical complications (Clavien-Dindo) in general surgery patients. Eur Surg. 2018;50(6):256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takiguchi N, Takahashi M, Ikeda M, et al. Long-term quality-of-life comparison of total gastrectomy and proximal gastrectomy by Postgastrectomy Syndrome Assessment Scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18(2):407–16. [DOI] [PubMed] [Google Scholar]
- 17.An JY, Youn HG, Choi MG, et al. The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg. 2008;196(4):587–91. [DOI] [PubMed] [Google Scholar]
- 18.Shen C, Yang H, Zhang B, Chen H, Chen Z, Chen J. Improved quality of life in patients with adenocarcinoma of esophagogastric junction after gastric tube reconstruction. Hepatogastroenterology. 2013;60(128):1985–9. [PubMed]
- 19.Sakamoto T, Fujimaki M, Tazawa K. Ileocolon interposition as a substitute stomach after total or proximal gastrectomy. Ann Surg. 1997;226(2):139–45. [DOI] [PMC free article] [PubMed]
- 20.Chen J, Wang F, Gao S, et al. Surgical outcomes of laparoscopic proximal gastrectomy for upper-third gastric cancer: esophagogastrostomy, gastric tube reconstruction, and double-tract reconstruction. BMC Surg. 2023;23(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanioka T, Waratchanont R, Fukuyo R, et al. Surgical and nutritional outcomes of laparoscopic proximal gastrectomy versus total gastrectomy: a meta-analysis. Surg Endosc. 2020;34(3):1061–9. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Zhao XK, Xu RH, et al. Transthoracic, thoracoabdominal, and transabdominal surgical approaches for gastric cardia adenocarcinomas: a survival evaluation based on a cohort of 7103 patients. World J Surg Oncol. 2022;20(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26(1):1–25. 10.1007/s10120-022-01331-8. [DOI] [PMC free article] [PubMed]
- 24.Liu K, Zhu YF, Yang YS, Chen LQ, Hu JK. [Interpretation of Chinese expert consensus on the surgical treatment for adenocarcinoma of esophagogastric junction(2024 edition)]. Zhonghua Wei Chang Wai Ke Za Zhi. 2024;27(2):127–31. [DOI] [PubMed]
- 25.Oh SE, Lee GH, An JY, et al. Comparison of transabdominal and transthoracic surgical approaches in the treatment of Siewert type II esophagogastric junction cancers: a propensity score-matching analysis. Eur J Surg Oncol. 2022;48(2):370–6. [DOI] [PubMed] [Google Scholar]
- 26.Zheng H, Yin X, Pan T, et al. Effect of different surgical approaches on the survival and safety of Siewert type II esophagogastric junction adenocarcinoma: a systematic review and meta-analysis. BMC Cancer. 2023;23(1):1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing J, Liu M, Kai Xu, et al. Short-term and long-term outcomes following transhiatal versus right thoracoabdominal resection of Siewert type II adenocarcinoma of the esophagogastric junction. Cancer Manag Res. 2020;12:11813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian Y, Lv H, Wang M, et al. A retrospective analysis of lymph node dissection in Siewert II adenocarcinoma of the esophagogastric junction. J Cardiothorac Surg. 2024;19(1):460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inada T, Yoshida M, Ikeda M, et al. Evaluation of QOL after Proximal Gastrectomy Using a Newly Developed Assessment Scale (PGSAS-45). World J Surg. 2014;38(12):3152–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in this study are available upon request from the corresponding author.