Abstract
Xerostomia, generally addressed as dry mouth, poses significant challenges to patients’ quality of life, particularly in the context of cancer treatment. Although various medications and interventions, including salivary substitutes and stimulants, muscarinic agonists, antineoplastic detoxifying agents, anti-inflammatory agents, superoxide dismutase mimetics, mesenchymal stem cells, submandibular gland transfer, intensity-modulated radiation therapy, dose fractionation, transcutaneous electrical nerve stimulation, hyperbaric oxygen therapy, photobiomodulation, acupuncture, and nutritional interventions, have been proposed for this condition, no approved or definite treatments are currently available. Moreover, the evidence supporting the efficacy of proposed interventions remains limited and subject to controversy in terms of safety, efficacy, and optimal protocol. This review provides a comprehensive insight into cancer treatment-related xerostomia, underlying its pathophysiology, etiology, clinical manifestation, and therapeutic options, providing a clinical guide for clinicians to adopt a patient-tailored approach to cancer treatment-related xerostomia and offering vision on current ongoing and future studies in the field.
Keywords: Cancer-related salivary dysfunction, Cancer treatment-associated xerostomia, Head and neck cancer, Muscarinic agonists, Palliative care, Radiotherapy, Xerostomia
Background
Xerostomia, commonly known as dry mouth, is a distressing condition characterized by a patient’s subjective sense of a reduced or absent saliva flow [1]. Xerostomia could be clinically accompanied by hyposalivation, also called salivary gland hypofunction [2]. It can result from various etiologies, including medications, radiation therapy, autoimmune disorders, and systemic conditions [3, 4]. This condition poses significant challenges to patients’ quality of life due to increased risk of mucositis, difficulties in speaking, painful swallowing, and predisposition to oral infections. In this review, we will explore the clinical insights of cancer treatment-related xerostomia, discuss its pathophysiology, evaluate the available treatment options and ongoing candidates, and provide future perspectives.
Etiology and pathophysiology
Saliva is pivotal in maintaining oral health by lubricating the oral mucosa, facilitating speech, bolus formation, digestion, and protection against dental caries and oral infections [5, 6]. Xerostomia disrupts this delicate balance, leading to a cascade of adverse effects. Three main salivary glands, parotid, submandibular, and sublingual, along with numerous minor salivary glands distributed throughout the oral cavity, carry the duty of saliva production. Under normal physiological conditions, saliva production is regulated by a complex interplay of neural, hormonal, and local factors, including autonomic nervous system innervation and stimulation by cholinergic and adrenergic neurotransmitters [7]. Various etiologies, such as medications, radiation therapy, autoimmune diseases, and systemic conditions, can disrupt salivary gland function, resulting in xerostomia [8].
The pathophysiology of xerostomia involves dysfunction in salivary gland secretion, either due to reduced saliva production or altered saliva composition [9–13]:
- Reduced saliva production: Several factors could contribute to reduced saliva production, including:
-
oRadiation therapy: As the leading cause of hyposalivation in cancer patients, radiation therapy to head, neck, and upper thoracic regions could irreversibly damage salivary gland tissues and impair their ability to produce saliva, leading to acute and chronic xerostomia.
-
oMedications: Certain medications, including anticholinergics, antidepressants, antihypertensives, and antihistamines, can inhibit salivary gland secretion by blocking muscarinic receptors or interfering with neurotransmitter release.
-
oSystemic diseases: Autoimmune disorders, such as Sjögren’s syndrome, and systemic conditions, such as diabetes mellitus, could cause immune-mediated destruction of salivary gland tissues, resulting in xerostomia.
-
o
Altered saliva composition: In addition to reduced saliva production, xerostomia can also result from altered saliva composition, including changes in electrolyte concentrations, pH levels, and protein content. Reduced saliva flow rates can lead to increased salivary viscosity and decreased buffering capacity, predisposing individuals to dental caries and oral infections. Altered saliva composition also affects oral mucosal integrity.
Several factors could affect the salivary glands and saliva composition, resulting in xerostomia. Table 1 summarizes the most important general causes of xerostomia. However, cancer treatment-related xerostomia could be classified to five main categories:
Table 1.
Causes of xerostomia
| Etiology | Description |
|---|---|
| Radiation therapy and radioisotopes | Radionuclides and external radiation therapy involving the head and neck region could reversibly or irreversibly damage the salivary glands |
| Systemic cytotoxic chemotherapy/immunotherapy | Chemotherapeutic agents such as 5-Fluorouracil result in altered salivary gland functions |
| Medications | Anticholinergics, antidepressants, antihypertensives, and antihistamines are common reasons for drug-induced xerostomia |
| Underlying systemic disease | Sjögren’s syndrome, diabetes mellitus, and autoimmune diseases can manifest with xerostomia |
| Dehydration | Inadequate fluid intake or conditions causing dehydration can result in transient xerostomia |
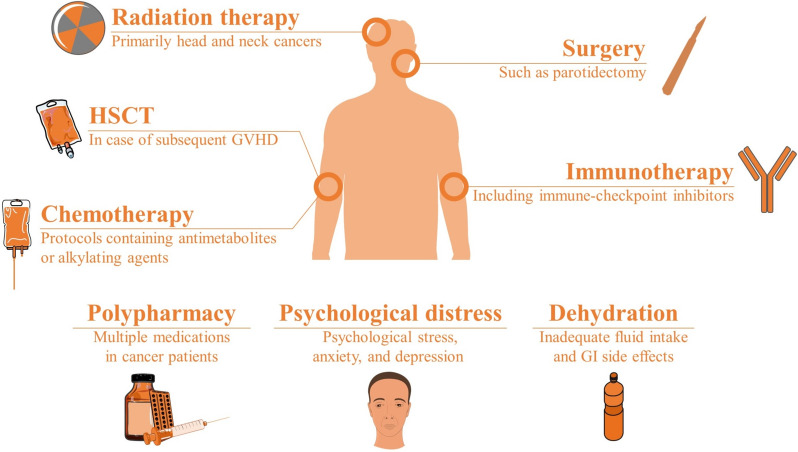
Radiation therapy-induced
Xerostomia is one of the most common adverse effects of radiation therapy [14, 15]. The salivary glands, including the major parotid, submandibular, and sublingual glands, are often exposed to radiation during treatment, leading to long-term and irreversible damage to the glandular tissues and impairment of saliva production. Previous studies have suggested that around two-third of the patients undergoing conventional two-dimensional radiotherapy techniques experience moderate-to-severe degrees of xerostomia due to irreversible damage to salivary glands [16, 17]. Newer approaches, such as intensity-modulated radiation therapy (IMRT), come with a reduced post-radiation xerostomia incidence and enhanced salivary recovery [18, 19]. A recent meta-analysis has reported an over 70% reduction in the long-term prevalence of post-radiation xerostomia, in favor of IMRT over three-dimensional conformal radiotherapy [20]. The extent and severity of xerostomia depend on various factors, including the radiation dose, treated volume, fractionation schedule, and individual patient factors, such as age and pre-existing salivary gland dysfunction [21]. Acinar cells are highly sensitive to radiation, and direct DNA damage rapidly escalates to cell death and reduced salivary flow. The repeated cell loss from radiation-induced apoptosis and necrosis can lead to glandular atrophy [22]. Radiation results in high-volume production of reactive oxygen species (ROS), damaging cellular components through oxidative stress. Preclinical studies have demonstrated a significant overexpression of genes associated with ROS, such as NOX4, following the exposure to radiation [23]. Apart from the acute impacts of radiation on salivary glands, chronic inflammation from radiation exposure can lead to excessive deposition of extracellular matrix (ECM) proteins, such as collagen, which replaces functional glandular tissue with non-functional fibrotic tissue [24, 25]. Less common, radiation-induced ductal changes may contribute to reduced salivary output [26].
Chemotherapy-induced
Chemotherapy agents can also contribute to the development of xerostomia, either directly or indirectly [27, 28]. The mechanisms of chemotherapy-induced xerostomia are also not exactly understood. Apart from the direct cytotoxic impacts of chemotherapy agents on salivary glands, the subsequent increase in ROS production and trigger of pro-inflammatory pathways potentially affects the salivary function through structural alterations, oxidative damage, and changes in vascular permeability [17, 29, 30]. Studies have shown the impact of systemic cytotoxic chemotherapy on oral microbiome composition, which in turn could lead to oral complications, such as xerostomia [31, 32]. Moreover, some agents, such as taxanes, are linked to reduced salivary function, possibly due to their neurotoxic properties [33]. The prevalence of chemotherapy-induced xerostomia is not known well, but previous studies have reported a range of 32–93% prevalence for hyposalivation [34, 35]. Certain chemotherapeutic drugs, such as cisplatin, 5-fluorouracil (5-FU), and methotrexate, may exert toxic effects on the salivary glands, leading to reduced saliva production [36–39]. In addition, chemotherapy-induced mucositis can result in pain, discomfort, and dryness in the mouth, further exacerbating xerostomia symptoms [40, 41].
Immunotherapy-induced
Immunotherapy has emerged as a promising treatment modality for various malignancies, including head and neck cancers [42]. However, immunotherapy agents, such as immune checkpoint inhibitors (ICIs), can cause immune-related adverse events, including xerostomia [43]. Xerostomia is the most common oral immune-related adverse event (irAE). Although most studies are still ongoing, an estimated xerostomia prevalence of as high as 53–58% has been linked with ICIs in some studies [44, 45]. ICIs increase the levels of inflammatory cytokines, such as interferon-gamma (IFN-γ) and interleukin-6 (IL-6) [46]. Glandular inflammation, vascular permeability changes, and edema are well-anticipated as a result of these changes. Reports are suggesting lymphocytic infiltration as a potential cause of damage to the salivary acini and ICI-related xerostomia [47, 48]. Xerostomia associated with immunotherapy may also result from autoimmune-mediated damage to the salivary glands or secondary effects of immune activation on oral mucosal tissues [49]. However, the exact mechanisms of these effects are still under investigation [50].
Surgery-associated
Although rare, salivary gland malignancies such as mucoepidermoid carcinomas are normally removed through surgery [51]. As a result of parotidectomy, saliva production decreases, and xerostomia symptoms appear in the patient.
Graft-versus-host disease
Graft-versus-host disease (GVHD) is a common complication of allogeneic hematopoietic stem cell transplantation (HSCT), occurring when donor-derived immune cells attack the recipient tissues [52]. Oral GVHD can manifest with a range of symptoms, including xerostomia, oral mucositis, and oral ulcerations [53, 54]. Xerostomia in GVHD may result from immune-mediated damage to the salivary glands, leading to decreased saliva production and oral dryness.
Other etiologies
Some other cancer treatment-related causes could indirectly lead to xerostomia [55, 56]:
Dehydration: Inadequate fluid intake could result in transient xerostomia, particularly in cancer patients undergoing aggressive treatments or experiencing gastrointestinal side effects, such as nausea/vomiting or diarrhea.
Polypharmacy: Cancer patients often receive multiple medications, including chemotherapy agents, supportive medications, and medications for comorbid conditions, many of which can contribute to xerostomia as a side effect.
Psychological factors: Psychological stress, anxiety, and depression commonly experienced by cancer patients can also exacerbate xerostomia symptoms through effects on autonomic nervous system regulation and saliva production.
Figure 1 demonstrates the underlying etiologies and causes of treatment-related xerostomia in people with cancer.
Fig. 1.
Underlying causes of treatment-related xerostomia in cancer patients (GI gastrointestinal, GVHD graft vs host disease, HSCT hematopoietic stem cell therapy)
Clinical manifestations
Patients with xerostomia may present with various accompanying symptoms, ranging from mild discomfort to severe impairment of oral functions. Common clinical manifestations include [29, 57–60]:
Dryness: Patients complain of persistent dryness in the mouth and throat, which can be exacerbated by environmental factors, such as dry air or mouth breathing.
Difficulty in chewing and swallowing: Reduced saliva impairs the lubrication and bolus formation, leading to difficulties in chewing and swallowing food.
Dysphagia: Severe cases of xerostomia may result in dysphagia, making it challenging for patients even to swallow liquids (Grade IV dysphagia).
Oral infections: Diminished saliva flow compromises the oral mucosal defense mechanism, increasing the risk of oral infections, such as candidiasis and bacterial overgrowth.
Dental caries: Saliva is crucial in remineralizing enamel and buffering oral pH. Xerostomia predisposes individuals to dental caries and tooth decay. Radiation caries can develop shortly after radiation. Radiation can weaken the enamel, making teeth more susceptible to decay. Without sufficient saliva due to radiation-induced xerostomia, enamel demineralization accelerates and caries appear. Radiation caries typically appear with enamel craze lines, black/brownish tooth discoloration, delamination, and rapid tooth destruction or crown amputation.
Tooth loss: Dryness, changes in the oral mucosa, and the development of dental caries may lead to permanent periodontal tissue damage and increase the risk of tooth loss.
The severity of xerostomia symptoms can vary depending on the underlying etiology, duration of the problem, and patient-centered factors, such as age, overall health status, and concomitant use of medications. Table 2 presents the standard clinical grading for xerostomia based on the Common Terminology Criteria for Adverse Events (CTCAE) [61].
Table 2.
Clinical grading of xerostomia, based on the common terminology criteria for adverse events (CTCAE)
| Grading | Clinical implication | Unstimulated saliva flow |
|---|---|---|
| Grade 1 (mild) | Symptomatic, without significant dietary changes | ≥ 0.2 mL/min |
| Grade 2 (moderate) | Symptomatic, with significant dietary changes (Large intake of water or use of other lubricants, or diet limited to soft food) | 0.1–0.2 mL/min |
| Grade 3 (severe) | Symptomatic, with oral feeding inability—requiring enteral or parenteral nutrition | < 0.1 mL/min |
| Grade 4 (life-threatening) | – | – |
| Grade 5 (fatal) | – | – |
Management
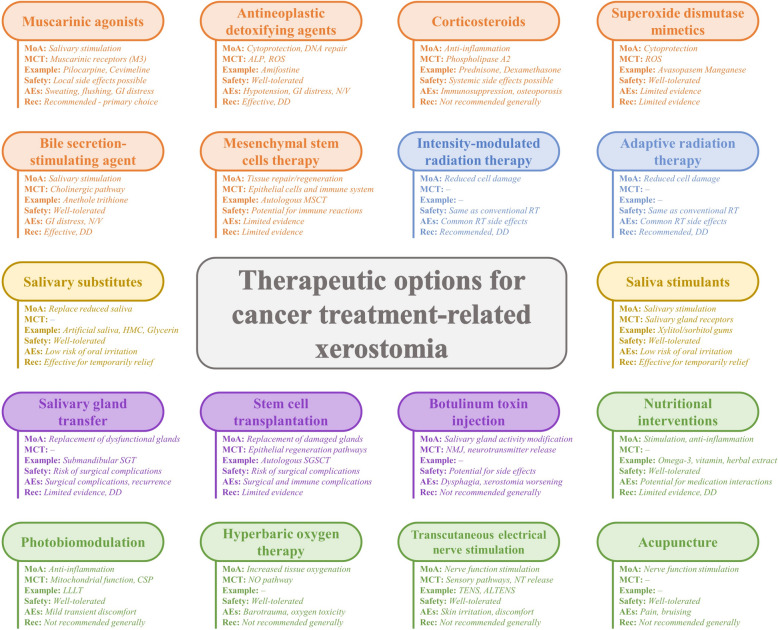
Currently, there are no approved treatments for cancer treatment-related xerostomia. However, several modalities have shown promise in preclinical and clinical studies [62]. Figure 2 presents an inclusive outline of currently suggested interventions for the management of cancer treatment-related xerostomia, their mechanisms of action, molecular/cellular targets, safety, and adverse effects, along with examples and clinical recommendations.
Fig. 2.
Comprehensive overview of proposed interventions for cancer treatment-related xerostomia, their mechanisms of action, molecular and cellular targets, available examples, safety, adverse effects, and general recommendation (AEs Adverse effects, ALP Alkaline phosphatase, ALTENS Acupuncture-like Transcutaneous electrical nerve stimulation, CSP Cellular signaling pathways, DD Discretionary decision (per physician’s judgement), GI Gastrointestinal, HMC Hydroxyethylcellulose, IMRT Intensity-modulated radiation therapy, LLLT Low-level laser therapy, MCT Molecular/cellular target, MoA Mechanism of action, MSCT Mesenchymal stem cells transplantation, N/V Nausea/Vomiting, NMJ Neuromuscular junction, NO Nitric Oxide, NT Neurotransmitter, Rec Recommendation, ROS Reactive oxygen species, RT Radiotherapy, SGSCT Salivary gland stem cells transplantation, SGT Salivary gland transfer, TENS Transcutaneous electrical nerve stimulation)
Pharmacological treatments
Muscarinic agonists
Muscarinic agonists, including pilocarpine and cevimeline, are currently the primary pharmacological candidates for cancer treatment-related xerostomia [63]. These agents activate the muscarinic acetylcholine receptors (mAChRs) located on the surface of salivary gland cells [64]. As a subdomain of the G protein-coupled receptors family, these receptors are predominantly of the M1 and M3 subtypes, primarily responsible for stimulating salivary gland secretion [64].
Pilocarpine is a non-selective muscarinic agonist. Upon binding to muscarinic receptors, pilocarpine activates intracellular signaling pathways that result in increased intracellular calcium levels and subsequent exocytosis of secretory vesicles [65]. This mechanism leads to enhanced saliva production and improved oral moisture, relieving symptoms of dry mouth. On the other hand, cevimeline is a selective muscarinic M1 and M3 receptor agonist that is expected to show a greater specificity for salivary gland tissue compared to pilocarpine [66]. By targeting the M3 subtype of muscarinic receptors, cevimeline specifically stimulates salivary gland secretion without significantly affecting other muscarinic receptor subtypes present in non-salivary tissues. This selective action reduces the likelihood of off-target adverse effects commonly associated with non-selective muscarinic agonists.
Both pilocarpine and cevimeline have demonstrated efficacy in increasing salivary flow rates and improving symptoms of xerostomia in patients undergoing cancer treatment [67, 68]. In general, some studies have suggested higher salivary flow in post-treatment cancer patients under cevimeline, but the differences have not been statistically significant [69]. On the other hand, apart from the etiology and considering other patients, including the ones with Sjögren’s syndrome, diabetes mellitus, and other contributing diseases, studies have suggested pilocarpine with slightly higher salivary flow, which is also statistically non-significant [70]. Moreover, muscarinic agonists have also been effective in palliating other post-cancer treatment xerostomia-associated complications, such as oral mucosal inflammation and dental caries [62].
Considering the high heterogeneity of the available data, the optimum dosage of pilocarpine and cevimeline has not been determined so far; however, 5 mg and 30 mg tablets are typically recommended, respectively, both three times per day [71]. Some studies have also suggested pilocarpine administration as a mouthwash solution [72]; however, the results seem inferior to systemic administration, though inconclusive [73]. However, considering the lower adverse effects frequency and better patient compliance, topical pilocarpine is still considered an equivalent to its systemic routes of administration [67]. Several common adverse effects, including sweating, gastrointestinal discomfort, flushing, and urinary symptoms, are associated with the use of these muscarinic agonists [65]. Both medications are well-tolerated and safe, though pilocarpine has been associated with slightly higher rates of sweating, flushing, and gastrointestinal discomfort adverse effects [69].
Overall, current studies cannot determine the superiority of either medication, and the choice between pilocarpine and cevimeline should be made according to each patients’ baseline characteristics, the clinical response to each medication, their subjective satisfaction with the treatment, and most importantly, the frequency and severity of adverse effects.
Antineoplastic detoxifying agents
Antineoplastic detoxifying agents are used to reduce the toxic effects of chemotherapy and radiation therapy on normal tissues while preserving the therapeutic efficacy against cancer cells. These agents function by various mechanisms, including scavenging free radicals, enhancing DNA repair mechanisms, and reducing inflammation and oxidative stress [74, 75]. As a promising antineoplastic detoxifying agent, amifostine transforms to free thiol metabolite by the mediating effect of alkaline phosphatase—which is observed to be significantly more active in the normal tissues than the tumor tissue—reducing radiation toxicity in non-tumor tissue [76]. In addition, amifostine is known to enhance the activity of DNA repair enzymes, reduce inflammation by suppressing the release of pro-inflammatory cytokines and chemokines, such as tumor necrosis factor-alpha (TNF-α) and IL-6, and improve tissue oxygenation by vasodilation, thereby reducing the radiation-induced hypoxic state [77]. Amifostine is generally well-tolerated among the patients [78].
Anti-inflammatory agents
Corticosteroids are one of the widely used interventions for Sjögren’s syndrome-associated xerostomia, suggesting the potential applicability of these anti-inflammatory agents to cancer treatment-related xerostomia [79]. Corticosteroids suppress the release of pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α, and increase the expression of lipocortin, therefore inhibiting the phospholipase A2 and the subsequent synthesis of inflammatory mediators, such as prostaglandins and leukotrienes [80].
Corticosteroids have been recently utilized for cancer treatment-related, mainly immunotherapy-related xerostomia [81]. However, corticosteroids are associated with a variety of potential adverse effects, ranging from hyperglycemia to osteoporosis [82]. Moreover, corticosteroids might not be administrable in some phases of cancer treatment; therefore, are less favored for this condition [83].
Superoxide dismutase mimetics
Superoxide dismutase enzymes catalyze the conversion of superoxide radicals () into oxygen (O2) and hydrogen peroxide (H2O2), thereby neutralizing ROS and preventing oxidative damage to cellular components [84]. Superoxide dismutase mimetics, such as MnTE-2-PyP manganese porphyrin and avasopasem manganese, have been previously studied for cancer treatment-related xerostomia, showing effectiveness in reducing post-radiation xerostomia and mucositis [85–87]. However, further studies are needed to establish the efficacy and safety of these agents.
Anethole trithione
As a bile secretion-stimulating drug and dithiole–thione derivative, anethole trithione could be used to treat xerostomia due to its parasympathomimetic effects and consequent impact on salivary secretion [88]. Anethole trithione is generally well-tolerated, with some cases of gastrointestinal discomfort [88].
Autologous mesenchymal stem cells
Autologous mesenchymal stem cells have attracted attention in regenerative medicine due to their unique ability in modulating immune responses and promote tissue repair. Several preliminary studies have demonstrated the safety and clinically significant efficacy of the autologous mesenchymal stem cells for cancer treatment-related xerostomia [89, 90]. Although is not currently an optimal approach, mesenchymal stem cells could be an interesting option for future research and practice.
Symptom-based care
Oral moisturizing products
Moisturizing oral products such as oral rinses, gels, mouthwashes, lip balms, and artificial saliva can temporarily relieve xerostomia symptoms. These products often contain humectants, such as glycerin or sorbitol, which attract and retain moisture in the oral cavity [91]. Honey, aloe vera, and seaweed could act as natural humectants, briefly relieving the symptoms of xerostomia (also see ‘complementary and alternative treatments’) [92, 93].
Salivary substitutes
Salivary substitutes mimic the composition and function of natural saliva, providing lubrication and moisture to the oral mucosa. These substitutes generally contain carboxymethylcellulose, hydroxyethylcellulose, mucin, or glycerin as active ingredients, and are available in different forms, including gels, sprays, and lozenges [94, 95]. Salivary substitutes are available in various formulations, including sprays, gels, and lozenges. Some herbal and natural extracts, such as thyme, honey, and rice bran oil, have shown positive potential in managing xerostomia by substituting saliva and increasing the salivary flow, which are further discussed in Table 3 and the ‘complementary and alternative treatments’ section.
Table 3.
Trials with complementary and integrative approaches to cancer treatment-related xerostomia
| Author (Reference) | Year | Country | Design | Intervention | Patients and participants | Study groups | Outcomes |
|---|---|---|---|---|---|---|---|
| Garcia et al. [134] | 2019 | USA and China | RCT | Acupuncture | Head and neck cancer patients (339 patients) |
- Standard care control (SCC): 112 patients - Intervention group (True acupuncture): 112 patients - Control group (Sham acupuncture): 115 patients |
Xerostomia in the intervention group was significantly lower than in the SCC group, but the difference with the control group was not statistically significant. Symptoms were fewer and less severe 1 year after treatment compared to SCC |
| Simcock et al. [135] | 2012 | UK | Crossover RCT | Acupuncture | Patients with chronic radiation-induced xerostomia (145 patients) |
- Educational oral care program - Weekly group acupuncture (8 sessions) |
Acupuncture provided significantly better symptom management for patients suffering from chronic radiation-induced xerostomia. However, no significant changes were observed in objective saliva measurements |
| Meng et al. [136] | 2011 | China | RCT | Acupuncture | Nasopharyngeal carcinoma patients undergoing radiotherapy (86 Patients) |
- Intervention group: 40 patients - Control group: 46 patients |
The acupuncture group had a significantly lower prevalence of xerostomia and showed a preventing potential, leading to improved quality of life |
| Forner et al. [137] | 2011 | Denmark | Uncontrolled pilot study | Hyperbaric oxygen | Irradiated head and neck cancer patients (80 patients) | - Intervention group: 80 patients | Subjective improvement was reported among the patients with hyposalivation and xerostomia |
| Palma et al. [138] | 2017 | Brazil | Clinical trial | Photobiomodulation | Head and neck cancer patients with radiation-induced xerostomia (29 patients) | - Intervention group (Low-level laser therapy for 24 sessions in 3 months): 29 patients | Salivary flow rates and quality of life were improved |
| Louzeiro et al. [139] | 2020 | Brazil | RCT | Photobiomodulation | Head and neck patients undergoing radiotherapy (21 patients) |
- Intervention group: 10 patients - Control group (sham group): 11 patients |
The salivary flow deteriorated in both groups. No difference was observed between groups regarding salivary flow and composition, xerostomia, or quality of life |
| de Carvalho e Silva et al. [140] | 2023 | Brazil | RCT | Photobiomodulation and artificial saliva | Patients with head and neck squamous cell carcinoma (53 patients) |
- Intervention group (artificial saliva and photobiomodulation) - Control group (artificial saliva and sham laser simulation) |
The intervention group experienced a significantly improved state of xerostomia and quality of life. The groups had no significant difference in the DMFT index or periodontal charts |
| Nuchit et al. [141] | 2020 | Thailand | RCT | Saliva substitutes (Oral moisturizing jelly versus a topical saliva gel) | Post-radiation head and neck cancer patients (62 patients) |
- Intervention group 1 (OMJ): 31 patients - Intervention group 2: 31 patients |
Continuous use of saliva substitutes (OMJ or SG) for over 1 month improves xerostomia, enhancing the swallowing ability. Edible OMJ is superior to topical SG |
| Apperley et al. [142] | 2017 | New Zealand | Crossover RCT | Emulsion of rice bran oil, soy lecithin, and propylene glycol | Patients treated with head and neck radiotherapy (40 patients) |
- Intervention group (emulsion) - Control group 1 (methylcellulose) - Control group 2 (water) |
None of the products showed a significant difference in patient outcomes |
| Rupe et al. [143] | 2023 | Italy | Crossover RCT | Sodium-hyaluronate mouthwash | Patients with head and neck cancer (39 patients) |
- Intervention group (GUM Hydral®) - Control group (Placebo) |
The intervention significantly reduced the symptoms of xerostomia. The intervention group had higher reported satisfaction levels among the patients |
| Beuth et al. [144] | 2013 | Germany | Clinical trial | A combination of sodium selenite, proteolytic plant enzymes (bromelain and papain), and Lens culinaris lectin | Breast cancer patients undergoing adjuvant hormone therapy (310 patients) | - Intervention group: 310 patients | Almost two-thirds of patients with severe mucosal dryness significantly benefited from complementary medicine. Side-effects of hormone therapy were significantly reduced after 4 weeks |
| Heydarirad et al. [145] | 2017 | Iran | RCT | Alcea digitata and Malva sylvestris | Head and neck cancer patients (60 patients) |
- Intervention group: 30 patients - Control group: 30 patients |
In the intervention group, a significant improvement in quality of life, pain, and ease of swallowing, speaking, and eating was observed |
| Quimbt et al. [146] | 2020 | Canada | Uncontrolled pilot study | Coconut oil | Post-radiation head and neck cancer patients (30 patients) | - Intervention group: 30 patients | No significant difference was observed before and after the intervention |
| Charalambou et al. [147] | 2018 | Cyprus | RCT | Thyme honey rinses | Head and neck cancer patients (72 patients) |
- Intervention group: 36 - Control group (saline rinses): 36 |
The intervention group had significantly lower grades of xerostomia. Patients’ quality of life was also significantly higher in the intervention group |
| Chamani et al. [148] | 2017 | Iran | RCT | Ginger capsule | Patients with post-radiotherapy xerostomia (61 patients) |
- Intervention group: 30 patients - Control group (Placebo): 31 patients |
Although the intervention group had a marginally improved status, no significant difference was observed between groups |
| Chung et al. [149] | 2016 | South Korea | RCT | Antioxidant supplements (vitamin E + vitamin C) | Head and neck cancer patients with radiotherapy-induced xerostomia (45 patients) |
- Intervention group: 25 patients - Control group (Placebo): 20 patients |
The intervention group showed significant long-term improvement compared to the control group |
| Heiser et al. [150] | 2016 | Germany | Clinical trial | Liposomal nose and mouth spray (LipoNasal, LipoSaliva) | Head and neck cancer patients (98 patients) | - Intervention groups (three subgroups per cancer treatment): 98 patients | A positive subjective outcome was observed, which could suggest liposomal sprays as a first-line treatment due to their safety |
| Steinmann et al. [151] | 2012 | Germany | Non-randomized trial | Homeopathy (Mouth rinses with either Traumeel S solution or Salvia officinalis) | Patients under radiation therapy for head and neck cancer (20 patients) |
- Intervention group (Traumeel S): 10 patients - Control group (sage tea or Salvia officinalis): 10 patients |
No significant difference was observed among the study groups |
| Dalbem Paim et al. [152] | 2019 | Brazil | RCT | Transcutaneous electrical nerve stimulation (TENS) | Post-radiation head and neck cancer patients (68 patients) |
- Intervention group (TENS): 37 patients - Control group: 31 patients |
The intervention group showed a progressive increase in salivary flow in long-term follow-up |
| Wong et al. [153] | 2015 | USA | Clinical trial | Acupuncture-like transcutaneous electrical nerve stimulation (ALTENS) | Patients with post-radiation xerostomia (146 patients) |
- Intervention group 1 (ALTENS): 75 patients - Intervention group 2 (Oral Pilocarpine): 73 patients |
No significant difference was observed between the groups. However, less toxicity was seen in patients receiving ALTENS. Radiation-induced xerostomia improved over time for all patients |
ALTENS Acupuncture-like transcutaneous electrical nerve stimulation, DMFT index decayed, missing, and filled teeth index, OMJ Oral moisturizing jelly, RCT randomized controlled trial, SG Saliva gel, TENS Transcutaneous electrical nerve stimulation
Saliva stimulants
The general concept behind using saliva-stimulating interventions is the same as the commonly prescribed muscarinic receptor agonists: relieving xerostomia symptoms by increasing saliva production. Gums, particularly the ones containing xylitol or sorbitol, natural products with citric acid, and some ginger-based supplementations have been proposed for this purpose (also see ‘complementary and alternative treatments’) [96].
Complementary and alternative treatments
Previous studies have suggested the potential role of several complementary and alternative treatments in ameliorating the symptoms of xerostomia and improving patients’ quality of life [97]. Table 3 broadly reviews the key trials from complementary and integrative approaches to cancer treatment-related xerostomia.
Acupuncture
Acupuncture, rooted in traditional Chinese medicine, involves precise insertion of needles into specific acupoints to stimulate physiological responses [98]. Several studies have investigated the efficacy of acupuncture in managing xerostomia, particularly in patients undergoing radiation therapy for head and neck cancer [99, 100]. Acupuncture is believed to modulate salivary gland function by activating neural pathways and promoting vasodilation, leading to increased saliva production and improved oral moisture [101]. While some clinical trials have reported positive outcomes regarding subjective symptom relief and objective measures of saliva flow, the evidence remains inconclusive due to methodological limitations and heterogeneity among study designs (Table 3) [102]. Moreover, recent evidence-based studies have revealed no clinically significant efficacy of acupuncture in the treatment of radiation-induced xerostomia [103]. Further well-designed randomized controlled trials (RCTs) are required to clarify the efficacy and optimal treatment protocols of acupuncture for xerostomia.
Nutritional interventions
Many nutraceuticals have garnered interest in managing xerostomia due to their antioxidative, anti-inflammatory, and mucoprotective properties. Examples of nutraceuticals studied for xerostomia include omega-3 fatty acids, vitamin C, vitamin E, and herbal extracts, such as green tea polyphenols and aloe vera [104–106]. These compounds are hypothesized to alleviate xerostomia symptoms by reducing oxidative stress, inflammation, and mucosal damage. While some preclinical and clinical studies have shown promising results in improving saliva flow rates and subjective symptom relief, the evidence remains limited, and further research is needed to establish their efficacy, optimal dosing regimens, and long-term safety profiles. Avoidance of caffeine, alcohol, and tobacco can also help mitigate xerostomia symptoms, as these substances can exacerbate dry mouth [107, 108].
Photobiomodulation
Photobiomodulation has been investigated for its potential to stimulate salivary gland function and increase saliva production in patients with xerostomia. Low-level laser therapy applied to the salivary glands may enhance cellular metabolism, promote tissue repair, and modulate inflammatory responses, leading to improved salivary flow rates and alleviation of xerostomia symptoms [109, 110].
Xerostomia often involves inflammatory changes in the salivary glands and oral mucosa and photobiomodulation has anti-inflammatory effects [111]. By modulating inflammatory mediators and cytokines, photobiomodulation may palliate tissue damage, enhance tissue repair processes, and alleviate symptoms associated with oral dryness and discomfort. In addition, photobiomodulation could promote minimum analgesic effects by modulating pain perception, reducing nerve sensitivity, and promoting the release of endogenous opioids, providing brief symptomatic relief of xerostomia-related pain and enhancing the patient’s quality of life [112, 113]. There are significant variations in procedures among studies regarding the number of points, energy, and density, but generally, parotid and submandibular glands were treated through extraoral protocols at 808 nm, while sublingual glands followed the extraoral protocols at 660 nm. Nevertheless, most clinical evidence only shows subjective improvement in patients’ symptoms (Table 3).
Transcutaneous electrical nerve stimulation
Transcutaneous electrical nerve stimulation (TENS) is a non-invasive modality involving the application of low-voltage electrical currents through electrodes placed on the skin. While TENS is primarily used for pain management, it has also been explored for its potential therapeutic effects in various medical conditions, including xerostomia [114]. The electrical stimulation delivered by TENS could directly stimulate nerve fibers innervating the salivary glands, potentially enhancing neural signaling and promoting salivary flow [115]. TENS could stimulate the release of endogenous opioids, relieving xerostomia-related symptoms and pain. It may also improve blood flow to the salivary glands and surrounding tissues [114]. TENS protocols also vary significantly among the studies, with frequency starting from 4 Hz, pulse width of 250 μs–250 ms, and sessions ranging from 5 to 20 min [116]. The current studies on TENS present lower levels of evidence with limited confidence in results for clinical use. Similar to photobiomodulation, TENS requires extensive future studies to ensure efficacy and become integrated into the conventional approach to this condition.
Hyperbaric oxygen therapy
Hyperbaric oxygen therapy delivers high concentrations of oxygen to tissues, reducing inflammation and improving the oxygenation of hypoxic tissues. While hyperbaric oxygen therapy is primarily used to treat conditions, such as decompression sickness and gas embolism, it has also been explored for its potential therapeutic effects in various medical conditions, including xerostomia. The primary idea behind the initial use of hyperbaric oxygen for this condition is the promotion of neovascularization and nitric oxide pathway in hypoxic tissues, suppressing pro-inflammatory cytokines, and modulating immune cell function in salivary glands [117].
Meanwhile, hyperbaric oxygen could also result in barotrauma, oxygen toxicity, and claustrophobia in occasional cases. Overall, this intervention could lead to long-term subjective satisfaction of patients, but the available evidence does not demonstrate objective clinical significance [118, 119].
Homeopathic treatments
Homeopathic treatments involve using highly diluted natural substances, typically derived from plants or minerals, to stimulate self-healing mechanisms and restore balance. In the context of xerostomia, homeopathic treatments aim to address the underlying causes of dry mouth symptoms and promote salivary gland function and oral moisture. Homeopathic remedies for oral complications of cancer may include substances, such as Hypericum, Arsenicum album, Matricaria chamomilla, or Salvia officinalis [120, 121]. Nevertheless, the impact of homeopathic treatments on xerostomia is a subject of serious debate, since the scientific basis and efficacy of these interventions are questionable, and the available studies are limited, doubtful, and inconclusive [122].
Surgical approach
Submandibular salivary gland transfer
One of the widely known suggested approaches to cancer treatment-related xerostomia is the submandibular salivary gland transfer [123]. The relocation of the healthy submandibular salivary gland from its original anatomical location to a site outside the radiation field, typically in the submental region, could improve the salivary flow rates [124]. However, studies have reported variable outcomes following surgery, with some patients experiencing significant improvements in oral moisture and quality of life, while others may have more modest or transient benefits. Several factors, including patients’ characteristics, timing of surgery relative to radiation therapy, surgical technique, and postoperative management, could impact the outcomes. Besides, this approach makes the patients prone to surgical complications, such as surgical site infection or hematoma, salivary fistula, vascular compromise of the transferred gland, or transient/permanent facial nerve injury.
Autotransplantation and stem cell transplantation
There are some reports of successful intervention for post-radiation xerostomia using autotransplantation of cryopreserved minor salivary glands [125]. Autologous salivary gland stem cell transplantation has also been around for over a decade, showing impressive outcomes [126]. However, lack of long-term follow-up data, financial burdens, and non-applicability for all patients have limited the clinical pertinence of these approaches.
Botulinum toxin injection
Although botulinum toxin injection has been suggested to prevent radiation-induced sialadenitis, studies have shown a generally reduced salivary flow, which would lead to worsening symptoms in patients with xerostomia [127]. In general, patients with cancer treatment-related xerostomia might not benefit from botulinum toxin injection.
Gland-preserving radiation therapy techniques
As an effective preventive measure, techniques such as IMRT or proton therapy may be employed to minimize radiation exposure to the salivary glands.
Intensity-modulated radiation therapy
IMRT is a radiation technique that delivers highly conformal doses of radiation to the tumor target while minimizing radiation exposure to surrounding normal tissues, such as salivary glands [128]. IMRT uses multiple radiation beams that can be modulated in intensity and directed from different angles to precisely shape the radiation dose distribution and spare critical structures, such as the salivary glands. By optimizing the radiation dose distribution, IMRT reduces the risk of radiation-induced damage to the salivary glands and preserves salivary function.
Proton therapy
Proton therapy is an advanced form of radiation therapy that uses protons, rather than conventional photons, to deliver radiation to the tumor target. Proton therapy offers the advantage of delivering radiation with greater precision and sparing healthy salivary glands from unnecessary radiation exposure [129].
Adaptive radiation therapy
Adaptive radiation therapy (ART) involves the real-time monitoring and adjustment of radiation therapy based on changes in tumor size, shape, and position during the course of treatment. This technique facilitates monitoring anatomical changes and could help minimize radiation exposure to salivary glands. However, recent studies have shown non-superiority of ART compared to IMRT [130].
Dose fractionation
Dose fractionation refers to delivering radiation therapy in smaller, divided doses over multiple treatment sessions, rather than in a single high-dose fraction. Fractionating the radiation dose allows for the repair of sublethal damage to normal tissues between treatment sessions, reducing the risk of acute and late radiation-induced xerostomia [131].
Salivary gland shields and positioning stents
During radiation therapy planning, customized shielding blocks or devices could be positioned to physically shield the salivary glands from direct radiation exposure. Positioning stents are simple and available options that have shown effectiveness in preventing mid-term complications, such as xerostomia, as higher salivary flow rates have been reported within 6 months after radiation [132, 133]. However, the level of evidence is still weak, and more studies are required [133].
Ongoing studies and future directions
Several studies are being conducted worldwide to find the optimum treatment of choice for cancer treatment-related xerostomia. Table 4 summarizes the ongoing trials of potential candidates for this condition.
Table 4.
Active and ongoing trials for management of cancer treatment-related xerostomia
| Country | Study design | Study population | Intervention(s) | Source ID (clinicaltrials.gov) |
|---|---|---|---|---|
| USA | Randomized, multicenter, double-blinded, controlled trial | Adults with radiation-induced late xerostomia |
AAV2-hAQP1 Gene therapy |
NCT05926765 |
| USA | Dose-escalation phase I trial | Post-radiation adults with head and neck cancer |
AAV2-hAQP1 Gene therapy |
NCT02446249 |
| Denmark | Randomized, single-center, double-blinded, controlled trial (phase II) | Post-radiation adults with head and neck cancer | Mesenchymal stem cells | NCT04776538 |
| Denmark | Non-randomized phase I | Post-radiation adults with oropharynx cancer | Mesenchymal stem cells | NCT03874572 |
| USA | Dose-escalation phase I trial | Adult head and neck cancer patients | Bone marrow cell transplantation into the salivary glands | NCT05820711 |
| Brazil | Randomized, multicenter, single-blinded controlled clinical trial | Post-radiation adults with head and neck cancer | Photobiomodulation (Intraoral and extraoral) | NCT05242991 |
| Brazil | Randomized, multicenter, single-blinded clinical trial | Adult patients undergoing hematopoietic cell transplantation | Photobiomodulation (Intraoral and extraoral) | NCT05759975 |
| Spain | Randomized, single-center, double-blinded controlled trial | Adult head and neck cancer patients | Photobiomodulation (Intraoral and extraoral—along with M-health tool) | NCT05106608 |
| USA | Single-arm prospective study | Post-radiation adults with head and neck cancer | Non-invasive acupuncture-like transcutaneous electrical nerve stimulation (ALTENS) | NCT04805528 |
| USA and China | Randomized, multicenter, controlled trial | Adults with head and neck cancer, scheduled to undergo IMRT | Acupuncture | NCT01266044 |
| France | Randomized, multicenter, single-blinded clinical trial | Adult head and neck cancer patients | Auriculotherapy | NCT04222478 |
| Denmark | Single-arm pilot trial | Post-radiation adults with head and neck cancer | Craniosacral therapy | NCT05882890 |
| France | Randomized, single-center, controlled trial | Post-radiation adults with head and neck cancer | Corticosteroids | NCT04584164 |
| Cyprus | Randomized, multicenter, double-blinded controlled trial | Adult head and neck cancer patients undergoing radiotherapy | Thyme and honey spray | NCT04880148 |
| China | Randomized, multicenter, non-inferiority, controlled trial | Adult patients with nasopharyngeal carcinoma | Intensity-modulated radiation therapy (IMRT) | NCT06282497 |
| Switzerland | Single-arm trial | Adults with head and neck cancer | Adaptive radiation therapy | NCT03972072 |
| USA | Randomized, single-center, controlled trial | Adults with squamous cell carcinoma of the head and neck | IMRT (margin-based vs. robust photon radiation therapy planning) | NCT03552965 |
AAV2-hAQP1 Adeno-associated viral vector encoding human Aquaporin-1, ALTENS Acupuncture-like transcutaneous electrical nerve stimulation, IMRT Intensity-modulated radiation therapy
Future studies, specifically, randomized controlled trials, with larger sample sizes and longer follow-up durations, are required to evaluate the efficacy, safety, and optimal treatment protocols of the discussed modalities. Considering the high prevalence of post-treatment xerostomia in cancer patients and the lack of approved treatments, this subject requires more attention and collaborative efforts, using multidisciplinary teams of medical, surgical, and radiation oncologists, palliative care physicians and nurses, dentists, and otolaryngologists. Standardized outcome measures are needed to consistently assess subjective symptom relief, objective salivary flow rates, and patient-reported outcomes across studies. Moreover, most of the current evidence with complementary and non-conventional interventions exclude patients with a history of cancer or prior head and neck radiation therapies, which results in limited insight into the management of cancer treatment-related xerostomia. While complementary therapies offer potential benefits, the limitations of the existing evidence should be considered, including the heterogeneity among study designs, lack of reproducibility due to subjective assessment, variability in treatment protocols, and potential placebo effects.
Conclusions
Cancer treatment-related xerostomia is a complex and multifactorial condition that significantly impacts patients’ quality of life. Clinicians should be skilled at recognizing serious cases of xerostomia and implementing appropriate management strategies tailored to individual patient needs. Muscarinic agonists, novel radiation therapy approaches, salivary substitutes, saliva stimulants, oral moisturizing products, and nutritional supplementations have shown the potential for improving xerostomia symptoms and patients’ oral health and well-being; however, the current evidence is limited and, in some cases, probably biased, due to protocol variabilities and potential placebo effects. Further research is required to ensure the efficacy and safety of the proposed treatments and to develop well-tolerated protocols for xerostomia management in patients with cancer.
Acknowledgements
We would like to express our appreciation for the cooperation of the Clinical Research Development Unit of Tabriz Valiasr Hospital, Tabriz University of Medical Sciences, Tabriz, Iran, in conducting this research. Some parts of the figures were created using Servier Medical Art, licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Author contributions
MSH: Conceptualization, Data Curation, Supervision, Visualization, Writing—Original Draft. SS: Conceptualization, Data Curation, Supervision, Writing—Original Draft. AM: Data Curation, Visualization, Writing—Original Draft. SJB: Conceptualization, Data Curation, Writing—Original Draft. HJB: Conceptualization, Data Curation, Writing—Review & Editing. SF: Data Curation, Writing—Review & Editing. FJ: Data Curation, Writing—Review & Editing. MZ: Data Curation, Writing—Original Draft. ARM: Data Curation, Writing—Original Draft. MKR: Data Curation, Writing—Review & Editing.
Funding
The study was supported by Students Research Committee of the Research Center for Integrative Medicine in Aging (Tabriz University of Medical Sciences—Tabriz, Iran).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The lead author is currently an associate editor of the European Journal of Medical Research. The authors declare no other competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohammad-Salar Hosseini and Sarvin Sanaie are equally contributing authors.
Contributor Information
Mohammad-Salar Hosseini, Email: hosseini.msalar@gmail.com, Email: hosseinim@tbzmed.ac.ir.
Sarvin Sanaie, Email: sarvin_so2000@yahoo.com, Email: sanaies@tbzmed.ac.ir.
References
- 1.Hopcraft MS, Ceaser T. Xerostomia: an update for clinicians. Aust Dental J. 2010;55(3):238–44. [DOI] [PubMed] [Google Scholar]
- 2.Niklander S, Veas L, Barrera C, Fuentes F, Chiappini G, Marshall M. Risk factors, hyposalivation and impact of xerostomia on oral health-related quality of life. Braz Oral Res. 2017;31: e14. 10.1590/1807-3107BOR-2017.vol31.0014. [DOI] [PubMed] [Google Scholar]
- 3.Mortazavi H, Baharvand M, Movahhedian A, Mohammadi M, Khodadoustan A. Xerostomia due to systemic disease: a review of 20 conditions and mechanisms. Ann Med Health Sci Res. 2014;4(4):503–10. 10.4103/2141-9248.139284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fornari CB, Bergonci D, Stein CB, Agostini BA, Rigo L. Prevalence of xerostomia and its association with systemic diseases and medications in the elderly: a cross-sectional study. Sao Paulo Med J. 2021;139:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen AML, Sørensen CE, Proctor GB, Carpenter GH, Ekström J. Salivary secretion in health and disease. J Oral Rehabil. 2018;45(9):730–46. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen AML, Belstrøm D. The role of natural salivary defences in maintaining a healthy oral microbiota. J Dentis. 2019;80:S3–12. [DOI] [PubMed] [Google Scholar]
- 7.Konttinen YT, Porcar AV, Porola P, Koskenpato K, Rodriguez ML, Pöllänen R, et al. Neurobiology and hormonal control of lacrimal and salivary gland function. In: Sjögren’s syndrome: practical guidelines to diagnosis and therapy. 2012:151-75.
- 8.Millsop JW, Wang EA, Fazel N. Etiology, evaluation, and management of xerostomia. Clin Dermatol. 2017;35(5):468–76. [DOI] [PubMed] [Google Scholar]
- 9.Saleh J, Figueiredo MAZ, Cherubini K, Salum FG. Salivary hypofunction: an update on aetiology, diagnosis and therapeutics. Arch Oral Biol. 2015;60(2):242–55. [DOI] [PubMed] [Google Scholar]
- 10.Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dental Assoc. 2003;134(1):61–9. [DOI] [PubMed] [Google Scholar]
- 11.Bhide SA, Miah AB, Harrington KJ, Newbold KL, Nutting CM. Radiation-induced xerostomia: pathophysiology, prevention and treatment. Clin Oncol. 2009;21(10):737–44. 10.1016/j.clon.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Einhorn OM, Georgiou K, Tompa A. Salivary dysfunction caused by medication usage. Physiol Int. 2020;107(2):195–208. [DOI] [PubMed] [Google Scholar]
- 13.Kim YJ. Xerostomia and its cellular targets. Int J Mol Sci. 2023;24(6):5358. 10.3390/ijms24065358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaguar GC, Prado JD, Campanhã D, Alves FA. Clinical features and preventive therapies of radiation-induced xerostomia in head and neck cancer patient: a literature review. Appl Cancer Res. 2017;37(1):1–8. [Google Scholar]
- 15.Brook I. Late side effects of radiation treatment for head and neck cancer. Radiat Oncol J. 2020;38(2):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijers OB, Levendag PC, Braaksma MM, Boonzaaijer M, Visch LL, Schmitz PI. Patients with head and neck cancer cured by radiation therapy: a survey of the dry mouth syndrome in long-term survivors. Head Neck. 2002;24(8):737–47. 10.1002/hed.10129. [DOI] [PubMed] [Google Scholar]
- 17.Vistoso MA, Polonsky G, Shiboski C, Sankar V, Villa A. Salivary gland dysfunction secondary to cancer treatment. Front Oral Health. 2022;3: 907778. 10.3389/froh.2022.907778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–36. 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Miao J, Xiao X, Hu J, Zhang G, Peng Y, et al. Impact on xerostomia for nasopharyngeal carcinoma patients treated with superficial parotid lobe-sparing intensity-modulated radiation therapy (SPLS-IMRT): a prospective phase II randomized controlled study. Radiothera Oncol. 2022;175:1–9. 10.1016/j.radonc.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 20.De Felice F, Pranno N, Papi P, Brugnoletti O, Tombolini V, Polimeni A. Xerostomia and clinical outcomes in definitive intensity modulated radiotherapy (IMRT) versus three-dimensional conformal radiotherapy (3D-CRT) for head and neck squamous cell carcinoma: a meta-analysis. In vivo (Athens, Greece). 2020;34(2):623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villa A, Abati S. Risk factors and symptoms associated with xerostomia: a cross-sectional study. Aust Dental J. 2011;56(3):290–5. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Li X, Pang R, Yang G, Tian M, Zhao T, et al. Diagnosis, prevention, and treatment of radiotherapy-induced xerostomia: a review. J Oncol. 2022;2022:7802334. 10.1155/2022/7802334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren J, Huang R, Li Y, Chen R, Tian H, Liu C. Radioprotective effects and mechanism of HL-003 on radiation-induced salivary gland damage in mice. Sci Rep. 2022;12(1):8419. 10.1038/s41598-022-12581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Z, Xu C, Song B, Zhang S, Chen C, Li C, et al. Tissue fibrosis induced by radiotherapy: current understanding of the molecular mechanisms, diagnosis and therapeutic advances. J Transl Med. 2023;21(1):708. 10.1186/s12967-023-04554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141(11):1985–94. 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jasmer KJ, Gilman KE, Muñoz Forti K, Weisman GA, Limesand KH. Radiation-induced salivary gland dysfunction: mechanisms, therapeutics and future directions. J Clin Med. 2020;9(12):4095. 10.3390/jcm9124095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Athanasios P, Petros P, Dimitrios A. Chemotherapy: oral side effects and dental interventions-a review of the literature. Stomatolog Dis Sci. 2017;1:35–49. [Google Scholar]
- 28.Epstein JB, Thariat J, Bensadoun R-J, Barasch A, Murphy BA, Kolnick L, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62(6):400–22. [DOI] [PubMed] [Google Scholar]
- 29.Sardellitti L, Bortone A, Filigheddu E, Serralutzu F, Milia EP. Xerostomia: from pharmacological treatments to traditional medicine—an overview on the possible clinical management and prevention using systemic approaches. Curr Oncol (Toronto, Ont). 2023;30(5):4412–26. 10.3390/curroncol30050336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russi EG, Raber-Durlacher JE, Sonis ST. Local and systemic pathogenesis and consequences of regimen-induced inflammatory responses in patients with head and neck cancer receiving chemoradiation. Mediators Inflamm. 2014;2014: 518261. 10.1155/2014/518261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klymiuk I, Bilgilier C, Mahnert A, Prokesch A, Heininger C, Brandl I, et al. Chemotherapy-associated oral microbiome changes in breast cancer patients. Front Oncol. 2022;12: 949071. 10.3389/fonc.2022.949071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseini M-S, Sanaie S, Akbarzadeh MA, Fattahi S, Jabbari S. The potential impact of oral dysbiosis on the pathogenesis of oral neoplasms: a systematic review. Microb Interact Health Dis. 2024; 125.
- 33.Ghosh-Laskar S, Pilar A, Prabhash K, Joshi A, Agarwal JP, Gupta T, et al. Taxane-based induction chemotherapy plus concurrent chemoradiotherapy in nasopharyngeal carcinoma: prospective results from a non-endemic cohort. Clin Oncol. 2019;31(12):850–7. 10.1016/j.clon.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Stolze J, Teepen JC, Raber-Durlacher JE, Loonen JJ, Kok JL, Tissing WJE, et al. Prevalence and risk factors for hyposalivation and xerostomia in childhood cancer survivors following different treatment modalities—a dutch childhood cancer survivor study late effects 2 clinical study (DCCSS LATER 2). Cancers. 2022;14(14):3379. 10.3390/cancers14143379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hitomi S, Ujihara I, Sago-Ito M, Nodai T, Shikayama T, Inenaga K, et al. Hyposalivation due to chemotherapy exacerbates oral ulcerative mucositis and delays its healing. Arch Oral Biol. 2019;105:20–6. 10.1016/j.archoralbio.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Al Mousa A, Abu-Hijlih R, Salem A, Sultan I, Mula-Hussain L, Ismael T, et al. Induction methotrexate, cisplatin, and 5-fluorouracil versus cisplatin and 5-fluorouracil followed by radiotherapy in pediatric nasopharyngeal carcinoma: a retrospective analysis in a tertiary cancer center. J Pediatr Hematol/Oncol. 2017;39(8):e437–42. [DOI] [PubMed] [Google Scholar]
- 37.Djuric M, Cakic S, Hadzi-Mihailovic M, Petrovic D, Jankovic L. Oral status in patients receiving 5-fluorouracil for colorectal cancer. J BUON. 2010;15(3):475–9. [PubMed] [Google Scholar]
- 38.Abdelzaher WY, Nassan MA, Ahmed SM, Welson NN, El-Saber BG, Khalaf HM. Xanthine oxidase inhibitor, febuxostat is effective against 5-fluorouracil-induced parotid salivary gland injury in rats via inhibition of oxidative stress, inflammation and targeting TRPC1/CHOP signalling pathway. Pharmaceuticals. 2022;15(2):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villa A, Sonis S. Toxicities associated with head and neck cancer treatment and oncology-related clinical trials. Curr Probl Cancer. 2016;40(5–6):244–57. [DOI] [PubMed] [Google Scholar]
- 40.Radvansky LJ, Pace MB, Siddiqui A. Prevention and management of radiation-induced dermatitis, mucositis, and xerostomia. Am J Health-Syst Pharm. 2013;70(12):1025–32. [DOI] [PubMed] [Google Scholar]
- 41.Abo El Fadel DM, Kamal Y, Hassouna AH, Ali A. Prevalence of oral side effects associated with chemo and radiotherapy in head and neck cancer treatment: a cross-sectional study in Egypt. Oral Oncol Rep. 2024;10:100177. 10.1016/j.oor.2024.100177. [Google Scholar]
- 42.Hosseini M-S, Akbarzadeh MA, Jadidi-Niaragh F. Myeloid-derived suppressor cells and macrophage polarization in cancer immunotherapy. critical developments in cancer immunotherapy. IGI Global. 2024. p. 157–204. 10.4018/979-8-3693-3976-3.ch005.
- 43.Klein BA, Shazib MA, Villa A, de Abreu AF, Vacharotayangul P, Sonis S, et al. Immune checkpoint inhibitors in cancer therapy: review of orofacial adverse events and role of the oral healthcare provider. Front Oral Health. 2022;3. 10.3389/froh.2022.968157. [DOI] [PMC free article] [PubMed]
- 44.Xu Y, Wen N, Sonis ST, Villa A. Oral side effects of immune checkpoint inhibitor therapy (ICIT): an analysis of 4683 patients receiving ICIT for malignancies at Massachusetts General Hospital, Brigham & Women’s Hospital, and the Dana-Farber Cancer Institute, 2011 to 2019. Cancer. 2021;127(11):1796–804. 10.1002/cncr.33436. [DOI] [PubMed] [Google Scholar]
- 45.Filho OVO, Medeiros YDL, Oliveira TBD, Gibbons IL, Treister NS, Alves FA. Prevalence of cutaneous and oral immune-related adverse events related to immune checkpoint inhibitor therapy in a single institution. Oral Surg Oral Med Oral Pathol Oral Radiol. 2023;136(5): e169. 10.1016/j.oooo.2023.07.043. [Google Scholar]
- 46.Zhang X, Lu X, Yu Y, Tan K, Cui H. Changes of IL-6 And IFN-γ before and after the adverse events related to immune checkpoint inhibitors: a retrospective study. Medicine. 2022;101(46): e31761. 10.1097/md.0000000000031761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kouji K, Saori F, Kayoko I, Kaname N, Noboru K, Masaki T, et al. Radiological imaging features of the salivary glands in xerostomia induced by an immune checkpoint inhibitor. Oral Radiol. 2021;37:531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warner BM, Baer AN, Lipson EJ, Allen C, Hinrichs C, Rajan A, et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist. 2019;24(9):1259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Zubidi N, Cody Page J, GombosDan S, Akanksha S, Eric A, Gidley PW, et al. Immune-related oral, otologic, and ocular adverse events. In: Immunotherapy. Cham: Springer; 2021. p. 399–416. [DOI] [PubMed] [Google Scholar]
- 50.Asan MF, Castelino RL, Babu SG, Rao K, Pandita V. Oral immune-related adverse events—current concepts and their management. Asia-Pac J Oncol Nurs. 2021;8(6):604–9. 10.4103/apjon.apjon-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ullah A, Khan J, Waheed A, Karki NR, Goodbee M, Yasinzai AQK, et al. Mucoepidermoid carcinoma of the salivary gland: demographics and comparative analysis in us children and adults with future perspective of management. Cancers. 2022;15(1):250. 10.3390/cancers15010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marco P, Federico B, Francesco B, Mario A, Giuseppe G, Piera V, et al. Incidence, risk factors and complications of ocular graft-versus-host disease following hematopoietic stem cell transplantation. Am J Ophthalmol. 2021;227:25–34. [DOI] [PubMed] [Google Scholar]
- 53.Elad S, Aljitawi O, Zadik Y. Oral graft-versus-host disease: a pictorial review and a guide for dental practitioners. Int Dental J. 2021;71(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mays JW, Fassil H, Edwards DA, Pavletic SZ, Bassim CW. Oral chronic graft-versus-host disease: current pathogenesis, therapy, and research. Oral Dis. 2013;19(4):327–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cherry-Peppers G, Fryer C, Jackson AD, Ford D, Glascoe A, Smith D, et al. A review of the risks and relationships between oral health and chronic diseases. J Nat Med Assoc. 2024. [DOI] [PubMed]
- 56.Marcott S, Dewan K, Kwan M, Baik F, Lee YJ, Sirjani D. Where dysphagia begins: polypharmacy and xerostomia. Fed Practition. 2020;37(5):234–41. [PMC free article] [PubMed] [Google Scholar]
- 57.Arakelyan MG, Tambovtseva NV, Arzukanyan AV. The main causes and clinical manifestations of xerostomia. Russian J Dentis. 2016;20(2):74–8. [Google Scholar]
- 58.Ristevska I, Armata RS, D’Ambrosio C, Furtado M, Anand L, Katzman MA. Xerostomia: understanding the diagnosis and the treatment of dry mouth. J Fam Med Dis Prev. 2015;1(008):1–5. [Google Scholar]
- 59.Somay E, Topkan E, Bascil S, Selek U. Influence of radiation-induced xerostomia on tooth loss in head and neck cancer patients. In: Interdisciplinary cancer research. Cham: Springer International Publishing; 2024. p. 1–19. [Google Scholar]
- 60.Palmier NR, Migliorati CA, Prado-Ribeiro AC, de Oliveira MCQ, Vechiato FAJ, de Goes MF, et al. Radiation-related caries: current diagnostic, prognostic, and management paradigms. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(1):52–62. 10.1016/j.oooo.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Health US Department of, Services Human. Common terminology criteria for adverse events (CTCAE). (No Title). 2010.
- 62.Riley P, Glenny AM, Hua F, Worthington HV. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst Rev. 2017;7(7):CD012744. 10.1002/14651858.cd012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mercadante V, Al Hamad A, Lodi G, Porter S, Fedele S. Interventions for the management of radiotherapy-induced xerostomia and hyposalivation: A systematic review and meta-analysis. Oral Oncol. 2017;66:64–74. 10.1016/j.oraloncology.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 64.Pronin AN, Wang Q, Slepak VZ. Teaching an old drug new tricks: agonism, antagonism, and biased signaling of pilocarpine through M3 muscarinic acetylcholine receptor. Mol Pharmacol. 2017;92(5):601–12. 10.1124/mol.117.109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bui T, Duong H. Muscarinic agonists. StatPearls: StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 66.Oleksak P, Novotny M, Patocka J, Nepovimova E, Hort J, Pavlik J, et al. Neuropharmacology of cevimeline and muscarinic drugs-focus on cognition and neurodegeneration. Int J Mol Sci. 2021;22(16):8908. 10.3390/ijms22168908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kapourani A, Kontogiannopoulos KN, Barmpalexis P. A review on the role of pilocarpine on the management of xerostomia and the importance of the topical administration systems development. Pharmaceuticals (Basel, Switzerland). 2022;15(6):762. 10.3390/ph15060762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braga MA, Tarzia O, Bergamaschi CC, Santos FA, Andrade ED, Groppo FC. Comparison of the effects of pilocarpine and cevimeline on salivary flow. Int J Dental Hyg. 2009;7(2):126–30. 10.1111/j.1601-5037.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- 69.Farag AM, Holliday C, Cimmino J, Roomian T, Papas A. Comparing the effectiveness and adverse effects of pilocarpine and cevimeline in patients with hyposalivation. Oral Dis. 2019;25(8):1937–44. 10.1111/odi.13192. [DOI] [PubMed] [Google Scholar]
- 70.Brimhall J, Jhaveri MA, Yepes JF. Efficacy of cevimeline vs. pilocarpine in the secretion of saliva: a pilot study. Special Care Dentis. 2013;33(3):123–7. [DOI] [PubMed] [Google Scholar]
- 71.Estafanos R. Extemporaneously compounded buccal pilocarpine preparations, acceptability and pilot testing for the treatment of xerostomia (dry mouth) in Australia. 2020.
- 72.Motamed B, Alaee A, Azizi A, Jahandar H, Fard MJK, Jafari A. Comparison of the 1 and 2% pilocarpine mouthwash in a xerostomic population: a randomized clinical trial. BMC Oral Health. 2022;22(1):548. 10.1186/s12903-022-02576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim JH, Ahn H-J, Choi J-H, Jung DW, Kwon J-S. Effect of 0.1% pilocarpine mouthwash on xerostomia: double-blind, randomised controlled trial. J Oral Rehabil. 2014;41(3):226–35. 10.1111/joor.12127. [DOI] [PubMed] [Google Scholar]
- 74.Eren H, Mercantepe T, Tumkaya L, Mercantepe F, Dil E, Horsanali MO, et al. Evaluation of the protective effects of amifostine and melatonin against cisplatin induced testis injury via oxidative stress and apoptosis in rats. Exp Mol Pathol. 2020;112:104324. [DOI] [PubMed] [Google Scholar]
- 75.Singh VK, Seed TM, Cheema AK. Metabolomics-based predictive biomarkers of radiation injury and countermeasure efficacy: current status and future perspectives. Exp Rev Mol Diagn. 2021;21(7):641–54. [DOI] [PubMed] [Google Scholar]
- 76.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 2007;12(6):738–47. 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 77.Arora A, Bhuria V, Hazari PP, Pathak U, Mathur S, Roy BG, et al. Amifostine analog, DRDE-30, attenuates bleomycin-induced pulmonary fibrosis in mice. Front Pharmacol. 2018;9:394. 10.3389/fphar.2018.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Culy CR, Spencer CM. Amifostine: an update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. Drugs. 2001;61(5):641–84. 10.2165/00003495-200161050-00012. [DOI] [PubMed] [Google Scholar]
- 79.Priori R, Mastromanno L, Izzo R. What about glucocorticoids in primary Sjögren’s syndrome? Clin Exp Rheumatol. 2020;38(Suppl 124):237–44. [PubMed] [Google Scholar]
- 80.Malcher-Lopes R, Franco A, Tasker JG. Glucocorticoids shift arachidonic acid metabolism toward endocannabinoid synthesis: a non-genomic anti-inflammatory switch. Eur J Pharmacol. 2008;583(2–3):322–39. 10.1016/j.ejphar.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bustillos H, Indorf A, Alwan L, Thompson J, Jung L. Xerostomia: an immunotherapy-related adverse effect in cancer patients. Support Care Cancer. 2022;30(2):1681–7. 10.1007/s00520-021-06535-9. [DOI] [PubMed] [Google Scholar]
- 82.Moghadam-Kia S, Werth VP. Prevention and treatment of systemic glucocorticoid side effects. Int J Dermatol. 2010;49(3):239–48. 10.1111/j.1365-4632.2009.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faggiano A, Mazzilli R, Natalicchio A, Adinolfi V, Argentiero A, Danesi R, et al. Corticosteroids in oncology: use, overuse, indications, contraindications. An Italian Association of Medical Oncology (AIOM)/ Italian Association of Medical Diabetologists (AMD)/ Italian Society of Endocrinology (SIE)/Italian Society of Pharmacology (SIF) multidisciplinary consensus position paper. Crit Rev Oncol/Hematol. 2022;180:103826. 10.1016/j.critrevonc.2022.103826. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217(6):1915–28. 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sonis ST. Superoxide dismutase as an intervention for radiation therapy-associated toxicities: review and profile of avasopasem manganese as a treatment option for radiation-induced mucositis. Drug Des Dev Ther. 2021;15:1021–9. 10.2147/dddt.s267400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sishc BJ, Ding L, Nam TK, Heer CD, Rodman SN, Schoenfeld JD, et al. Avasopasem manganese synergizes with hypofractionated radiation to ablate tumors through the generation of hydrogen peroxide. Sci Transl Med. 2021;13(593):eabb3788. 10.1126/scitranslmed.abb3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chatterjee A, Kosmacek EA, Shrishrimal S, McDonald JT, Oberley-Deegan RE. MnTE-2-PyP, a manganese porphyrin, reduces cytotoxicity caused by irradiation in a diabetic environment through the induction of endogenous antioxidant defenses. Redox Biol. 2020;34: 101542. 10.1016/j.redox.2020.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pinna R, Campus G, Cumbo E, Mura I, Milia E. Xerostomia induced by radiotherapy: an overview of the physiopathology, clinical evidence, and management of the oral damage. Therapeut Clin Risk Manage. 2015:171–88. [DOI] [PMC free article] [PubMed]
- 89.Lynggaard CD, Grønhøj C, Christensen R, Fischer-Nielsen A, Melchiors J, Specht L, et al. Intraglandular off-the-shelf allogeneic mesenchymal stem cell treatment in patients with radiation-induced xerostomia: a safety study (MESRIX-II). Stem Cells Transl Med. 2022;11(5):478–89. 10.1093/stcltm/szac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blitzer GC, Glazer T, Burr A, Gustafson S, Ganz O, Meyers R, et al. Marrow-derived autologous stromal cells for the restoration of salivary hypofunction (MARSH): a pilot, first-in-human study of interferon gamma-stimulated marrow mesenchymal stromal cells for treatment of radiation-induced xerostomia. Cytotherapy. 2023;25(11):1139–44. 10.1016/j.jcyt.2023.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Awuchi CG, Echeta KC. Current developments in sugar alcohols: chemistry, nutrition, and health concerns of sorbitol, xylitol, glycerol, arabitol, inositol, maltitol, and lactitol. Int J Adv Acad Res. 2019;5(11):1–33. [Google Scholar]
- 92.Taheri JB, Azimi S, Rafieian N, Zanjani HA. Herbs in dentistry. Int Dental J. 2011;61(6):287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agung I, Wedagama DM. Angular cheilitis: malnutrition, diet and home remedies in children. Lambert Academic Publishing. 2019.
- 94.Kapourani A, Kontogiannopoulos KN, Manioudaki A-E, Poulopoulos AK, Tsalikis L, Assimopoulou AN, et al. A review on xerostomia and its various management strategies: the role of advanced polymeric materials in the treatment approaches. Polymers. 2022;14(5):850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu J, Andablo-Reyes E, Mighell A, Pavitt S, Sarkar A. Dry mouth diagnosis and saliva substitutes—a review from a textural perspective. J Texture Stud. 2021;52(2):141–56. [DOI] [PubMed] [Google Scholar]
- 96.Kontogiannopoulos KN, Kapourani A, Gkougkourelas I, Anagnostaki M-E, Tsalikis L, Assimopoulou AN, et al. A review of the role of natural products as treatment approaches for xerostomia. Pharmaceuticals. 2023;16(8):1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ritschel M-L, Hübner J, Wurm-Kuczera R, Büntzel J. Phytotherapy known and applied by head-neck cancer patients and medical students to treat oral discomfort in Germany: an observational study. J Cancer Res Clin Oncol. 2023;149(5):2057–70. 10.1007/s00432-022-04200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan MWC, Wu XY, Wu JCY, Wong SYS, Chung VCH. Safety of acupuncture: overview of systematic reviews. Sci Rep. 2017;7(1):3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhuang L, Yang Z, Zeng X, Zhua X, Chen Z, Liu L, et al. The preventive and therapeutic effect of acupuncture for radiation-induced xerostomia in patients with head and neck cancer: a systematic review. Integr Cancer Thera. 2013;12(3):197–205. [DOI] [PubMed] [Google Scholar]
- 100.Lovelace TL, Fox NF, Sood AJ, Nguyen SA, Day TA. Management of radiotherapy-induced salivary hypofunction and consequent xerostomia in patients with oral or head and neck cancer: meta-analysis and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(5):595–607. [DOI] [PubMed] [Google Scholar]
- 101.Davies A. Salivary gland dysfunction. In: Oral complications of cancer and its management. Oxford: Oxford University Press; 2010. p. 203–23. [Google Scholar]
- 102.Hubner J, Dorfler J, Freuding M, Zaiser C, Buntzel J, Keinki C, et al. Methodological review: summary of findings for acupuncture as treatment for cancer therapy-induced xerostomia. In vivo (Athens, Greece). 2022;36(6):2579–97. 10.21873/invivo.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dörfler J, Freuding M, Zaiser C, Büntzel J, Keinki C, Käsmann L, et al. Umbrella review: summary of findings for acupuncture as treatment for radiation-induced xerostomia. Head Neck. 2023;45(4):1026–44. 10.1002/hed.27297. [DOI] [PubMed] [Google Scholar]
- 104.Marino P, Pepe G, Basilicata MG, Vestuto V, Marzocco S, Autore G, et al. Potential role of natural antioxidant products in oncological diseases. Antioxidants. 2023;12(3):704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jennifer D, Amanda A, Hui-Ling Y, Kate D, Wasek F. Vitamin E in cancer treatment: a review of clinical applications in randomized control trials. Nutrients. 2022;14(20):4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Indre S, Jolanta A, Alina P, Lina S-M. Association between diet and xerostomia: is xerostomia a barrier to a healthy eating pattern? Nutrients. 2021;13(12):4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Müller F, Chebib N, Maniewicz S, Genton L. The impact of xerostomia on food choices—a review with clinical recommendations. J Clin Med. 2023;12(14):4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Visvanathan V, Nix P. Managing the patient presenting with xerostomia: a review. Int J Clin Pract. 2010;64(3):404–7. [DOI] [PubMed] [Google Scholar]
- 109.Galiano-Castillo N, Liu L, Lozano-Lozano M, Tumilty S, Cantarero-Villanueva I, Baxter GD. Acute and cumulative benefits of photobiomodulation for xerostomia: a systematic review and meta-analysis. Oral Dis. 2021;27(5):1115–26. [DOI] [PubMed] [Google Scholar]
- 110.Heiskanen V, Zadik Y, Elad S. Photobiomodulation therapy for cancer treatment-related salivary gland dysfunction: a systematic review. Photobiomodul Photomed Laser Surg. 2020;38(6):340–7. [DOI] [PubMed] [Google Scholar]
- 111.Oliveira SV, Batista JVF, Gutierres GG, Silva NP, Lino-dos-Santos-Franco A, Rodrigues MFSD, et al. The supportive use of photobiomodulation on salivary glands: a narrative review and meta-analysis. Eur Arch Oto-Rhino-Laryngol. 2024;281:2793. [DOI] [PubMed] [Google Scholar]
- 112.de Lima LCA, Guimarães DM, ESilva ES, Do Couto MFN, Oliveira GL, Alves MSA, et al. Photobiomodulation in the treatment of oral diseases. Res Soc Dev. 2023;12(3):e9512338070. [Google Scholar]
- 113.Cronshaw M, Mylona V. photobiomodulation therapy within clinical dentistry: theoretical and applied concepts. In: Lasers in dentistry—current concepts. Cham: Springer; 2024. p. 173–236. [Google Scholar]
- 114.Chandra R, Bhakta P, Beniwal J, Dhanda R, Saxena V, Sinha S. Evaluation of the efficacy of transcutaneous electrical nerve stimulation (TENS) on salivary flow rate in patients with xerostomia—a case control study. J Fam Med Prim Care. 2022;11(2):767–71. 10.4103/jfmpc.jfmpc_922_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aggarwal H, Pal-Singh M, Mathur H, Astekar S, Gulati P, Lakhani S. Evaluation of the effect of transcutaneous electrical nerve stimulation (TENS) on whole salivary flow rate. J Clin Exp Dent. 2015;7(1):e13–7. 10.4317/jced.51828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Salimi F, Saavedra F, Andrews B, FitzGerald J, Winter SC. Trans-cutaneous electrical nerve stimulation to treat dry mouth (xerostomia) following radiotherapy for head and neck cancer. A systematic review. Ann Med Surg. 2021;63:102146. 10.1016/j.amsu.2021.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hadley T, Song C, Wells L, Lehnhardt J, Rogers MW, Anderson J, et al. Does hyperbaric oxygen therapy have the potential to improve salivary gland function in irradiated head and neck cancer patients? Med Gas Res. 2013;3(1):15. 10.1186/2045-9912-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fox NF, Xiao C, Sood AJ, Lovelace TL, Nguyen SA, Sharma A, et al. Hyperbaric oxygen therapy for the treatment of radiation-induced xerostomia: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(1):22–8. 10.1016/j.oooo.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 119.Sherlock S, Way M, Tabah A. Hyperbaric oxygen treatment for the management of radiation-induced xerostomia. J Med Imaging Radiat Oncol. 2018;62(6):841–6. 10.1111/1754-9485.12789. [DOI] [PubMed] [Google Scholar]
- 120.Premalatha BR, Ravindra S, Hegde U, Chandavarkar V, Mishra MN, Sangeetha R. AYUSH in oral health and diseases: an overview. J Nat Remedies. 2023;23(3):811–23. 10.18311/jnr/2023/31848. [Google Scholar]
- 121.Haila S, Koskinen A, Tenovuo J. Effects of homeopathic treatment on salivary flow rate and subjective symptoms in patients with oral dryness: a randomized trial. Homeopathy J Faculty Homeopathy. 2005;94(3):175–81. 10.1016/j.homp.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 122.Anna W, Jennifer D, Maren F, Lena J, Jutta H. Homeopathy effects in patients during oncological treatment: a systematic review. J Cancer Res Clin Oncol. 2023;149(5):1785–810. 10.1007/s00432-022-04054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xiangmin Z, Folin L, Lan Xiaolin Yu, Wei LW, Xiuhong Wu, et al. Clinical observation of submandibular gland transfer for the prevention of xerostomia after radiotherapy for nasopharyngeal carcinoma: a prospective randomized controlled study of 32 cases. Radiat Oncol. 2014;9(1):62. 10.1186/1748-717X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jana R, Hadi S, Naresh J, Jeffrey H, David W, Richard L, et al. Submandibular gland transfer for prevention of xerostomia after radiation therapy: swallowing outcomes. Arch Otolaryngol Head Neck Surg. 2005;131(2):140–5. 10.1001/archotol.131.2.140. [DOI] [PubMed] [Google Scholar]
- 125.Kolahi J, Mansourian M. Autotransplantation of cryopreserved minor salivary glands: A new approach for management of radiation-induced xerostomia. Med Hypotheses. 2010;74(1):29–30. 10.1016/j.mehy.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 126.Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE. 2008;3(4): e2063. 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nieri CA, Benaim EH, Zhang YH, Garcia-Godoy F, Herr MJ, Zhang W, et al. Botox for the prevention of radiation-induced Sialadenitis and xerostomia in head and neck cancer patients: a pilot study. Head Neck. 2023;45(9):2198–206. 10.1002/hed.27449. [DOI] [PubMed] [Google Scholar]
- 128.Cao J, Zhang X, Jiang B, Chen J, Wang X, Wang L, et al. Intensity-modulated proton therapy for oropharyngeal cancer reduces rates of late xerostomia. Radiothera Oncol. 2021;160:32–9. 10.1016/j.radonc.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Snider JW, Paine CC. Sticky stuff: xerostomia in patients undergoing head and neck radiotherapy—prevalence, prevention, and palliative care. Ann Palliative Med. 2020;9(3):1340–50. [DOI] [PubMed] [Google Scholar]
- 130.Castelli J, Thariat J, Benezery K, Hasbini A, Gery B, Berger A, et al. Weekly adaptive radiotherapy vs standard intensity-modulated radiotherapy for improving salivary function in patients with head and neck cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2023;9(8):1056–64. 10.1001/jamaoncol.2023.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lou J, Huang P, Ma C, Zheng Y, Chen J, Liang Y, et al. Parotid gland radiation dose-xerostomia relationships based on actual delivered dose for nasopharyngeal carcinoma. J Appl Clin Med Phys. 2018;19(3):251–60. 10.1002/acm2.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mall P, Chand P, Singh BP, Rao J, Siddarth R, Srivastava K. Effectiveness of positioning stents in radiation-induced xerostomia in patients with tongue carcinoma: a randomized controlled trial. Int J Prosthodont. 2016;29(5):455–60. 10.11607/ijp.4499. [DOI] [PubMed] [Google Scholar]
- 133.Chen D, Chen X, Chen X, Jiang N, Jiang L. The efficacy of positioning stents in preventing oral complications after head and neck radiotherapy: a systematic literature review. Radiat Oncol (London, England). 2020;15(1):90. 10.1186/s13014-020-01536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garcia MK, Meng Z, Rosenthal DI, Shen Y, Chambers M, Yang P, et al. Effect of true and sham acupuncture on radiation-induced xerostomia among patients with head and neck cancer: a randomized clinical trial. JAMA Netw Open. 2019;2(12): e1916910. 10.1001/jamanetworkopen.2019.16910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Simcock R, Fallowfield L, Monson K, Solis-Trapala I, Parlour L, Langridge C, et al. ARIX: a randomised trial of acupuncture v oral care sessions in patients with chronic xerostomia following treatment of head and neck cancer. Ann Oncol. 2013;24(3):776–83. 10.1093/annonc/mds515. [DOI] [PubMed] [Google Scholar]
- 136.Meng Z, Garcia MK, Hu C, Chiang J, Chambers M, Rosenthal DI, et al. Randomized controlled trial of acupuncture for prevention of radiation-induced xerostomia among patients with nasopharyngeal carcinoma. Cancer. 2012;118(13):3337–44. 10.1002/cncr.26550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Forner L, Hyldegaard O, von Brockdorff AS, Specht L, Andersen E, Jansen EC, et al. Does hyperbaric oxygen treatment have the potential to increase salivary flow rate and reduce xerostomia in previously irradiated head and neck cancer patients? A pilot study. Oral Oncol. 2011;47(6):546–51. 10.1016/j.oraloncology.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 138.Palma LF, Gonnelli FAS, Marcucci M, Dias RS, Giordani AJ, Segreto RA, et al. Impact of low-level laser therapy on hyposalivation, salivary pH, and quality of life in head and neck cancer patients post-radiotherapy. Lasers Med Sci. 2017;32(4):827–32. 10.1007/s10103-017-2180-3. [DOI] [PubMed] [Google Scholar]
- 139.Louzeiro GC, Cherubini K, de Figueiredo MAZ, Salum FG. Effect of photobiomodulation on salivary flow and composition, xerostomia and quality of life of patients during head and neck radiotherapy in short term follow-up: a randomized controlled clinical trial. J Photochem Photobiol, B. 2020;209: 111933. 10.1016/j.jphotobiol.2020.111933. [DOI] [PubMed] [Google Scholar]
- 140.de Carvalho e Silva RM, Mendes FM, Degasperi GR, Pinheiro SL. Photobiomodulation for the management of xerostomia and oral mucositis in patients with cancer: a randomized clinical trial. Lasers Med Sci. 2023;38(1):101. 10.1007/s10103-023-03760-y. [DOI] [PubMed] [Google Scholar]
- 141.Nuchit S, Lam-Ubol A, Paemuang W, Talungchit S, Chokchaitam O, Mungkung OO, et al. Alleviation of dry mouth by saliva substitutes improved swallowing ability and clinical nutritional status of post-radiotherapy head and neck cancer patients: a randomized controlled trial. Support Care Cancer. 2020;28(6):2817–28. 10.1007/s00520-019-05132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Apperley O, Medlicott N, Rich A, Hanning S, Huckabee ML. A clinical trial of a novel emulsion for potential use as a saliva substitute in patients with radiation-induced xerostomia. J Oral Rehabil. 2017;44(11):889–95. 10.1111/joor.12545. [DOI] [PubMed] [Google Scholar]
- 143.Rupe C, Basco A, Gioco G, Patini R, Lucchese A, Micciché F, et al. Sodium-hyaluronate mouthwash on radiotherapy-induced xerostomia: a randomised clinical trial. Support Care Cancer. 2023;31(12):644. 10.1007/s00520-023-08090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Beuth J, van Leendert R, Schneider B, Uhlenbruck G. Complementary medicine on side-effects of adjuvant hormone therapy in patients with breast cancer. In vivo (Athens, Greece). 2013;27(6):869–71. [PubMed] [Google Scholar]
- 145.Heydarirad G, Rezaeizadeh H, Choopani R, Mosavat SH, Ameri A. Efficacy of a traditional Persian medicine preparation for radiation-induced xerostomia: a randomized, open-label, active-controlled trial. J Integr Med. 2017;15(3):201–8. 10.1016/s2095-4964(17)60333-9. [DOI] [PubMed] [Google Scholar]
- 146.Quimby AE, Hogan D, Khalil D, Hearn M, Nault C, Johnson-Obaseki S. Coconut oil as a novel approach to managing radiation-induced xerostomia: a primary feasibility study. Int J Otolaryngol. 2020;2020:8537643. 10.1155/2020/8537643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Charalambous M, Raftopoulos V, Paikousis L, Katodritis N, Lambrinou E, Vomvas D, et al. The effect of the use of thyme honey in minimizing radiation-induced oral mucositis in head and neck cancer patients: a randomized controlled trial. Eur J Oncol Nurs. 2018;34:89–97. 10.1016/j.ejon.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 148.Chamani G, Zarei MR, Mehrabani M, Nakhaee N, Kalaghchi B, Aghili M, et al. Assessment of systemic effects of ginger on salivation in patients with post-radiotherapy xerostomia. J Oral Health Oral Epidemiol. 2017;6(3):130. [Google Scholar]
- 149.Chung MK, Kim DH, Ahn YC, Choi JY, Kim EH, Son Y-I. Randomized trial of vitamin C/E complex for prevention of radiation-induced xerostomia in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2016;155(3):423–30. [DOI] [PubMed] [Google Scholar]
- 150.Heiser C, Hofauer B, Scherer E, Schukraft J, Knopf A. Liposomal treatment of xerostomia, odor, and taste abnormalities in patients with head and neck cancer. Head Neck. 2016;38(Suppl 1):E1232–7. 10.1002/hed.24198. [DOI] [PubMed] [Google Scholar]
- 151.Steinmann D, Eilers V, Beynenson D, Buhck H, Fink M. Effect of traumeel S on pain and discomfort in radiation-induced oral mucositis: a preliminary observational study. Altern Ther Health Med. 2012;18(4):12–8. [PubMed] [Google Scholar]
- 152.Dalbem PÉ, Batista CBB, Zanella GV, Martins VB, Macagnan FE. Effects of transcutaneous electrical nerve stimulation on the salivary flow of patients with hyposalivation induced by radiotherapy in the head and neck region—a randomised clinical trial. J Oral Rehabil. 2019;46(12):1142–50. 10.1111/joor.12851. [DOI] [PubMed] [Google Scholar]
- 153.Wong RK, Deshmukh S, Wyatt G, Sagar S, Singh AK, Sultanem K, et al. Acupuncture-like transcutaneous electrical nerve stimulation versus pilocarpine in treating radiation-induced xerostomia: results of RTOG 0537 phase 3 study. Int J Radiat Oncol Biol Phys. 2015;92(2):220–7. 10.1016/j.ijrobp.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.