Abstract
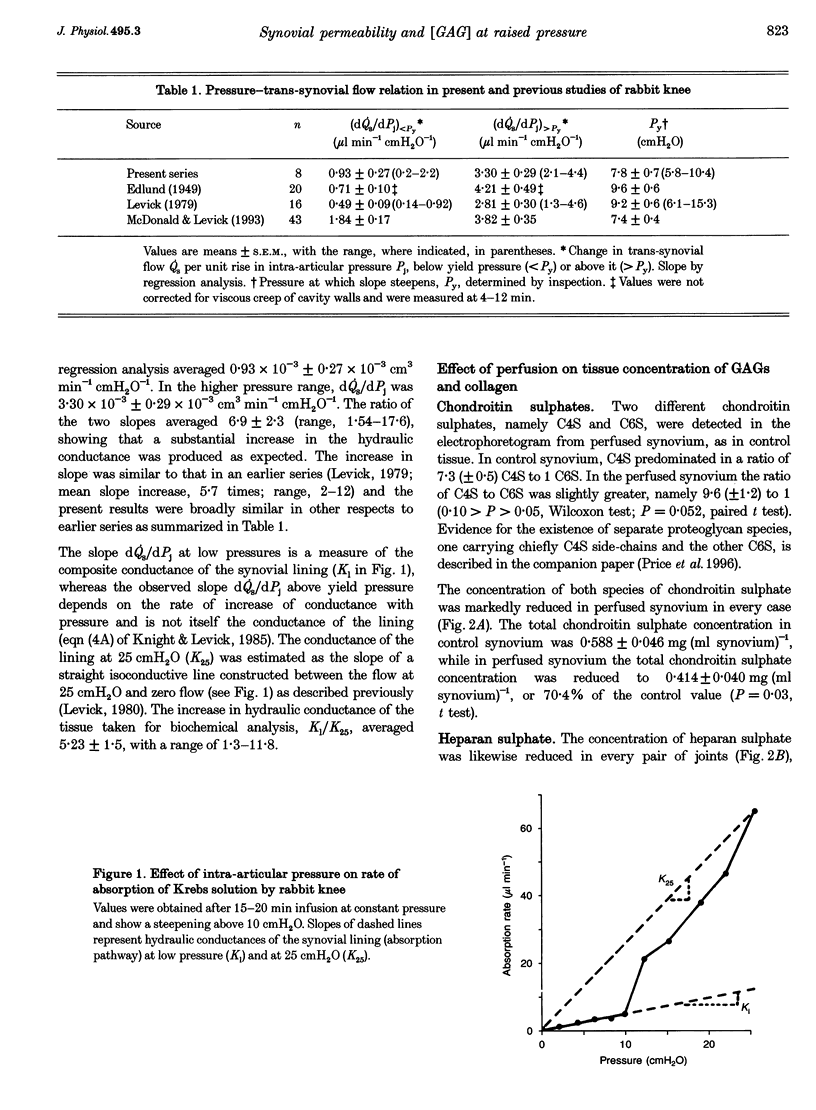
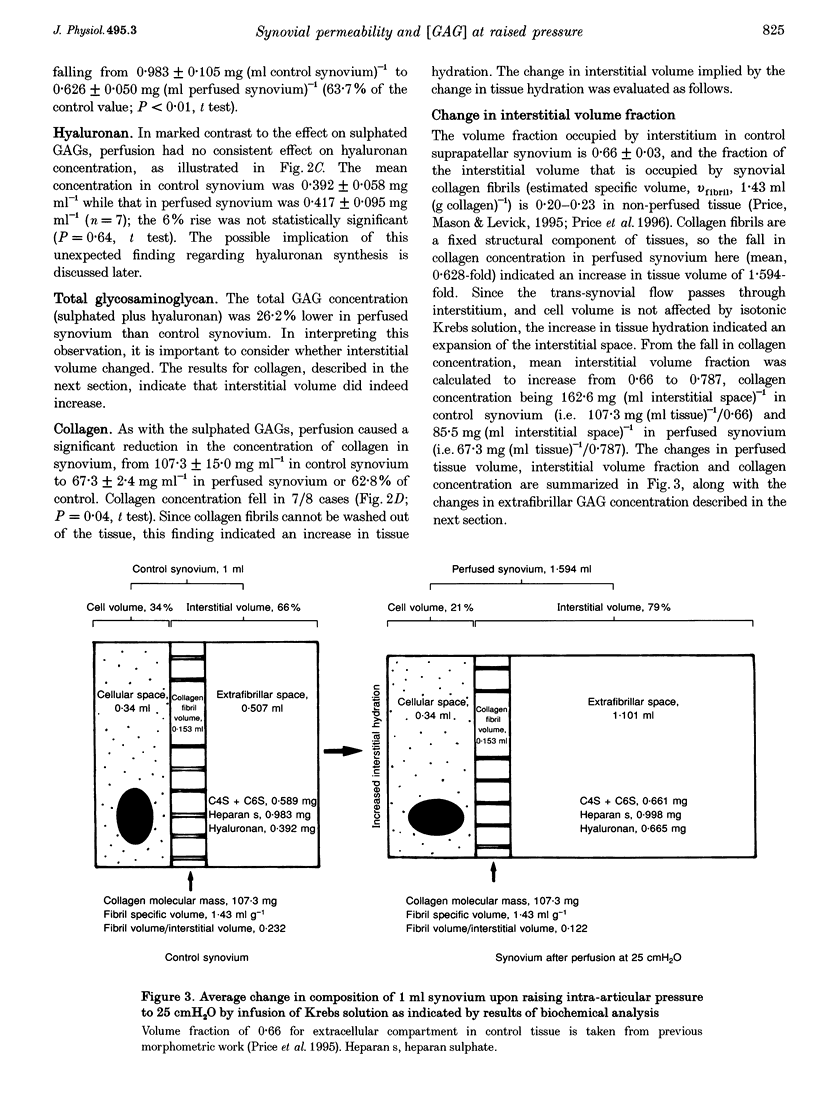
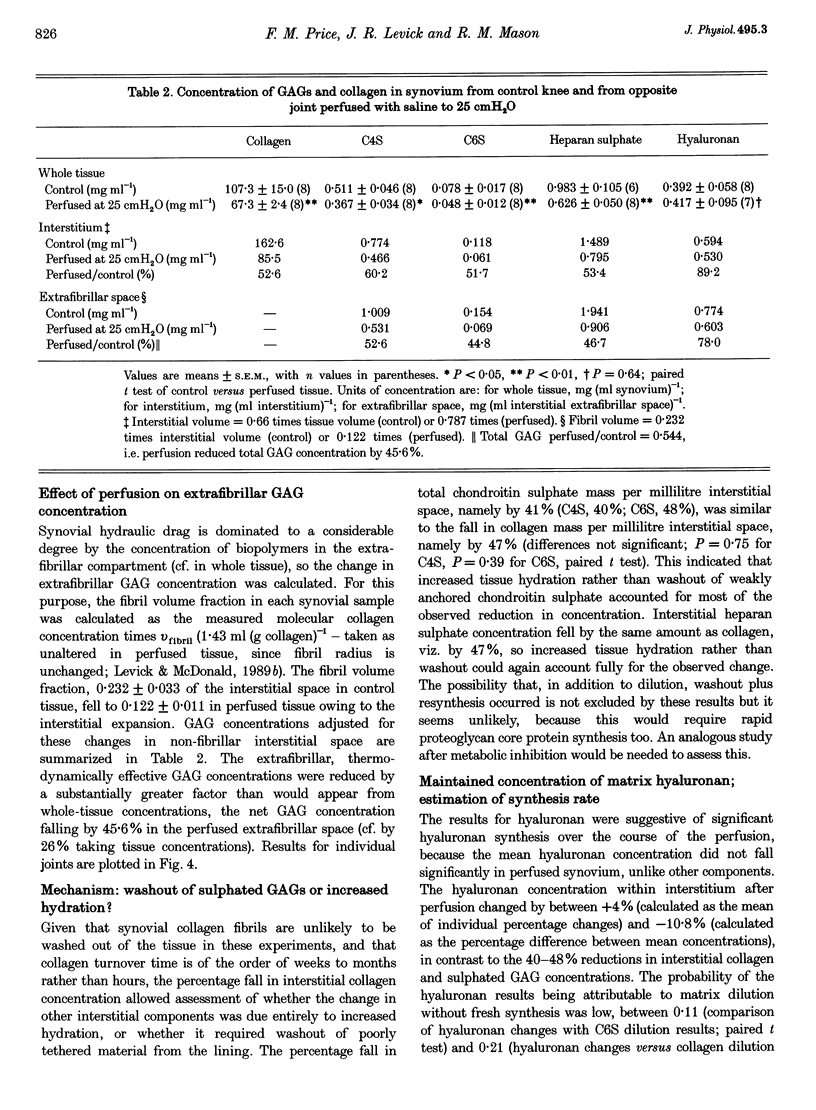
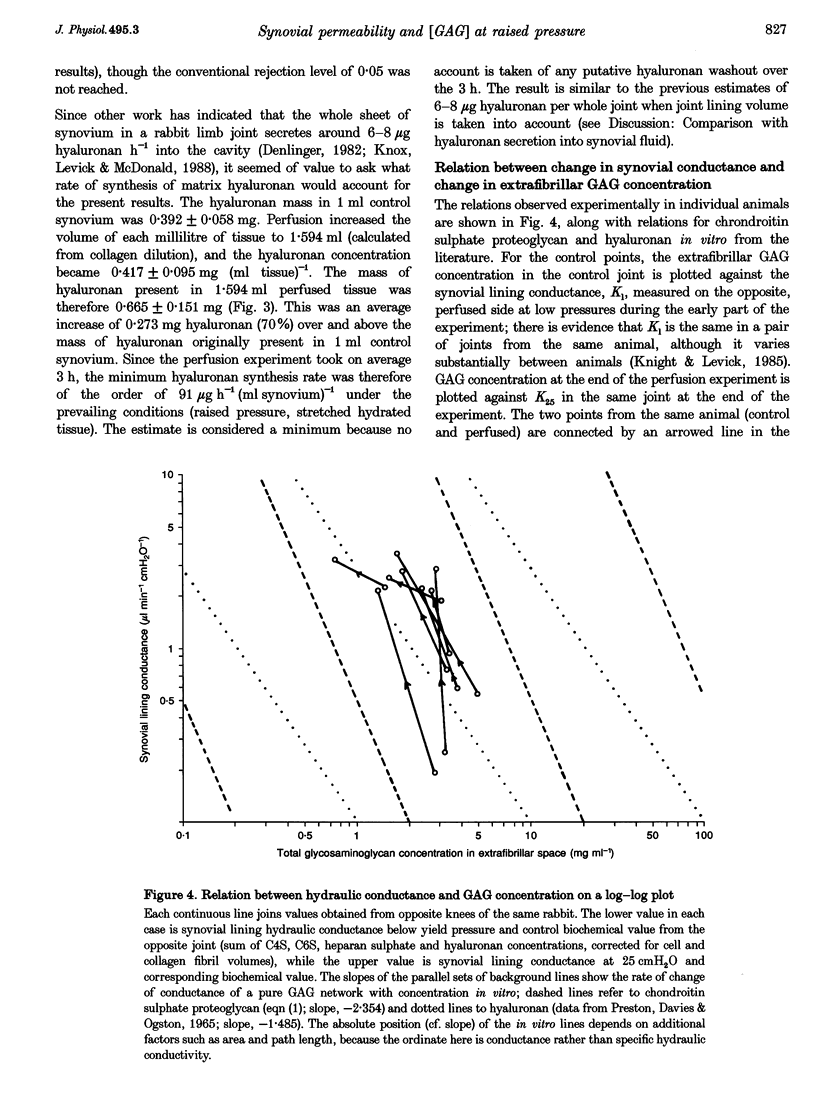
1. When intra-articular pressure is raised to pathological values (> 9 cmH2O) by saline, the hydraulic conductance of the synovial lining increases manyfold. The increase at 25 cmH2O is only partially accounted for by stretching of the tissue and has been ascribed to washout and/or dilution of interstitial matrix biopolymers. This suggestion was tested in this study by sampling synovium from control joints (rabbit knees) and from joints perfused with saline to 25 cmH2O, and analysing them quantitatively for collagen, chondroitin sulphate, heparan sulphate and hyaluronan. 2. Pressure and trans-synovial flow measurements showed that in samples taken at 25 cmH2O the conductance of the synovial lining had increased by a factor of 5.23 +/- 1.5 (mean +/- S.E.M.) over the conductance at low pressures (just above atmospheric pressure). 3. The tissue concentrations of collagen and the sulphated glycosaminoglycans (GAGs) were reduced by similar amounts after perfusion to 25 cmH2O, namely to 62.8-70.4% of control. The hyaluronan concentration by contrast was not significantly reduced (106% of control). 4. The reduction in collagen concentration (fixed material) indicated increased interstitial hydration. The closely similar reduction in sulphated GAGs indicated that dilution rather than washout of these components was occurring. The hyaluronan results could be explained by synthesis in vivo at a rate of > or = 91 micrograms h-1 (ml synovium)-1 (possibly a non-basal rate under the conditions of the experiment, i.e. raised pressure and a stretched hydrated membrane). 5. Because interstitial hydraulic drag is related to biopolymer concentration by a power function, the overall matrix dilution observed here was more than sufficient to explain the rise in synovial lining hydraulic conductance at 25 cmH2O when taken in conjunction with stretching of the synovial lining (increased area, reduced thickness).
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidanset D. J., Guidry C., Rosenberg L. C., Choi H. U., Timpl R., Hook M. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992 Mar 15;267(8):5250–5256. [PubMed] [Google Scholar]
- Castor C. W., Roberts D. J., Hossler P. A., Bignall M. C. Connective tissue activation. XXV. Regulation of proteoglycan synthesis in human synovial cells. Arthritis Rheum. 1983 Apr;26(4):522–527. doi: 10.1002/art.1780260411. [DOI] [PubMed] [Google Scholar]
- Clarris B. J., Fraser J. R., Muirden K. D., Malcolm L. P., Holmes M. W., Rogers K. Stimulation of glycosaminoglycan production and lysosomal activity of human synovial cells in culture by low environmental pH. Ann Rheum Dis. 1984 Apr;43(2):313–319. doi: 10.1136/ard.43.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daireaux M., Redini F., Loyau G., Pujol J. P. Effects of associated cytokines (IL-1, TNF-alpha, IFN-gamma and TGF-beta) on collagen and glycosaminoglycan production by cultured human synovial cells. Int J Tissue React. 1990;12(1):21–31. [PubMed] [Google Scholar]
- Edwards J. C. Synovial intimal fibroblasts. Ann Rheum Dis. 1995 May;54(5):395–397. doi: 10.1136/ard.54.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. C. The nature and origins of synovium: experimental approaches to the study of synoviocyte differentiation. J Anat. 1994 Jun;184(Pt 3):493–501. [PMC free article] [PubMed] [Google Scholar]
- Fisher R. F. The water permeability of basement membrane under increasing pressure: evidence for a new theory of permeability. Proc R Soc Lond B Biol Sci. 1982 Nov 22;216(1205):475–496. doi: 10.1098/rspb.1982.0087. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Wang C., Lange L. A., Turley E. A. Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J Cell Biol. 1994 Jul;126(2):575–588. doi: 10.1083/jcb.126.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubeck H. D., Kock R., Fischer D. C., Van de Leur E., Hoffmeister K., Greiling H. Transforming growth factor beta 1, a major stimulator of hyaluronan synthesis in human synovial lining cells. Arthritis Rheum. 1995 May;38(5):669–677. doi: 10.1002/art.1780380515. [DOI] [PubMed] [Google Scholar]
- Henderson K. J., Edwards J. C., Worrall J. G. Expression of CD44 in normal and rheumatoid synovium and cultured synovial fibroblasts. Ann Rheum Dis. 1994 Nov;53(11):729–734. doi: 10.1136/ard.53.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Dorfman A. Glycosaminoglycan synthesis by cultured human skin fibroblasts after transformation with simian virus 40. J Biol Chem. 1977 Jul 25;252(14):4777–4785. [PubMed] [Google Scholar]
- Kielty C. M., Whittaker S. P., Grant M. E., Shuttleworth C. A. Type VI collagen microfibrils: evidence for a structural association with hyaluronan. J Cell Biol. 1992 Aug;118(4):979–990. doi: 10.1083/jcb.118.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. Effect of fluid pressure on the hydraulic conductance of interstitium and fenestrated endothelium in the rabbit knee. J Physiol. 1985 Mar;360:311–332. doi: 10.1113/jphysiol.1985.sp015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R., McDonald J. N. Relation between trans-synovial flow and plasma osmotic pressure, with an estimation of the albumin reflection coefficient in the rabbit knee. Q J Exp Physiol. 1988 Jan;73(1):47–65. doi: 10.1113/expphysiol.1988.sp003122. [DOI] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. The density and distribution of capillaries around a synovial cavity. Q J Exp Physiol. 1983 Oct;68(4):629–644. doi: 10.1113/expphysiol.1983.sp002753. [DOI] [PubMed] [Google Scholar]
- Knox P., Levick J. R., McDonald J. N. Synovial fluid--its mass, macromolecular content and pressure in major limb joints of the rabbit. Q J Exp Physiol. 1988 Jan;73(1):33–45. doi: 10.1113/expphysiol.1988.sp003121. [DOI] [PubMed] [Google Scholar]
- Levick J. R. A two-dimensional morphometry-based model of interstitial and transcapillary flow in rabbit synovium. Exp Physiol. 1991 Nov;76(6):905–921. doi: 10.1113/expphysiol.1991.sp003553. [DOI] [PubMed] [Google Scholar]
- Levick J. R. An analysis of the interaction between interstitial plasma protein, interstitial flow, and fenestral filtration and its application to synovium. Microvasc Res. 1994 Jan;47(1):90–125. doi: 10.1006/mvre.1994.1007. [DOI] [PubMed] [Google Scholar]
- Levick J. R. Contributions of the lymphatic and microvascular systems to fluid absorption from the synovial cavity of the rabbit knee. J Physiol. 1980 Sep;306:445–461. doi: 10.1113/jphysiol.1980.sp013406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R., McDonald J. N. Synovial capillary distribution in relation to altered pressure and permeability in knees of anaesthetized rabbits. J Physiol. 1989 Dec;419:477–492. doi: 10.1113/jphysiol.1989.sp017881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R., McDonald J. N. Ultrastructure of transport pathways in stressed synovium of the knee in anaesthetized rabbits. J Physiol. 1989 Dec;419:493–508. doi: 10.1113/jphysiol.1989.sp017882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R. The influence of hydrostatic pressure on trans-synovial fluid movement and on capsular expansion in the rabbit knee. J Physiol. 1979 Apr;289:69–82. doi: 10.1113/jphysiol.1979.sp012725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. M., Crossman M. V., Sweeney C. Hyaluronan and hyaluronan-binding proteins in cartilaginous tissues. Ciba Found Symp. 1989;143:107-16; discussion 117-20, 281-5. doi: 10.1002/9780470513774.ch7. [DOI] [PubMed] [Google Scholar]
- McDonald J. N., Levick J. R. Effect of extravascular plasma protein on pressure-flow relations across synovium in anaesthetized rabbits. J Physiol. 1993 Jun;465:539–559. doi: 10.1113/jphysiol.1993.sp019692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. N., Levick J. R. Morphology of surface synoviocytes in situ at normal and raised joint pressure, studied by scanning electron microscopy. Ann Rheum Dis. 1988 Mar;47(3):232–240. doi: 10.1136/ard.47.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y., Matsumura F., Motoyoshi H., Yamasaki H., Fukuda K., Tanaka S. Secretion of hyaluronic acid from synovial fibroblasts is enhanced by histamine: a newly observed metabolic effect of histamine. J Lab Clin Med. 1992 Nov;120(5):707–712. [PubMed] [Google Scholar]
- Ostgaard G., Reed R. K. Increased lymphatic hyaluronan output and preserved hyaluronan content of the rat small intestine in prolonged hypoproteinaemia. Acta Physiol Scand. 1994 Sep;152(1):51–56. doi: 10.1111/j.1748-1716.1994.tb09783.x. [DOI] [PubMed] [Google Scholar]
- Preston B. N., Davies M., Ogston A. G. The composition and physicochemical properties of hyaluronic acids prepared from ox synovial fluid and from a case of mesothelioma. Biochem J. 1965 Aug;96(2):449–474. doi: 10.1042/bj0960449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price F. M., Levick J. R., Mason R. M. Glycosaminoglycan concentration in synovium and other tissues of rabbit knee in relation to synovial hydraulic resistance. J Physiol. 1996 Sep 15;495(Pt 3):803–820. doi: 10.1113/jphysiol.1996.sp021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price F. M., Mason R. M., Levick J. R. Radial organization of interstitial exchange pathway and influence of collagen in synovium. Biophys J. 1995 Oct;69(4):1429–1439. doi: 10.1016/S0006-3495(95)80012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. K., Townsley M. I., Zhao Z., Ishibashi M., Laurent T. C., Taylor A. E. Lymphatic hyaluronan flux from skin increases during increased lymph flow induced by intravenous saline loading. Int J Microcirc Clin Exp. 1994 Jan-Apr;14(1-2):56–61. doi: 10.1159/000178207. [DOI] [PubMed] [Google Scholar]
- Sisson J. C., Castor C. W., Klavons J. A. Connective tissue activation. XVIII. Stimulation of hyaluronic acid synthetase activity. J Lab Clin Med. 1980 Aug;96(2):189–197. [PubMed] [Google Scholar]
- Zamparo O., Comper W. D. Hydraulic conductivity of chondroitin sulfate proteoglycan solutions. Arch Biochem Biophys. 1989 Oct;274(1):259–269. doi: 10.1016/0003-9861(89)90438-4. [DOI] [PubMed] [Google Scholar]


