Abstract
Background
Resolvins, which are divided into series D (RvD) and E (RvE), originate from omega-3 fatty acids, DHA and EPA and were recently found to be involved in the modulation of inflammation in some tumors, including breast cancer (BC). We aimed to assess the resolvin profiles (RvD1, RvD2, RvD3 and RvE1) in the plasma of BC patients compared with those of controls and to determine differences in their concentrations according to BC presentation and immunohistochemical characteristics.
Methods
We considered BC patients (sporadic, familiar and BRCA1/2-mutated forms) naïve to any anticancer treatment and controls affected by nonmalignant breast disease. According to the BC immunohistochemical characteristics, we identified the luminal-A, luminal-B, HER2 + and triple-negative subtypes. The levels of RvD1, RvD2, RvD3 and RvE1 in the plasma of all the participants were measured via ELISA kits.
Results
We enrolled 64 women, 53 with BC (age 51 ± 10 y) and 11 controls (age 49 ± 7 y). Twenty-seven patients presented with sporadic BC, 16 with a positive history of BC (familiar), and 10 with BRCA 1/2 gene mutations. Compared with control patients, BC patients presented higher levels of RvD1 (p = 0.015), and no differences were detected for RvD2, 3 or RvE1. In BC, all resolvin levels were positively correlated with each other (p < 0.001). The expression of RvD1 and RvD3 was lower in the mutated group than in the familiar and sporadic groups (p < 0.05). The expression of RvD2 and RvE1 tended to be lower in the BRCA 1/2-mutated group than in the sporadic and familiar BC patients (p = 0.051 and p = 0.062, respectively). No differences in plasma resolvin levels were observed according to immunohistochemical characteristics (luminal A, luminal B, HER2+, triple-negative). However, RvD1 was lower in triple-negative patients than in patients with the other BC subtypes (p = 0.023). In terms of Ki-67 expression, RvD3 expression was lower in patients with high Ki-67 expression (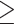 20%) than in those with low Ki-67 expression (<20%) (p = 0.034).
20%) than in those with low Ki-67 expression (<20%) (p = 0.034).
Conclusion
This is the first human study profiling specific plasma resolvin levels in BC patients, which revealed low plasma levels of some resolvins in patients with BRCA1/2 mutations, triple-negative subtypes and high Ki-67 expression, potentially impacting treatment response and prognosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02386-5.
Keywords: Plasma resolvins, Breast cancer, BRCA 1/2, Triple-negative, Ki-67
Introduction
The clinical course of breast cancer is often associated with changes in inflammatory status, which involves several interactions between different cell types and chemical mediators [1]. Omega-3 polyunsaturated fatty acids (PUFAs), mainly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), participate in the resolution of inflammation and have proresolving effects by inhibiting different pathways involved in the development of inflammation [3], including leukocyte chemotaxis, adhesion molecule expression and interactions, and inflammatory cytokine production, through the action of specialized proresolving lipid mediators (SPMs) [2, 3]. These molecules act to modulate the inflammatory process and stimulate the phases of inflammation resolution. The resolution of inflammation is considered an active process to be pursued in cancer, and these potent proresolution mediators, such as resolvins (Rv), protectins, neuroprotectins, and maresins, are able to resolve and actively contribute to homeostasis [4].
Resolvins, which are divided into series E (RvE) and D (RvD), originate from EPA and DHA, respectively, and both act by decreasing leukocyte infiltration and reducing cellular debris, leading to the cessation of the inflammatory state [5, 6]. In particular, resolvins D1, D2, D3 and E1 have been implicated in the inflammatory process during cancer [7].
Recent findings revealed that omega-6 fatty acids are increased and that omega-3 fatty acids EPA and DHA are decreased in most cancer tissues and that an increase in omega-3 PUFA consumption and the consequent omega-6/omega-3 ratio of 2–4:1 are associated with a reduced risk of cancer development and progression, including breast cancer [4]. In this context, omega-3 PUFAs may be considered potent modulators of the mechanisms regulating the onset, prolongation, and resolution of inflammation, and in the setting of breast cancer, they may also be protective against the inflammatory response.
In this study, we aimed to assess a resolvin profile in breast cancer patients naïve to any anticancer treatment, measuring the circulating plasma levels of resolvins D1, D2, D3 and E1, which are molecules found to be modulated in cancer [4–7], compared with those in healthy controls.
We then verified differences in plasma resolvin levels according to the type of presentation of breast cancer and according to immunohistochemical characteristics among the breast cancer groups.
Methods
Participants
Patients with a new diagnosis of primary breast cancer, on the basis of imaging methods and histologically confirmed by core-needle biopsy, and patients with benign breast disease (control group), who were followed at the Department of Surgery (formerly Department of Surgical Sciences), Sapienza University of Rome, were considered for this study and consecutively enrolled, with the approval of the local Ethics Committee (protocol n. 588/13) (Sapienza University, Policlinico Umberto I, Rome, Italy). The inclusion criteria were age ≥ 18 years, eligibility for surgery for breast cancer or nonmalignant diseases, and ability to provide informed consent. We excluded participants who self-reported the consumption of omega-3 polyunsaturated fatty acid supplements and omega-3 polyunsaturated fatty acid-supplemented foods in the prior six months and those with other concomitant comorbidities associated with acute/subacute inflammation.
On the basis of their familiar and medical history, we divided breast cancer patients into sporadic (no family history of breast cancer), familiar (history of familial breast cancer presentation without BRCA1/2 gene mutation) and mutated (patients with documented BRCA1/2 gene mutation) groups, as previously described [8].
In addition, we administered a questionnaire including questions on seafood intake in the diet, addressing portion size and frequency to account for omega-3/6 polyunsaturated fatty acid intake, as previously described [8, 9].
Immunohistochemical characteristics of breast cancer patients
Data on immunohistochemical characteristics were collected. Immunohistochemical subtypes were defined as follows: luminal A, luminal B, HER2 + and triple negative. We collected data on the percentage of Ki-67-expressing (ranging from 0 to 100%) tumor cells that were positive for anti–Ki-67 antibodies. As previously described, high Ki-67 expression was defined as  20% [10].
20% [10].
Blood samples for plasma resolvin measurements
Blood samples were taken from each participant (breast cancer patients and controls) before surgery, centrifuged at the laboratory of the Department of Translational and Precision Medicine, Sapienza University of Rome, and subsequently stored at -80 °C to determine the plasma levels of resolvins D1, D2, D3, and E1. Human Resolvin D1, human Resolvin D2, human Resolvin D3 and human Resolvin E1 were assessed via the ELISA “Double Antibody Sandwich” technique, which allows the formation of the antibody-antigen complex. The procedure included mixing the plasma and antibody, incubating, washing, and adding the final substrate. All samples were analyzed via the commercially available ELISA kits (Mybiosource, Inc., San Diego, CA, USA): Human Resolvin D1 (RvD1) (Cat. No: MBS053145, sensitivity 5.0 pg/mL); Human Resolvin D2 (RvD2) (Cat. No: MBS051498, sensitivity 5.0 pg/mL); Human Resolvin D3 (RvD3) (Cat. No: MBS056544, sensitivity 2.0 pg/mL); and Resolvin E1 (RvE1) (Cat. No: MBS025958, sensitivity 5.0 pg/mL), in accordance with the manufacturer’s instructions reporting high sensitivity and excellent specificity (https://www.mybiosource.com).
Statistical analyses
The anthropometric and laboratory data, including resolvins, were compared between the group of breast cancer patients and controls. Categorical variables were compared via Pearson’s nonparametric chi-square test or Fisher’s exact test, as appropriate. Continuous variables are expressed as the mean ± standard error. Significant differences were evaluated by Student’s t test or the Mann‒Whitney nonparametric test to assess differences between two groups (e.g., breast cancer patients vs. controls). Analysis of variance (ANOVA) and the Kruskal‒Wallis test were performed to assess differences between more than two groups (e.g., sporadic vs. familiar vs. mutated groups), according to a normal or nonnormal distribution, as appropriate. The correlations were assessed via Pearson’s correlation coefficient or Spearman’s rho coefficient, according to the type of distribution. The observed differences were considered statistically significant when the p value was < 0.05. SPSS version 28 was used to perform the statistical analyses.
Results
Participant characteristics
We enrolled a total of 64 women, 53 affected by breast cancer and 11 affected by nonmalignant breast diseases, both of whom were eligible for surgery. The mean age (years) of the breast cancer patients was 51.04 ± 10.1 years, and the mean BMI (kg/m2) was 24.4 ± 0.6 (Table 1). Among the breast cancer patients, 27 presented with sporadic breast cancer (sporadic group), 16 with a positive history of breast cancer (familiar group) and 10 with a mutation in the BRCA 1/2 gene (mutated group) (Table 1). On the basis of their immunohistochemical characteristics, 24 breast cancer patients were classified as luminal-A, 6 as luminal-B, 14 as HER2 + and 9 as triple-negative. The most frequent comorbidity of breast cancer patients was dyslipidemia (9/53, 17%), followed by diabetes (4/53, 7.5%). The control group presented a mean age (years) of 48.6 ± 6.6 years and a mean BMI (kg/m2) of 23.6 ± 2.8 years (Table 1). Additional characteristics of the participants are shown in Table 1.
Table 1.
Patient characteristics
| Parameter | Breast cancer patients (N = 53) | Controls (N = 11) |
|---|---|---|
| Age, years | 51.0 ± 10.1 | 48.6 ± 6.6 |
| Female, n (%) | 53 (100) | 11 (100) |
| Body mass index, kg/m2 | 24.36 ± 3.95 | 23.59 ± 2.77 |
| Creatinine, mg/dl | 0.79 ± 0.14 | 0.74 ± 0.09 |
| Fasting plasma glucose, mg/dl | 93 (88; 99.1) | 86.5 (82.9; 93) |
| Total cholesterol, mg/dl | 203 ± 33 | 206 ± 23 |
| Triglycerides, mg/dl | 92 (74; 119) | 72 (69; 90) |
| Good seafood consumer*, n (%) | 32 (71) | 9 (82) |
| Comorbidities | ||
| Diabetes, n (%) | 4 (8) | 0 (0) |
| Dyslipidemia, n (%) | 9 (17) | 1 (9) |
| Type of presentation | ||
| Sporadic, n (%) | 27 (51) | / |
| Familiar, n (%) | 16 (30) | / |
| BRCA1/2 Mutated, n (%) | 10 (19) | / |
| Immunohistochemistry | ||
| Luminal-A, n (%) | 24 (45) | / |
| Luminal-B, n (%) | 6 (11) | / |
| HER2+, n (%) | 14 (26) | / |
| Triple-negative, n (%) | 9 (17) | / |
Ki-67%  20%, n (%) 20%, n (%) |
34 (64) | / |
Data are shown as the mean ± standard deviation and median (25th; 75th percentiles) for normally and nonnormally distributed variables, respectively
*Data available for 45 breast cancer patients
Plasma resolvin levels among breast cancer patients and controls
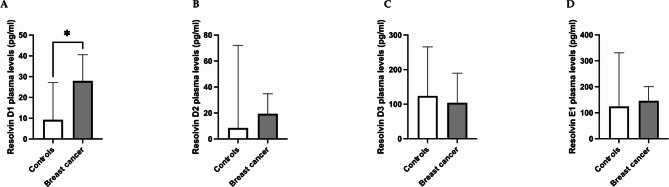
Compared with the control group, the breast cancer group presented increased median levels of resolvin D1 (p = 0.015) (Fig. 1, panel A), whereas no differences were observed for plasma resolvins D2, D3 and E1 between the two groups (Fig. 1, panels B-D). Among breast cancer patients, resolvin levels positively correlated with each other (Table 2). In the breast cancer group, no differences in resolvin levels were found according to comorbidities, including dyslipidemia and diabetes. No differences were observed in resolvin levels between good seafood consumers and bad seafood consumers among breast cancer patients or controls.
Fig. 1.
Differences in plasma resolvin levels between patients with breast cancer and controls. The data revealed higher levels of resolvin D1 in breast cancer patients than in controls (p = 0.015) (panel A). No differences were observed in resolvin D2, D3 or E1 levels between cancer patients and controls (panel B, C). Data are expressed as pg/ml and are presented as the median (95% CI). * p < 0.05
Table 2.
Linear correlations between plasma resolvin levels in breast cancer patients
| Resolvin D1 | Resolvin D2 | Resolvin D3 | Resolvin E1 | |
|---|---|---|---|---|
| Resolvin D1 | / |
rho = 0.800 p < 0.001 |
rho = 0.700 p < 0.001 |
rho = 0.754 p < 0.001 |
| Resolvin D2 |
rho = 0.800 p < 0.001 |
/ |
rho = 0.709 p < 0.001 |
rho = 0.776 p < 0.001 |
| Resolvin D3 |
rho = 0.700 p < 0.001 |
rho = 0.709 p < 0.001 |
/ |
rho = 0.706 p < 0.001 |
| Resolvin E1 |
rho = 0.754 p < 0.001 |
rho = 0.776 p < 0.001 |
rho = 0.706 p < 0.001 |
/ |
Plasma resolvin levels among breast cancer patients according to the different forms of presentation (sporadic, familiar, or BRCA 1/2-mutated)
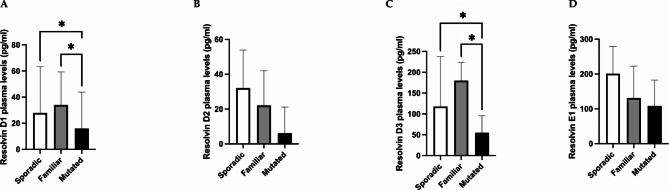
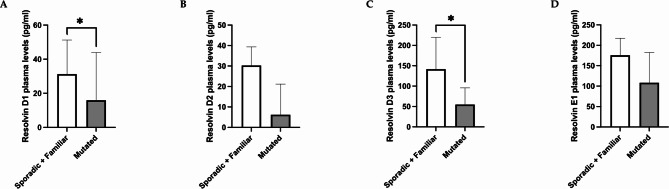
Among breast cancer patients, the plasma levels of resolvin D1 were lower in the mutated BRCA1/2 group than in the familiar (p = 0.031) and sporadic groups (p = 0.020), whereas no differences were detected between the sporadic and familiar groups (p = 0.979) (Fig. 2, panel A). Additionally, plasma resolvin D3 levels were found to be lower in the mutated group than in the familiar (p = 0.031) and sporadic (p = 0.013) groups (Fig. 2, panel C). No differences were detected in the resolvin D2 and E1 levels among the three groups (Fig. 2, panels B and D). Moreover, resolvin D1 and D3 levels were lower in the mutated group than in the sporadic and familiar patients (p = 0.014 and p = 0.010, respectively) (Fig. 3, panels A and C). Additionally, resolvin D2 and E1 tended to be lower in the mutated group than in the sporadic and familiar breast cancer patients (p = 0.051 and p = 0.062, respectively) (Fig. 3, panels B and D).
Fig. 2.
Differences in plasma resolvin levels in the cancer group according to the form of presentation (sporadic, familiar and BRCA1/2 mutated groups). The data revealed lower levels of resolvin D1 in BRCA1/2-mutated patients than in the familiar and sporadic patients (p = 0.031 and p = 0.020, respectively) (panel A). The data revealed lower levels of resolvin D3 in BRCA1/2-mutated patients than in the familiar and sporadic patients (p = 0.031 and p = 0.013, respectively) (panel C). No difference was observed in the resolvins D2 and E1 levels among the three groups (panel B, D). Data are expressed as pg/ml and are presented as the median (95% CI). * p < 0.05
Fig. 3.
Differences in plasma resolvin levels between the sporadic plus familiar group and the BRCA1/2 mutated patient group. The data revealed lower levels of resolvin D1 and resolvin D3 in BRCA1/2-mutated patients than in the familiar plus sporadic patients (p = 0.014 and p = 0.010, respectively) (panels A, C). Resolvin D2 and E1 levels tended to be lower in the BRCA1/2-mutated group than in the sporadic plus familiar group (p = 0.051 and p = 0.062, respectively). Data are expressed as pg/ml and are presented as the median (95% CI). * p < 0.05
Plasma resolvin levels among breast cancer patients according to immunohistochemical characteristics (luminal A, luminal B, HER2 + and triple-negative) and Ki-67 expression
No differences in plasma resolvin levels were observed according to the immunohistochemical characteristics (luminal A, luminal B, HER2+, or triple-negative).
The resolvin D1 levels were lower in triple-negative patients than in nontriple-negative patients (p = 0.023) (Fig. 4); no difference in the other resolvin levels was observed between the two groups (Supplementary Fig. 1). In terms of Ki-67 expression, resolvin D3 plasma levels were lower in patients with high Ki-67 expression ( 20%) than in those with lower Ki-67 expression (< 20%) (p = 0.034) (Fig. 5); no difference in the other resolvin levels was observed between the two groups (Supplementary Fig. 2).
20%) than in those with lower Ki-67 expression (< 20%) (p = 0.034) (Fig. 5); no difference in the other resolvin levels was observed between the two groups (Supplementary Fig. 2).
Fig. 4.
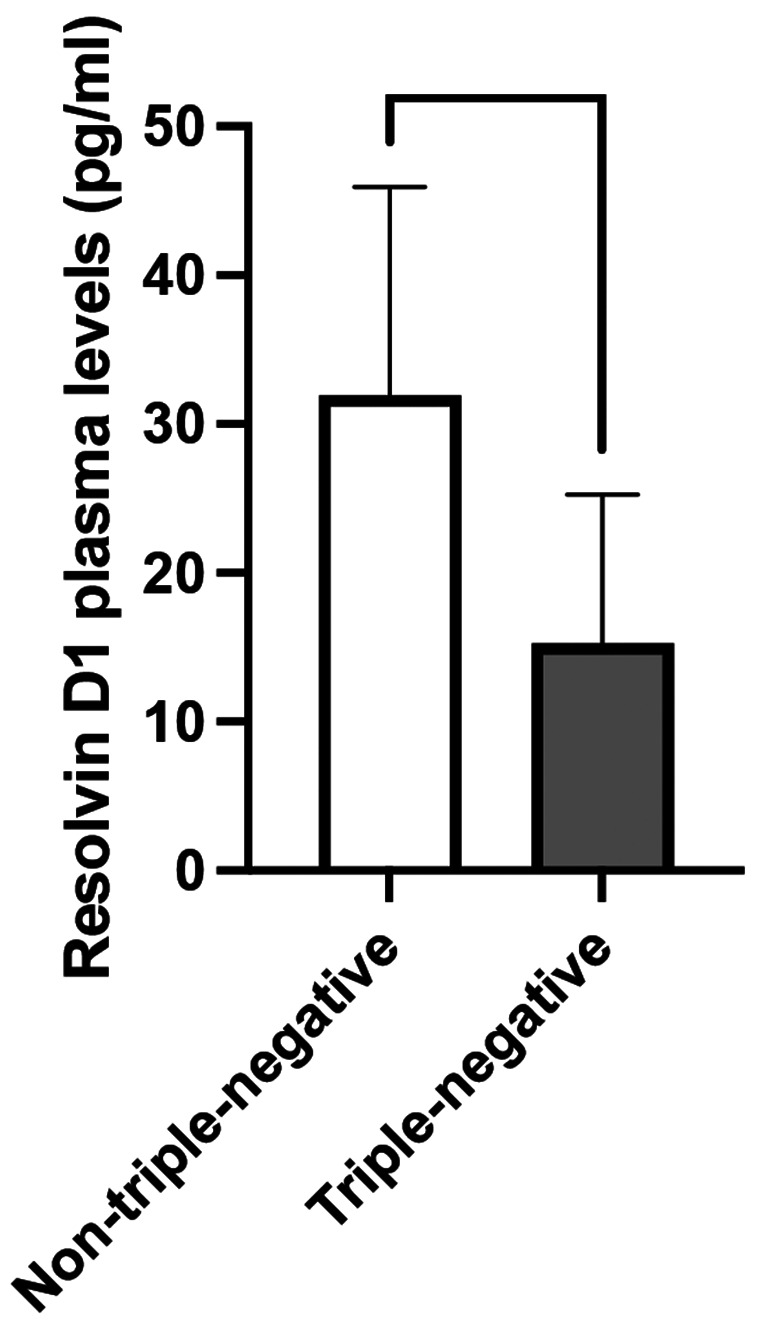
Differences in plasma resolvin D1 levels between triple-negative and nontriple-negative breast cancer patients. The data revealed lower levels of resolvin D1 in triple-negative patients than in non-triple-negative patients (p = 0.023). Data are expressed as pg/ml and are presented as the median (95% CI). * p < 0.05
Fig. 5.
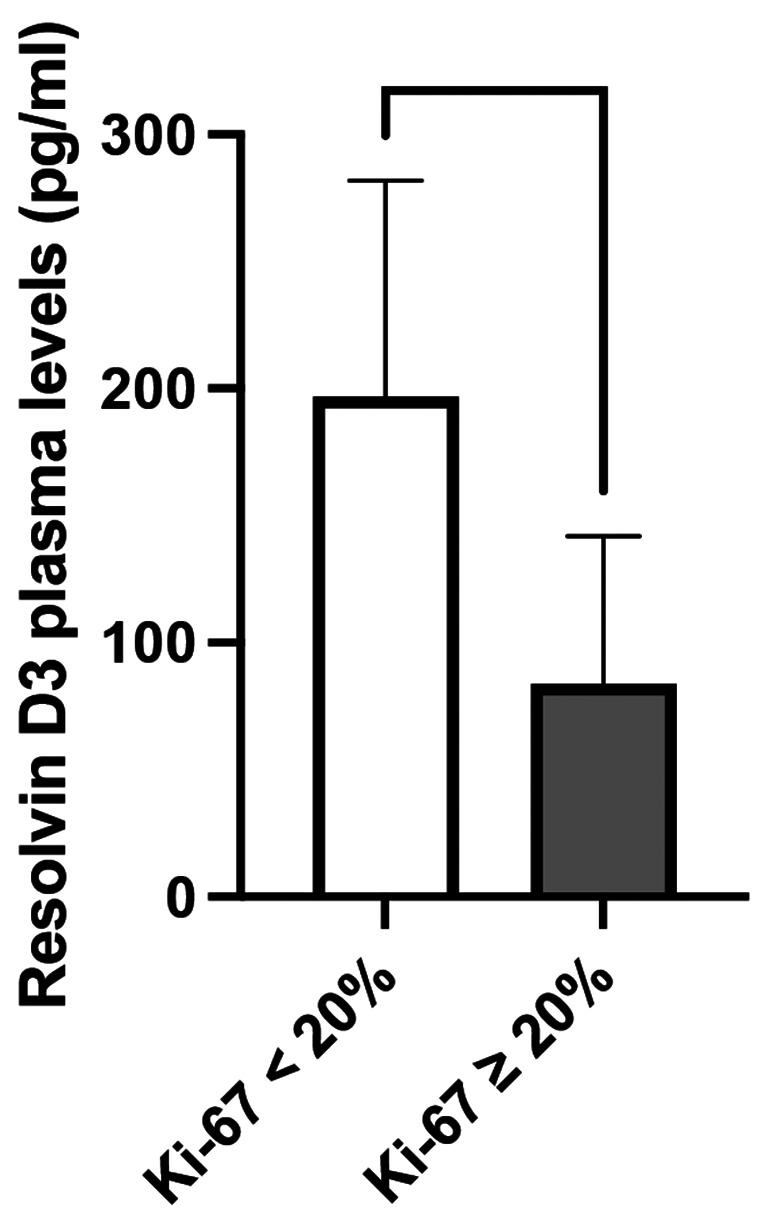
Differences in plasma resolvin D3 levels according to Ki-67% expression. The data revealed lower levels of resolvin D3 in breast cancer patients with high Ki-67 expression (≥ 20%) than in those with low Ki-67 expression (p = 0.034). Data are expressed as pg/ml and are presented as the median (95% CI). * p < 0.05
Discussion
Our results indicate that some plasma resolvin levels are altered in breast cancer patients who are naïve to anticancer therapies and eligible for surgery. In particular, among the entire cohort of breast cancer patients, resolvin D1 was higher in patients than in controls.
Notably, among the resolvins that we tested, the main differences were detected when their concentrations were analyzed in accordance with the form of presentation of breast cancer. In fact, resolvin D1 was lower in the mutated BRCA1/2 patients than in both the familiar and sporadic groups. This is of particular interest considering the possible anti-inflammatory and anticancer properties of this molecule. Recently, Mattoscio D et al. reported that in in vivo models of HPV tumors, resolvin D1 was able to impair cancer growth, enhancing the activity of tumor-associated neutrophils and the recruitment of antitumor monocytes [11]. Another study evaluated the role of resolvins, including D1 and E1, in counteracting debris-stimulated cancer progression and revealed that resolvins interfere with the release of specific cytokines stimulated by cell debris, impairing cancer progression [12].
Moreover, in our study, the resolvin D3 levels were lower in the mutated group than in the familiar and sporadic groups, suggesting a different proresolving status in patients with BRCA1/2 mutations.
Interestingly, resolvin D3 was investigated in association with resolvins D2 and D4 in Lewis lung carcinoma (LLC) models, and these molecules were able to inhibit metastases via the resolution of inflammation in the preoperative period [7].
With respect to E1, we did not observe significant differences among the sporadic, familiar and mutated forms but only a tendency toward a reduction in E1 in the mutated group compared with the nonmutated patients, in parallel with resolvin D2 concentrations.
The potential role of resolvin E1 in preventing tumor growth was investigated. Kantarci et al. showed the capacity of resolvin E1 to inhibit inflammation and tumor growth in LLC models [13].
With respect to the properties of resolvin D2, in an experimental model represented by ER-positive breast tumor (MCF-7) cells, Al Zaubai et al. described a proliferative effect of resolvin D2 on this cell line, suggesting a potential effect on estrogen-dependent breast cancer proliferation [14].
On the basis of this evidence, resolvins represent promising candidates as biomarkers of inflammation/resolution of inflammation not only in the presence of breast cancer but also according to the different presentations of the disease.
In addition, taking into account our findings, resolvin levels also appear to be modulated according to some immunohistochemical characteristics of breast cancer, including hormone receptor and Ki-67% expression. In fact, we observed a significant reduction in resolvin D1 in triple-negative breast cancer patients compared with patients with the other subtypes.
In a large prospective study, omega-3 PUFA intake via food was inversely correlated with the development of estrogen receptor-positive and progesterone receptor-positive breast cancer, whereas the intake of omega-6 PUFAs was positively associated with the development of this bioprofile of breast cancer [15]. These data may suggest a possible link between PUFA intake and circulating plasma resolvin levels. In our cohort, we did not find differences in the plasma concentrations of resolvins between good and bad seafood consumers. However, we do not have data on the exact amount of omega-3/6 PUFA intake and their circulating levels; therefore, we cannot completely exclude the possible effects of PUFA intake on plasma resolvin levels.
Moreover, we observed a reduction in resolvin D3 expression in patients with high Ki-67% expression. These data are clinically relevant considering the findings of others who reported a correlation between Ki-67% expression and the 21-gene recurrence score in patients with breast cancer (ER+/ERBB−), as well as a significant association between patients with low recurrence scores and high Ki-67% expression ( 20%) and recurrence-free survival beyond 3 years [10].
20%) and recurrence-free survival beyond 3 years [10].
Taken together, these results suggest a possible prognostic role of low resolvin levels in breast cancer, considering the worse prognosis associated with these phenotypes (triple-negative and high Ki-67% expression). However, longitudinal studies are mandatory to confirm this association and the possible role of resolvins as biomarkers in the setting of breast cancer.
Study strengths and limitations
This is the first human study revealing a specific plasma resolvins profile in BC patients, according to the type of presentation of breast cancer and Ki-67 expression, with potential implications on breast cancer treatment response and prognosis.
However, our study has several limitations. When performing the analyses on the basis of the different forms of breast cancer, the number of participants in each group was small. Although we measured different resolvins mostly implicated in cancer, we did not analyze other resolvins of the D and E series. Owing to the specific aim of our study, we did not include patients undergoing anticancer treatments (i.e., neoadjuvant treatments) in the analysis, limiting the results to patients who were naïve to therapy. For this reason, longitudinal studies including patients receiving anticancer treatments may add novel and intriguing data on the role of resolvins and their prognostic value.
Conclusions
Our study is the first to profile plasma resolvins in breast cancer patients naïve to anticancer treatments, revealing lower plasma levels of specific resolvins in patients with BRCA1/2 mutations, triple-negative subtypes and high Ki-67 expression. These observations appear to be clinically relevant, especially for suggesting the prognostic significance of proresolving mediators in breast cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank FoodAR Srl for the unconditional support providing the ELISA Kit for the plasma resolvins measurements.
Author contributions
AM and MIA developed and supervised the project, conducted the investigation, wrote the manuscript, and analyzed the data. GI wrote the manuscript and analyzed the data. GS, LL and MS designed and performed experiments. ADL and MLC enrolled the patients and conducted the investigation. MM supervised the study.All the authors read and approved the final version of the manuscript.
Funding
This study was supported by an intramural research grant from Sapienza University of Rome, Italy (Grant n. RM12117A86CCB2C6).
Data availability
The raw datasets generated, used, and analyzed in the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the local Ethics Committee (Sapienza University, Policlinico Umberto I, Rome, Italy— prot. n. 588/13). Written informed consent was obtained from all patients and controls enrolled in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alessio Molfino, Email: alessio.molfino@uniroma1.it.
Maurizio Muscaritoli, Email: maurizio.muscaritoli@uniroma1.it.
References
- 1.Brown KA. Metabolic pathways in obesity-related breast cancer. Nat Rev Endocrinol. 2021;17:350–63. 10.1038/s41574-021-00487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang X, Ge K, Song C, Ge Y, Zhang J. Effects of n-3PUFAs on autophagy and inflammation of hypothalamus and body weight in mice. Biochem Biophys Res Commun. 2018;501:927–32. 10.1016/j.bbrc.2018.05.084. [DOI] [PubMed] [Google Scholar]
- 3.Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, Spencer D, et al. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer Oxf Engl 1990. 2009;45:2077–86. 10.1016/j.ejca.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Zárate R, El Jaber-Vazdekis N, Tejera N, Pérez JA, Rodríguez C. Significance of long chain polyunsaturated fatty acids in human health. Clin Transl Med. 2017;6:e25. 10.1186/s40169-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–27. 10.1146/annurev-nutr-071811-150726. [DOI] [PubMed] [Google Scholar]
- 7.Panigrahy D, Gartung A, Yang J, Yang H, Gilligan MM, Sulciner ML, et al. Preoperative stimulation of resolution and inflammation blockade eradicates micrometastases. J Clin Invest. 2019;129:2964–79. 10.1172/JCI127282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molfino A, Amabile MI, Mazzucco S, Biolo G, Farcomeni A, Ramaccini C, et al. Effect of oral docosahexaenoic acid (DHA) supplementation on DHA levels and Omega-3 index in red blood cell membranes of breast Cancer patients. Front Physiol. 2017;8:549. 10.3389/fphys.2017.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl L, Mæland CA, Bjørkkjær T. A short food frequency questionnaire to assess intake of seafood and n-3 supplements: validation with biomarkers. Nutr J. 2011;10:127. 10.1186/1475-2891-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J, Lee Y-J, Bae SJ, Baek SH, Kook Y, Cha YJ, et al. Ki-67, 21-Gene recurrence score, Endocrine Resistance, and survival in patients with breast Cancer. JAMA Netw Open. 2023;6:e2330961. 10.1001/jamanetworkopen.2023.30961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattoscio D, Isopi E, Lamolinara A, Patruno S, Medda A, De Cecco F, et al. Resolvin D1 reduces cancer growth stimulating a protective neutrophil-dependent recruitment of anti-tumor monocytes. J Exp Clin Cancer Res CR. 2021;40:129. 10.1186/s13046-021-01937-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulciner ML, Serhan CN, Gilligan MM, Mudge DK, Chang J, Gartung A, et al. Resolvins suppress tumor growth and enhance cancer therapy. J Exp Med. 2018;215:115–40. 10.1084/jem.20170681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantarci A, Kansal S, Hasturk H, Stephens D, Van Dyke TE. Resolvin E1 reduces Tumor Growth in a xenograft model of Lung Cancer. Am J Pathol. 2022;192:1470–84. 10.1016/j.ajpath.2022.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Zaubai N, Johnstone CN, Leong MM, Li J, Rizzacasa M, Stewart AG. Resolvin D2 supports MCF-7 cell proliferation via activation of estrogen receptor. J Pharmacol Exp Ther. 2014;351:172–80. 10.1124/jpet.114.214403. [DOI] [PubMed] [Google Scholar]
- 15.Kiyabu GY, Inoue M, Saito E, Abe SK, Sawada N, Ishihara J, et al. Fish, n – 3 polyunsaturated fatty acids and n – 6 polyunsaturated fatty acids intake and breast cancer risk: the Japan Public Health Center-based prospective study. Int J Cancer. 2015;137:2915–26. 10.1002/ijc.29672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw datasets generated, used, and analyzed in the current study are available from the corresponding author upon reasonable request.