Abstract
Background
Patients with major depressive disorder (MDD) experience depressive symptoms such as anhedonia as well as cognitive dysfunction which can subsequently impair their work performance.
Purpose
To assess the effectiveness and safety of vortioxetine in working patients with MDD in South Korea.
Patients and Methods
This was a subgroup analysis of a prospective, multicenter, non-interventional, non-comparative post-marketing surveillance (PMS) study. Vortioxetine-naïve patients aged >18 years who were administered with vortioxetine were followed for up to 24±2 weeks. Working patients were defined as those who were working or studying full- (≥6 hours/day) or part-time (<6 hours/day) at baseline. Effectiveness and adverse events (AEs), assessed by both clinician and patient-reported measured, were analyzed.
Results
A total of 1082 working patients (mean age: 39.56 years) were included in the subgroup analysis. Clinically significant improvements in depressive symptoms, including anhedonia, were observed over the 24 weeks of follow-up, with mean scores for the total Montgomery-Asberg Depression Rating Scale (MADRS) and anhedonia subscale both significantly decreasing from baseline by mean±standard deviation (SD) of 9.73±9.08 and 5.37±5.24 points, respectively, at 24 weeks (both p<0.001 vs baseline). The vast majority of patients (80.01%) treated with vortioxetine also showed improvements in mental health symptoms over the 24 weeks, measured using the Clinical Global Impression – Improvement (CGI-I) scores. Significant improvements in cognitive symptoms were also observed over the study period, measured by the Korean Version of the Perceived Deficits Questionnaire-Depression as well as Digit Symbol Substitution Test (all p<0.0001 from baseline at Visits 2 and 3). Vortioxetine was well tolerated in working patients, with the respective rates of any AEs and serious AEs being 18.67% and 1.20%.
Conclusion
Working patients treated with vortioxetine had improvements in their depressive symptoms (including anhedonia), cognitive function and performance. Vortioxetine was found to be well tolerated in this study.
Keywords: major depressive disorder, non-interventional, post-marketing surveillance, South Korea, vortioxetine, work performance
Introduction
Major Depressive Disorder (MDD) is a common psychiatric disorder in adults, with approximately 5% of adults having experienced depression globally.1 MDD is one of the leading causes of health burden among the working-age population worldwide.2,3 In 2019, depressive disorders were the 6th highest cause of burden, measured using disability-adjusted life years, among those aged 25–49 years.2,3
In South Korea, the burden of MDD is also increasing; between 1990 and 2017, South Korea had the 3rd highest increase in age-standardized incidence of MDD among 195 countries.4 MDD is associated with cognitive symptoms that have detrimental impacts on work performance, such as reduced work productivity and increased sick leave. Such adverse work-related outcomes due to MDD could pose substantial economic burden personally and to society. A modelling study in South Korea reported that MDD was estimated to result in 11,740 excess deaths in people of working age when compared with those without MDD, which translated to 55,126 years of life lost due to MDD.5 The same study estimated that 1,627,333 productivity-adjusted life years were lost in the MDD cohort compared with those without MDD, equating to loss of approximately US$ 112 billion in the nation’s gross domestic product as of 2019.5 The impact of MDD on patients of working age was further demonstrated in a cross-sectional study in Korea, known as Epidemiological Research on Functioning Outcomes Related to Major depressive disorder in South Korea (PERFORM-K), which reported that the majority of patients experienced cognitive dysfunction as well as work productivity losses, work time missed (absenteeism) and actual work impaired (presenteeism) due to MDD.6 Therefore, there is a need to address the symptoms of MDD with an impact on the prognosis of working patients with MDD.6,7
A particularly debilitating core feature of MDD is anhedonia, defined as a loss of pleasure and lack of reactivity to pleasurable stimuli.8 It occurs in approximately 75% of MDD patients,9 with anhedonia severity being directly related to severity of MDD.10 A network-based analysis of MDD patients in China reported that compared with MDD patients with low anhedonia, those with high anhedonia presented with higher risk of suicidal ideation and depressive symptoms.11 As an aspect of MDD, anhedonia might have an impact on workplace productivity in working patients by mediating symptoms of MDD.12–15 In addition to being associated with non-response to antidepressants and cognitive dysfunction,13,14 anhedonia has also been reported to have a potential mediator effect on different functional domains, including work/school, amongst MDD patients.15 Anhedonia can also have deleterious effects on the conduct of day-to-day activities, such as functional impaired reward functioning to acknowledge one’s achievements at the workplace.16 As such, anhedonia has been associated with a high risk of being unemployed, demonstrating the negative impact of anhedonia in people of working age at a societal level.17 Therefore, treatments that can improve both anhedonia and cognitive function could help to address these impacts on work productivity and potentially reduce the societal burden of MDD.
Vortioxetine is a novel antidepressant that has shown clinically meaningful improvements in both depressive and cognitive symptoms for the treatment of MDD patients.18 Its unique multimodal mechanism allows the drug to exhibit high affinity for the serotonin blocker transporter as well as modulate pre- and post-synaptic 5-HT receptor activity.18 Several global and regional randomized controlled trials and real-world studies of MDD patients have demonstrated that vortioxetine was tolerable and improved several aspects of depressive symptoms,19–23 including anhedonia, as well as cognitive function.15,24 This led to subsequent improvements in daily and social functioning, including work productivity.21–23 The benefit of vortioxetine on working patients with MDD was further reinforced by multiple randomized clinical trials and real-world studies that demonstrated the safety and efficacy/effectiveness, including on work-related measures, of vortioxetine amongst working patients with MDD.12,24–30
A South Korean post-marketing surveillance (PMS) study of patients with MDD receiving vortioxetine over a 6-year period from 2014 to 2020 supported that vortioxetine was efficacious and safe in patients with MDD in the real world.31 This article presents the effectiveness and safety data on vortioxetine for a subgroup analysis of working patients from the PMS study.
Materials and Methods
Study Design and Participants
This was a prospective, multicenter, non-interventional, non-comparative PMS cohort study in South Korea. The study design, eligibility criteria, study procedures and statistical analysis of the study have been previously reported in detail.31
Briefly, data were collected via electronic case report forms (eCRF) at a first baseline visit (Visit 1, week 0), a second visit (Visit 2; 8±2 weeks after Visit 1) and at an optional third visit (Visit 3, 24±2 weeks after Visit 1) that was arranged at the investigator’s discretion. Patients were followed for up to 24±2 weeks after enrollment. Eligibility criteria for the PMS study were as follows: Patients aged ≥19 years who were naïve to vortioxetine for the treatment of MDD; who received at least one administration of vortioxetine based on eCRF records between 13th June 2016 and 14th August 2020; without hypersensitivity to any ingredient of the drug; and without any history of monoamine oxidase inhibitors within 14 days prior to enrollment.
The subgroup analysis defined working patients as those who were recorded as working or studying full- (≥6 hours/day) or part-time (<6 hours/day) at baseline, based on the definition used in other studies of vortioxetine in working patients.30,32
Study Procedures
Baseline Characteristics
The same set of baseline characteristics recorded in the primary analysis were captured for the working subgroup analysis: general demographics, presence of comorbidities, duration of MDD episodes, concomitant medication usage, and the dosage, duration, and reason for initiating vortioxetine.
Effectiveness
The Montgomery-Asberg Depression Rating Scale (MADRS) was used to assess the severity of depressive symptoms at baseline, Visit 2 and Visit 3.33 MADRS is a 10-item tool assessing depressive symptoms on a seven-point scale, ranging from 0 indicating the absence of symptoms to 6 indicating severe symptoms.33 The MADRS anhedonia subscale score was derived from the sum of the following items of the MADRS: Q1 (apparent sadness); Q2 (reported sadness); Q6 (concentration difficulties); Q7 (lassitude); Q8 (inability to feel), in alignment with previous literature.34 Treatment response was defined as a ≥50% reduction in MADRS total score and assessed at Visit 2 or Visit 3 compared to baseline score. Remission was defined as MADRS score ≤10 at Visit 2 or Visit 3.
The Clinical Global Impression-Severity of Illness (CGI-S) is a seven-point scale that assesses a patient’s severity of symptoms related to mental health, with a higher score indicating more severe illness.35,36 CGI-S was used to assess the severity of MDD at baseline. Clinical Global Impression-Improvement (CGI-I) is a seven-point scale that allows investigators to assess the degree of change in a patient’s symptoms and ranges from 1, being very much improved, to 7, being very much worse.36 Proportions of patients showing improvements at Visit 2 and Visit 3 were calculated by summing the number of patients who were categorized as very much improved, much improved and minimally improved by CGI-I at each visit.
Patient-reported cognitive impairment was assessed at baseline, Visit 2, and Visit 3 using the Korean Version of the Perceived Deficits Questionnaire-Depression (PDQ-K, with a higher score indicating more severe cognitive dysfunction) and the Digit Symbol Substitution Test (DSST, with a higher score indicating better cognitive function).
Safety and Tolerability
All adverse events (AEs) were assessed and recorded using World Health Organization Adverse Reactions Terminology (WHOART). Serious AEs (SAEs) were defined as AEs that caused death or were life-threatening; required hospitalization or prolonged hospitalization; caused persistent or significant handicap or hypofunction; caused congenital deformity or anomaly; or caused serious medical issues. Patients with non-serious AEs were followed up until AEs were resolved or stabilized; those with SAEs were followed up until SAEs were resolved. As in the primary analysis, actions taken to AEs were recorded as discontinuation; temporary discontinuation; dose reduction; dose increase or no change.31
Statistical Analysis
Descriptive analyses were conducted to evaluate patients’ baseline characteristics and data on effectiveness and safety. The effectiveness assessment set included patients who were included in the safety assessment set (ie a record of evaluation for safety through follow-up observation after administration of vortioxetine at least once), and had at least one CGI-I score available at either Visit 2 or Visit 3. Patients with unknown or missing AE status or missing CGI-I information were excluded from the safety and effectiveness analyses, respectively. Paired t-tests were used to compare outcomes at baseline, Visit 2 and Visit 3. A p-value <0.05 was considered statistically significant. All statistical analyses were carried out using the SAS Software version 9.4 (SAS Institute, North Carolina, US) and R version 4.3.0.
Results
Baseline Characteristics
Of 3263 included in the PMS study, 1082 patients who were classified as working at baseline were included in this subgroup analysis. Baseline characteristics of the safety assessment set in the working patient subgroup are presented in Table 1. Briefly, the mean age of the working patients was 39.56 years, ranging from 19 to 88 years. The majority of the patients (60.9%) had moderate, mild, or borderline MDD assessed using CGI-S, which aligned with the mean MADRS score (26.26 points).37
Table 1.
Baseline Patient Characteristics of Working Patients
| Patient Characteristics | Safety Population (N=1082) |
|---|---|
| Age in years, mean±SD | 39.56±15.49 |
| Aged ≥65 years, n (%) | 77 (7.12) |
| Female, n (%) | 516 (47.69) |
| Mean weight, kg±SD | 65.22±14.46 |
| Presence of other medical history, n (%) | 450 (41.59) |
| Severity of MDD using CGI-S, n (%) | |
| Normal | 3 (0.28) |
| Borderline | 38 (3.52) |
| Mild | 175 (16.20) |
| Moderate | 445 (41.20) |
| Markedly ill | 298 (27.59) |
| Severe | 116 (10.74) |
| Extreme | 5 (0.46) |
| Duration of MDD in days, mean±SDa | 632.68±1244.43 |
| MADRS total score, mean±SD | 26.26±9.56 |
| Duration of MDD episode in days, mean±SD | 287.28±722.61 |
| Reason for initiating vortioxetine, n (%) | |
| Treatment-naïve | 539 (49.82) |
| Ineffective previous antidepressants | 504 (46.58) |
| AEs of previous antidepressants | 28 (2.59) |
| Lack of compliance | 9 (0.83) |
| Others | 2 (0.18) |
| Duration of vortioxetine use in days, mean±SD | 76.11±60.57 |
| Long-term use (≥154 days/22 weeks), n (%) | 178 (21.09) |
| Starting dose, n (%)b | |
| 10 mg/day | 858 (79.30) |
| 5 mg/day | 224 (20.70) |
| Starting dose, mg/day, mean±SD | 8.96±2.03 |
| Concomitant medications, n (%)c | 629 (58.13) |
| Concomitant antidepressants, n (%) | 730 (67.47) |
Notes: aDuration of MDD was calculated as follows: Duration of MDD=[First administration start date] - [Initial Diagnosis Date of MDD]+1. bThe starting dose of vortioxetine in adult patients aged <65 years was 10 mg once daily. Additionally, adult patients aged <65 years who began administration once daily with 5 mg and increased the dose to more than 10 mg once a day were also included in the study. The starting dose of vortioxetine in patients aged ≥65 years was 5 mg once daily. cAny medications besides major depressive disorder treatments during the study period or major depressive disorder treatment drugs within 1 month before study participation and/or during the study period.
Abbreviations: AE, adverse event; CGI-S, Clinical Global Impression-Severity of Illness; MDD, major depressive disorder; SD, standard deviation.
Effectiveness
MADRS
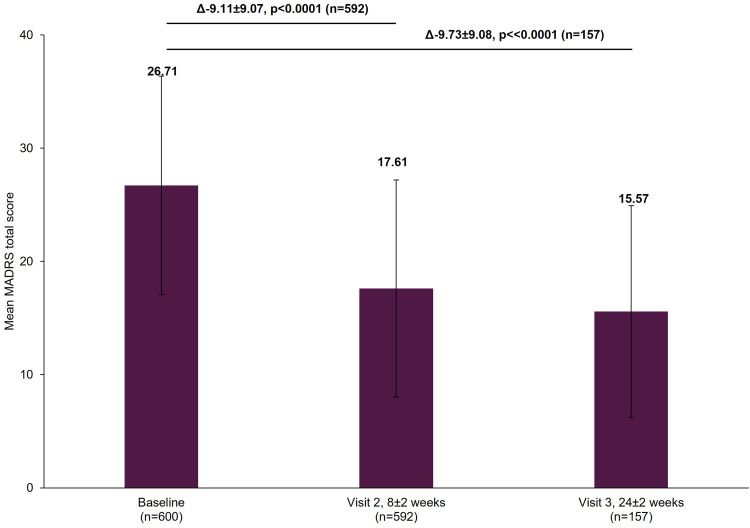
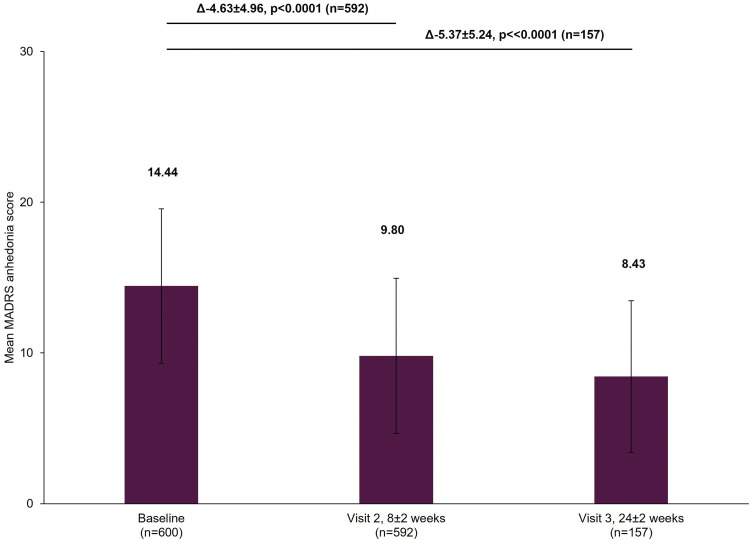
The working patients’ MADRS total scores decreased significantly from baseline by a mean±standard deviation (SD) of 9.11±9.07 points at Visit 2 and 9.73±9.08 points at Visit 3 (both p<0.0001; Figure 1). The patients’ MADRS anhedonia subscale scores similarly had significant decreases, with a mean±SD change from baseline of 4.63±4.96 points at Visit 2 and 5.37±5.24 points at Visit 3 (both p<0.0001; Figure 2). Rates of treatment response increased from 33.61% (95% confidence interval [CI]: 29.82–37.58) at Visit 2 to 39.49% (95% CI: 31.79–47.59) by Visit 3. The rates of remission increased from 26.69% (95% CI: 23.17–30.45) at Visit 2 to 30.57% (95% CI: 23.48–38.42) by Visit 3.
Figure 1.
Change in MADRS total score from baseline.
Notes: The mean MADRS total score at Visit 1 was based on all patients with data available at baseline, not just those that also had data at Visit 2 or Visit 3. However, when analyzing the difference between the visits (Visit 1 to Visit 2 and Visit 1 to Visit 3), scores of patients who had scores at both visits were included. Therefore, paired t-tests assessed the difference at Visit 2 (8±2 week) versus Visit 1 (0 week) in 592 patients and difference at Visit 3 (24±2 week) versus Visit 1 (0 week) in 157 patients. The bold texts above the graph bars represent the mean difference (∆), SD and the statistical significance of the mean scores between the visits. Error bars represent SD.
Abbreviations: MADRS, Montgomery-Asberg Depression Rating Scale; SD, standard deviation.
Figure 2.
Change in MADRS anhedonia subscale score from baseline.
Notes: The mean MADRS anhedonia score at Visit 1 was based on all patients with data available at baseline, not just those that also had data at Visit 2 or Visit 3. However, when analyzing the difference between the visits (Visit 1 to Visit 2 and Visit 1 to Visit 3), scores of patients who had scores at both visits were included. Therefore, paired t-tests assessed the difference at Visit 2 (8±2 week) versus Visit 1 (0 week) in 592 patients and difference at Visit 3 (24±2 week) versus Visit 1 (0 week) in 157 patients. The bold texts above the graph bars represent the mean difference (∆), SD and the statistical significance of the mean scores between the visits. Error bars represent SD.
Abbreviations: MADRS, Montgomery-Asberg Depression Rating Scale; SD, standard deviation.
CGI-I
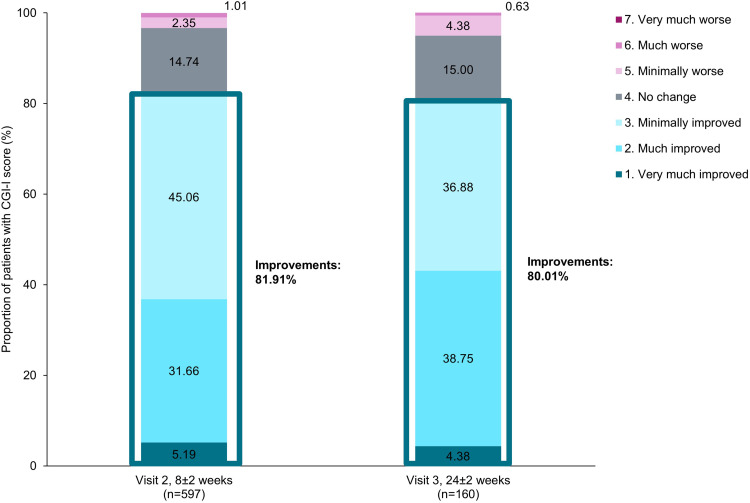
More than 80% of patients achieved improvements in mental-health associated symptoms from baseline at Visit 2 and these improvements were maintained at Visit 3 (Figure 3).
Figure 3.
Improvements from baseline using CGI-I.
Notes: Proportion of patients showing improvements at Visits 2 (8±2 week) and (24±2 week) was calculated by summing the proportions of patients classified as “Improved” categories, represented in bold text.
Abbreviation: CGI-I, Clinical Global Impression-Improvement.
PDQ-K and DSST
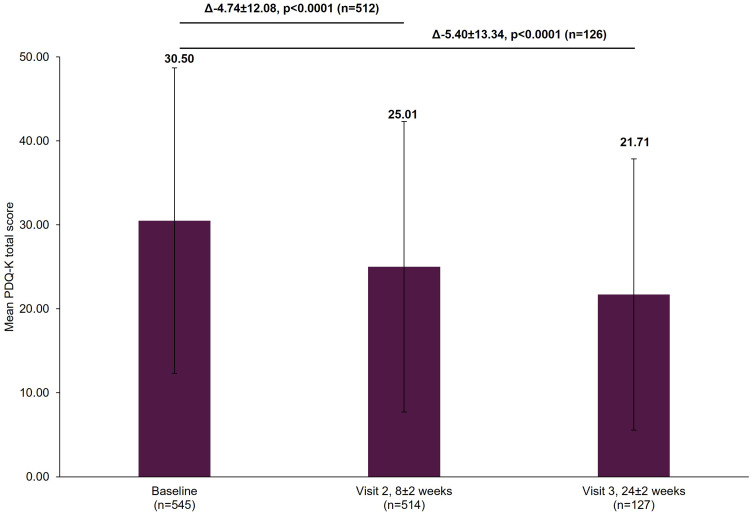
The mean PDQ-K score decreased significantly from baseline by a mean±SD of 4.74±12.08 points (p<0.0001) at Visit 2 and by 5.40±13.34 points (p<0.0001) at Visit 3 (Figure 4).
Figure 4.
Change in PDQ-K total score from baseline.
Notes: The mean PDQ-K total score at Visit 1 was based on all patients with data available at baseline, not just those that also had data at Visit 2 or Visit 3. However, when analyzing the difference between the visits (Visit 1 to Visit 2 and Visit 1 to Visit 3), scores of patients who had scores at both visits were included. Therefore, paired t-test assessed the difference at Visit 2 (8±2 week) versus Visit 1 (0 week) in 512 patients and difference at Visit 3 (24±2 week) versus Visit 1 (0 week) in 126 patients. The bold texts above the graph bars represent the mean difference (∆), SD and the statistical significance of the mean scores between the visits. Error bars represent SD.
Abbreviations: PDQ-K, Korean version of Perceived Deficits Questionnaire-Depression; SD, standard deviation.
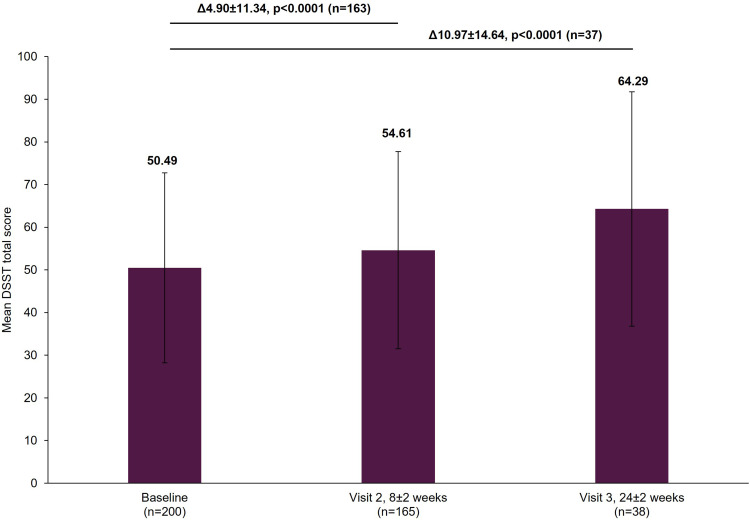
The mean DSST score improved significantly from baseline by a mean±SD of 4.90±11.34 points (p<0.0001) at Visit 2 and by 10.97±14.64 points (p<0.0001) at Visit 3 (Figure 5).
Figure 5.
Change in DSST total score from baseline.
Notes: The mean DSST total score at Visit 1 was based on all patients with data available at baseline, not just those that also had data at Visit 2 or Visit 3. However, when analyzing the difference between the visits (Visit 1 to Visit 2 and Visit 1 to Visit 3), scores of patients who had scores at both visits were included. Therefore, paired t-test assessed the difference at Visit 2 (8±2 week) versus Visit 1 (0 week) in 163 patients and difference at Visit 3 (24±2 week) versus Visit 1 (0 week) in 37 patients. The bold texts above the graph bars represent the mean difference (∆), SD and the statistical significance of the mean scores between the visits. Error bars represent SD.
Abbreviations: DSST, Digit Symbol Substitution Test; SD, standard deviation.
Safety and Tolerability
The rates of any AEs including SAEs are presented in Table 2. The most frequent types of AEs by preferred term were nausea, followed by headache, dizziness and insomnia. There were only 2 cases of sexual dysfunction in males (such as impotence or erectile dysfunction) during the course of treatment.
Table 2.
Summary of AEs
| Types of AEs | Number of Patients (%) (N=1082) | 95% CI (for %) | Number of Events |
|---|---|---|---|
| Any AEs | 202 (18.67) | 16.39–21.12 | 269 |
| Any AEs with frequency of ≥1% | |||
| Nausea | 77 (7.12) | 5.66–8.81 | 77 |
| Headache | 20 (1.85) | 1.13–2.84 | 20 |
| Dizziness | 13 (1.20) | 0.64–2.05 | 13 |
| Insomnia | 12 (1.11) | 0.57–1.93 | 12 |
| Serious AEs | 13 (1.20) | 0.64–2.05 | 17 |
Abbreviations: AE, adverse event; CI, confidence interval.
Mean change in weight from baseline was less than 0.5 kg at both Visit 2 (0.28±1.74 kg) and Visit 3 (0.39±3.65 kg).
The majority of AEs did not lead to any change in drug administration. The rate of discontinuation due to AEs (including SAEs) was 29.74% (80/269 events).
Discussion
Importance of Treating MDD Among Working Patients
Our study demonstrated that working patients in South Korea treated with vortioxetine had improved cognitive function and depressive symptoms. In particular, the treatment was well-tolerated, with low incidence of treatment-emergent AEs that could impair work function, such as insomnia or dizziness, highlighting the value of vortioxetine for the treatment of MDD among working patients.
MDD has posed a substantial clinical and economic burden in South Korea, and there is a particularly urgent need for treating MDD in working patients. PERFORM-K reported that 52.4% of MDD patients of working age in South Korea experienced a loss in overall work productivity, of whom 20% experienced absenteeism and 47.1% experienced presenteeism due to MDD.6 An analysis of the National Health Insurance Service database reported that the socio-economic cost of depression due to a decrease in work performance was 3.1 trillion Korean Won (approximately US$2.7 billion) in 2019.38 Therefore, the treatment of working patients with MDD should aim to improve depressive symptoms as well as cognitive function, considering the impact of these symptoms on work performance.39
Effectiveness of Vortioxetine in Working Patients
MDD patients treated with vortioxetine showed improvement in several aspects of work-related outcomes in the real-world setting, including depressive symptoms such as anhedonia, as well as cognitive function.15,21–23 In our analysis, depressive symptoms improved among working patients with MDD who were treated with vortioxetine. This aligns with findings from previous international clinical trials in MDD that included working patients, which have demonstrated the impact of vortioxetine on depressive symptoms as measured by change in mean MADRS total score from baseline (VIVRE: 13.6 points at 8 weeks;24 ReMind WORK: 15.15 points at 8 weeks;26 FOCUS: 15.6 points at 8 weeks [approximately 60% of the study population were working]).25 The magnitude of improvement in MADRS total score in this real-world analysis (9.11 points at 8±2 weeks) was similar to a Japanese real-world study of vortioxetine in working patients (VGOAL-J: 9.2 points at 8 weeks).29 The improvement in MADRS score in clinical trials appears greater than the improvement seen in our current real-world analysis. One possible reason for this may be that the mean dose of vortioxetine in our analysis (10.67 mg) was lower than the dose prescribed in trials or even the real-world VGOAL-J study, where patients predominantly received 20 mg/day of vortioxetine.24,34 These results may reflect the well-established dose–response relationship of vortioxetine in improving MDD symptoms that has been reported elsewhere,19,40,41 which demonstrated that vortioxetine 20 mg/day is more effective than vortioxetine 10 mg/day.41 The results may also highlight the real-world prescribing practice in South Korea, where physicians and patients may be more conscious of side effects such as nausea,42,43 and therefore may prefer a lower dose in order to prioritize the minimization of such side effects.
Presence of anhedonia has been identified as a predictor of non-response to many antidepressants and has been negatively associated with psychosocial functioning, highlighting the importance of treating anhedonia in MDD patients.14,44 A pooled analysis of randomized controlled trials of vortioxetine in MDD patients showed that vortioxetine was effective in treating anhedonia, which was associated with improvements in patient functioning.15 Our subgroup analysis is the first long-term, real-world study to demonstrate the effectiveness of vortioxetine in improving anhedonia measured by MADRS in working patients with MDD at the current time. The improvement in MADRS anhedonia score of 4.63 points at 8±2 weeks was also observed in a Canadian clinical trial of vortioxetine in MDD patients aged 18–65 years (7.1 points at 8 weeks);34 in our analysis, the anhedonia score additionally continued to improve over the longer term (5.37 points at 24±2 weeks).
With the association between anhedonia and cognitive dysfunction that negatively impacts social, occupational, and global functioning, improvement in these symptoms remains one of the key MDD treatment goals.45–50 Despite this, anhedonia and cognitive dysfunction can persist despite treatment, with a recent systematic literature review of 14 different conventional antidepressants, including clomipramine, sertraline and mirtazapine, demonstrating no significant improvement in cognitive function between antidepressants and placebo.51 This highlights an unmet need for effective treatments to improve both anhedonia and cognitive dysfunction. This subgroup analysis indicates an effect in patients treated with vortioxetine on cognitive function in the real-world, where patients using vortioxetine showed significant decreases in mean PDQ-K score (−4.74 points at 8±2 weeks and −5.40 points at 24±2 weeks) from baseline, as well as increases in mean DSST score (+4.90 points at 8±2 weeks and +10.97 at 24±2 weeks) from baseline. This aligns with findings from a Chinese real-world study of vortioxetine in working patients (a decrease in mean PDQ-D score from baseline in RELIEVE study: −9.7 points at 8 weeks and −16.8 points at 24 weeks), underscoring the real-world effectiveness of vortioxetine in improving cognitive function.30 The real-world improvements reported by our study also support similar results from placebo-controlled randomized trials of vortioxetine in which significant improvements in both subjective cognitive symptoms and objective cognitive performance relative to placebo in working patients with MDD have been reported.26,52 The findings of our study are therefore in line with PDQ and DSST results from clinical trials and other real-world studies of vortioxetine in working patients.12,25,26,28,52
When reviewing the results of the current working patient subgroup analysis with the previously published results in the overall study population,31 it is worth noting that the working patients included in the subgroup analysis were younger (mean age: 39.56 vs 51.28 years) and consisted of a smaller proportion of females (47.69% vs 61.97%) when compared with the overall study population. The difference in the mean age and proportion of females reflects the demographics of working patients in the general population in South Korea.53 Nonetheless, the extent of improvements in depressive and cognitive symptoms was similar across the populations, supporting the finding that vortioxetine was beneficial irrespective of age and gender.31 Other clinical characteristics were comparable between the total cohort and working patient subgroup.
Safety and Tolerability of Vortioxetine in Working Patients
The tolerability profile of vortioxetine in working patients was consistent with the profile from previous clinical trials and real-world studies, characterized by low rates of commonly observed AEs among the MDD patients.19 Consistent with the reported tolerability profile of vortioxetine in clinical trials as well as Japanese and Chinese real-world studies,20,29,30 nausea, headache, dizziness and insomnia were most commonly reported AEs in our analysis. The rates of these commonly reported AEs were ≤2%, except nausea (7.12%). It is important to note that most AEs emerging during vortioxetine treatment are reported to be transient.20
The impact of insomnia on work productivity and performance is considerable.54 In our analysis, a small proportion of patients (1.11%) experienced insomnia. This is lower than the rate observed in AtWoRC (9.1–9.7%),12,28 and is also lower than levels reported in clinical trials of vortioxetine in MDD patients aged 18–75 years irrespective of working status (2.0%–5.1%).20 Sexual dysfunction is also commonly observed during the course of antidepressants;55 in our study, the occurrence of male sexual dysfunction was low (0.18%). Weight gain is another undesirable phenomenon observed during antidepressant treatment. In our analysis, change in weight from baseline in the working subgroup was negligible (<0.5 kg at both 8±2 weeks and 24±2 weeks).
The safety of vortioxetine in working patients was similar to what was observed in the overall study population, with the comparable rates of AEs (18.67% vs 17.13%) and serious AEs (1.20% vs 1.56%).
Strengths and Limitations
This was the first analysis reporting the long-term, real-world effectiveness and safety of vortioxetine in a large number of working patients with MDD (N=1082) in South Korea, reflecting the real-world clinical practice of treating working patients with vortioxetine. Of particular importance, improvements in anhedonia and cognitive function with vortioxetine were observed, which may be important for reducing impairments in work function.17
Nonetheless, our study also had limitations. Firstly, this was a single-arm study without a control group to act as a comparator for the data on vortioxetine. Although our study followed up patients for up to 24 weeks, this duration may fall short in capturing vortioxetine’s longer-term effectiveness and safety in the real world. Additionally, as this was a subgroup analysis of a PMS study, work productivity was not directly measured, and impacts on work performance were inferred based on measures of anhedonia and cognition measured in this study. Thus, evidence that directly links improvements in anhedonia and cognitive function to work productivity remains limited. Future research should focus on interventional and longitudinal studies that assess the impact of targeted treatments such as vortioxetine on work-related measures in South Korea in order to confirm our findings. Lastly, the results of the subgroup analysis may not be generalizable to the broader working population of South Korea.
Conclusion
The current study suggests that vortioxetine is an effective treatment option for working patients with MDD, improving their depressive symptoms (including anhedonia) and cognitive symptoms, while also being generally well tolerated.
Acknowledgments
The authors thank the patients, the investigators and their teams who took part in this study. The authors also acknowledge Min Hee Choi, Ph.D., Yan Ran Wee, MSc, and Jennifer Evans, Ph.D., from Costello Medical Singapore Pte Ltd, for medical writing and editorial assistance based on the authors’ input and direction.
Funding Statement
This study was sponsored by H. Lundbeck A/S. Support for third-party writing assistance for this article, provided by Min Hee Choi, Ph.D., Yan Ran Wee, MSc, and Jennifer Evans, Ph.D., from Costello Medical Singapore Pte Ltd, was funded by H. Lundbeck A/S in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki and the regulations of the Ministry of Health and Welfare in Korea (MFDS notification number 2014-61). It was approved by Institutional Review Boards (IRBs) at each study site, or a centralized IRB designated by the MFDS when a study site did not have its own IRB. The official names of the IRBs for each study site are provided in Supplementary Table 1. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
This was a non-interventional study with the protocol and all data, including all adverse events, having been shared with and reviewed by MFDS. The study was funded by H. Lundbeck A/S, whose personnel contributed to the data analysis, review of the data, and review of the manuscript: Elin Heldbo Reines and Daniel Oudin Astrom are employees of H. Lundbeck A/S, Valby, Denmark. Minah Lee and Gayoung Kim are employees of Lundbeck Korea Co., Ltd., Seoul, Korea. Michael Adair was a former employee of H. Lundbeck A/S, Valby, Denmark during the course of the study. The authors report no other conflicts of interest in this work.
References
- 1.World Health Organisation. Depressive disorder (Depression); 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/depression. Accessed March 8, 2024.
- 2.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/s0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Psychiatry. 2022;9(2):137–150. doi: 10.1016/s2215-0366(21)00395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J Psychiatr Res. 2020;126:134–140. doi: 10.1016/j.jpsychires.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 5.Zomer E, Rhee Y, Liew D, Ademi Z. The health and productivity burden of depression in South Korea. Appl Health Econ Health Policy. 2021;19(6):941–951. doi: 10.1007/s40258-021-00649-1 [DOI] [PubMed] [Google Scholar]
- 6.Kim JM, Chalem Y, Di Nicola S, Hong JP, Won SH, Milea D. A cross-sectional study of functional disabilities and perceived cognitive dysfunction in patients with major depressive disorder in South Korea: the PERFORM-K study. Psychiatry Res. 2016;239:353–361. doi: 10.1016/j.psychres.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 7.Jaeger J. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol. 2018;38(5):513–519. doi: 10.1097/jcp.0000000000000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35. doi: 10.1016/j.neubiorev.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS). J Affect Disord. 2007;99(1–3):83–89. doi: 10.1016/j.jad.2006.08.020 [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Mou X, Jiang W, et al. A comparative study of anhedonia components between major depression and schizophrenia in Chinese populations. Ann Gen Psychiatry. 2015;14(1):24. doi: 10.1186/s12991-015-0061-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Xia Y, Yan R, et al. The relationship between disrupted anhedonia-related circuitry and suicidal ideation in major depressive disorder: a network-based analysis. Neuroimage Clin. 2023;40:103512. doi: 10.1016/j.nicl.2023.103512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chokka P, Bougie J, Rampakakis E, Proulx J. Assessment in work productivity and the relationship with cognitive symptoms (AtWoRC): primary analysis from a Canadian open-label study of vortioxetine in patients with major depressive disorder (MDD). CNS Spectr. 2019;24(3):338–347. doi: 10.1017/s1092852918000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khazanov GK, Xu C, Dunn BD, Cohen ZD, DeRubeis RJ, Hollon SD. Distress and anhedonia as predictors of depression treatment outcome: a secondary analysis of a randomized clinical trial. Behav Res Ther. 2020;125:103507. doi: 10.1016/j.brat.2019.103507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vrieze E, Demyttenaere K, Bruffaerts R, et al. Dimensions in major depressive disorder and their relevance for treatment outcome. J Affect Disord. 2014;155:35–41. doi: 10.1016/j.jad.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntyre RS, Loft H, Christensen MC. Efficacy of vortioxetine on anhedonia: results from a pooled analysis of short-term studies in patients with major depressive disorder. Neuropsychiatr Dis Treat. 2021;17:575–585. doi: 10.2147/ndt.S296451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barkus E. The effects of anhedonia in social context. Curr Behav Neurosci Reports. 2021;8(3):77–89. doi: 10.1007/s40473-021-00232-x [DOI] [Google Scholar]
- 17.Rizvi SJ, Cyriac A, Grima E, et al. Depression and employment status in primary and tertiary care settings. Can J Psychiatry. 2015;60(1):14–22. doi: 10.1177/070674371506000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Diego-Adeliño J, Crespo JM, Mora F, et al. Vortioxetine in major depressive disorder: from mechanisms of action to clinical studies. An updated review. Expert Opin Drug Saf. 2022;21(5):673–690. doi: 10.1080/14740338.2022.2019705 [DOI] [PubMed] [Google Scholar]
- 19.Baldwin DS, Florea I, Jacobsen PL, Zhong W, Nomikos GG. A meta-analysis of the efficacy of vortioxetine in patients with major depressive disorder (MDD) and high levels of anxiety symptoms. J Affect Disord. 2016;206:140–150. doi: 10.1016/j.jad.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 20.Baldwin DS, Chrones L, Florea I, et al. The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. 2016;30(3):242–252. doi: 10.1177/0269881116628440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G, Xiao L, Ren H, et al. Effectiveness and safety of vortioxetine for major depressive disorder in real-world clinical practice: results from the single-Arm RELIEVE China study. Neuropsychiatr Dis Treat. 2022;18:1939–1950. doi: 10.2147/ndt.S358253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang YK, Chen CS, Tsai CF, et al. A Taiwanese study on real-world evidence with vortioxetine in patients with major depression in Asia (TREVIDA). Curr Med Res Opin. 2021;37(12):2163–2173. doi: 10.1080/03007995.2021.1980869 [DOI] [PubMed] [Google Scholar]
- 23.Chin CN, Zain A, Hemrungrojn S, et al. Results of a real-world study on vortioxetine in patients with major depressive disorder in South East Asia (REVIDA). Curr Med Res Opin. 2018;34(11):1975–1984. doi: 10.1080/03007995.2018.1477746 [DOI] [PubMed] [Google Scholar]
- 24.McIntyre RS, Florea I, Pedersen MM, Christensen MC. Head-To-Head comparison of vortioxetine versus desvenlafaxine in patients with major depressive disorder with partial response to SSRI therapy: results of the VIVRE study. J Clin Psychiatry. 2023;84(4). doi: 10.4088/JCP.23m14780 [DOI] [PubMed] [Google Scholar]
- 25.McIntyre RS, Florea I, Tonnoir B, Loft H, Lam RW, Christensen MC. Efficacy of vortioxetine on cognitive functioning in working patients with major depressive disorder. J Clin Psychiatry. 2017;78(1):115–121. doi: 10.4088/JCP.16m10744 [DOI] [PubMed] [Google Scholar]
- 26.Baune BT, Sluth LB, Olsen CK. The effects of vortioxetine on cognitive performance in working patients with major depressive disorder: a short-term, randomized, double-blind, exploratory study. J Affect Disord. 2018;229:421–428. doi: 10.1016/j.jad.2017.12.056 [DOI] [PubMed] [Google Scholar]
- 27.Chokka P, Tvistholm AH, Bougie J, Clerzius G, Ettrup A. Improvements in workplace productivity in working patients with major depressive disorder: results from the AtWoRC study. J Occup Environ Med. 2020;62(3):e94–e101. doi: 10.1097/jom.0000000000001805 [DOI] [PubMed] [Google Scholar]
- 28.Chokka P, Bougie J, Proulx J, Tvistholm AH, Ettrup A. Long-term functioning outcomes are predicted by cognitive symptoms in working patients with major depressive disorder treated with vortioxetine: results from the AtWoRC study. CNS Spectr. 2019;24(6):616–627. doi: 10.1017/s1092852919000786 [DOI] [PubMed] [Google Scholar]
- 29.Watanabe K, Sumiyoshi T, Kato M, et al. Long-term effectiveness of vortioxetine on achievement of personal goals and work productivity in patients with major depressive disorder: the VGOAL-J study. 2023.
- 30.Wang G, Si T, Rieckmann A, Ma J, Christensen MC. Effectiveness of vortioxetine in working patients with major depressive disorder in china: a subgroup analysis of the RELIEVE China study. Neuropsychiatr Dis Treat. 2024;20:1211–1223. doi: 10.2147/ndt.S460408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon SW, Kim JW, Kim DH, et al. Safety and effectiveness of vortioxetine for major depressive disorder: real-world evidence from a population-based study in South Korea. Original Res Front Psychiatry. 2023;14:1075939. doi: 10.3389/fpsyt.2023.1075939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen MC, Loft H, Florea I, McIntyre RS. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr Apr. 2019;24(2):249–257. doi: 10.1017/s1092852917000761 [DOI] [PubMed] [Google Scholar]
- 33.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. doi: 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 34.Cao B, Park C, Subramaniapillai M, et al. The efficacy of vortioxetine on anhedonia in patients with major depressive disorder. Front Psychiatry. 2019;10:17. doi: 10.3389/fpsyt.2019.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 36.Guy W. Clinical global impression. Assess Manual Psychopharmacol. 1976;217–222. [Google Scholar]
- 37.Müller MJ, Himmerich H, Kienzle B, Szegedi A. Differentiating moderate and severe depression using the Montgomery-Asberg depression rating scale (MADRS). J Affect Disord. 2003;77(3):255–260. doi: 10.1016/s0165-0327(02)00120-9 [DOI] [PubMed] [Google Scholar]
- 38.Lee JG, Seonwoo SM, Choi MJ, et al. Calculation of socioeconomic cost of depression in Korea in 2019. J Korean Soc Biol Therapies Psychiatry. 2021;27(3):224–237. [Google Scholar]
- 39.Chokka P, Bender A, Brennan S, et al. Practical pathway for the management of depression in the workplace: a Canadian perspective. Rev Front Psychiatry. 2023;14:1207653. doi: 10.3389/fpsyt.2023.1207653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Florea I, Loft H, Danchenko N, et al. The effect of vortioxetine on overall patient functioning in patients with major depressive disorder. Brain Behav. 2017;7(3):e00622. doi: 10.1002/brb3.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christensen MC, McIntyre RS, Adair M, Florea I, Loft H, Fagiolini A. Clinical benefits of vortioxetine 20 mg/day in patients with major depressive disorder. CNS Spectr. 2023;28(6):693–701. doi: 10.1017/s1092852923002249 [DOI] [PubMed] [Google Scholar]
- 42.Jang SH, Bahk WM, Woo YS, et al. The Korean Medication Algorithm Project for Depressive Disorder (KMAP-DD): changes in preferred treatment strategies and medications over 20 years and five editions. J Clin Med. 2023;12(3):1146. doi: 10.3390/jcm12031146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim N, Kim K, Lee S, Paik J, Lee B, Hwang J. Health services utilization and depression care quality among depressed patients. Seoul: Health Insurance Rev Assess Service. 2008. [Google Scholar]
- 44.Buckner JD, Joiner TE, Pettit JW, Lewinsohn PM, Schmidt NB. Implications of the DSM’s emphasis on sadness and anhedonia in major depressive disorder. Psychiatry Res. 2008;159(1–2):25–30. doi: 10.1016/j.psychres.2007.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McIntyre RS, Woldeyohannes HO, Soczynska JK, et al. Anhedonia and cognitive function in adults with MDD: results from the international mood disorders collaborative project. CNS Spectr. 2016;21(5):362–366. doi: 10.1017/s1092852915000747 [DOI] [PubMed] [Google Scholar]
- 46.Cambridge OR, Knight MJ, Mills N, Baune BT. The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: a systematic review. Psychiatry Res. 2018;269:157–171. doi: 10.1016/j.psychres.2018.08.033 [DOI] [PubMed] [Google Scholar]
- 47.Haro JM, Hammer-Helmich L, Saragoussi D, Ettrup A, Larsen KG. Patient-reported depression severity and cognitive symptoms as determinants of functioning in patients with major depressive disorder: a secondary analysis of the 2-year prospective PERFORM study. Neuropsychiatr Dis Treat. 2019;15:2313–2323. doi: 10.2147/ndt.S206825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cha DS, Carmona NE, Subramaniapillai M, et al. Cognitive impairment as measured by the THINC-integrated tool (THINC-it): association with psychosocial function in major depressive disorder. J Affect Disord. 2017;222:14–20. doi: 10.1016/j.jad.2017.06.036 [DOI] [PubMed] [Google Scholar]
- 49.Woo YS, Rosenblat JD, Kakar R, Bahk WM, McIntyre RS. Cognitive deficits as a mediator of poor occupational function in remitted major depressive disorder patients. Clin Psychopharmacol Neurosci. 2016;14(1):1–16. doi: 10.9758/cpn.2016.14.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McIntyre RS, Soczynska JZ, Woldeyohannes HO, et al. The impact of cognitive impairment on perceived workforce performance: results from the international mood disorders collaborative project. Compr Psychiatry. 2015;56:279–282. doi: 10.1016/j.comppsych.2014.08.051 [DOI] [PubMed] [Google Scholar]
- 51.He Y, Li H, Huang J, et al. Efficacy of antidepressant drugs in the treatment of depression in Alzheimer disease patients: a systematic review and network meta-analysis. J Psychopharmacol. 2021;35(8):901–909. doi: 10.1177/02698811211030181 [DOI] [PubMed] [Google Scholar]
- 52.Smith J, Browning M, Conen S, et al. Vortioxetine reduces BOLD signal during performance of the N-back working memory task: a randomised neuroimaging trial in remitted depressed patients and healthy controls. Mol Psychiatry. 2018;23(5):1127–1133. doi: 10.1038/mp.2017.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korean Statistical Information Service. 연령별 경제활동인구 총괄 [Total economically active population by age]; 2023. Available from: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1DA7002S&conn_path=I2. Accessed March 8, 2024.
- 54.Rosekind MR, Gregory KB, Mallis MM, Brandt SL, Seal B, Lerner D. The cost of poor sleep: workplace productivity loss and associated costs. J Occup Environ Med. 2010;52(1):91–98. doi: 10.1097/JOM.0b013e3181c78c30 [DOI] [PubMed] [Google Scholar]
- 55.Segraves RT, Balon R. Antidepressant-induced sexual dysfunction in men. Pharmacol Biochem Behav. 2014;121:132–137. doi: 10.1016/j.pbb.2013.11.003 [DOI] [PubMed] [Google Scholar]