Abstract
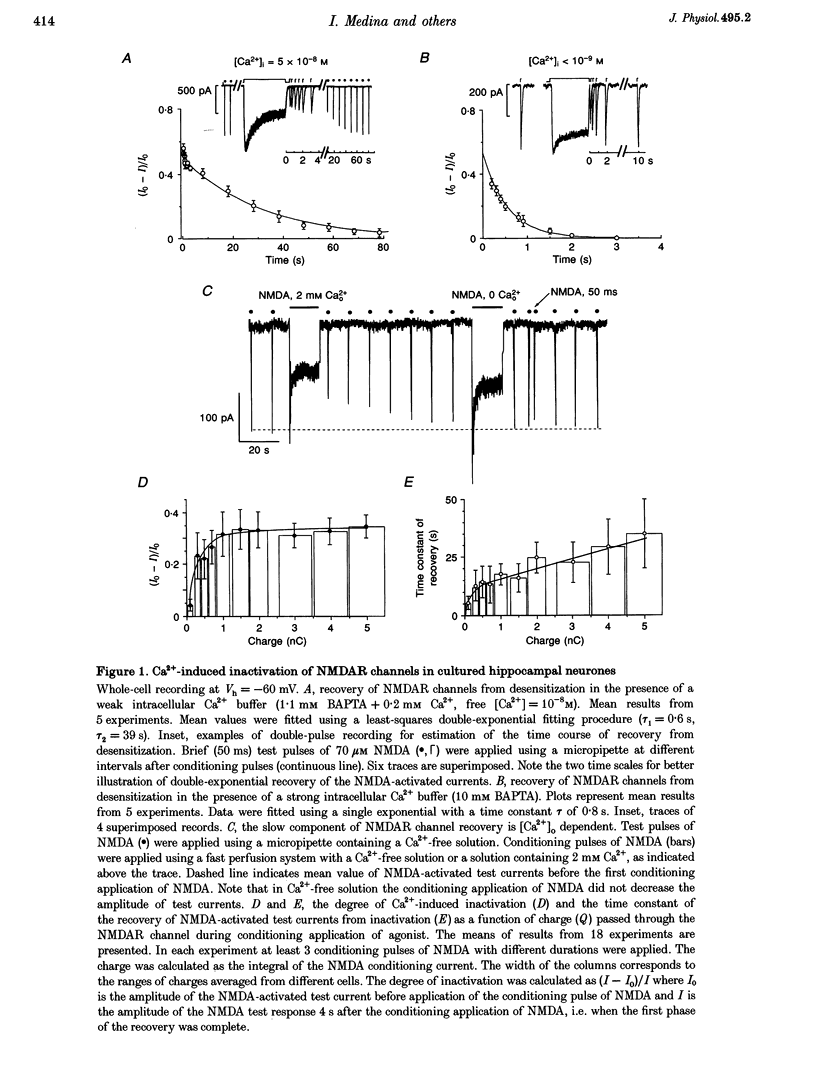
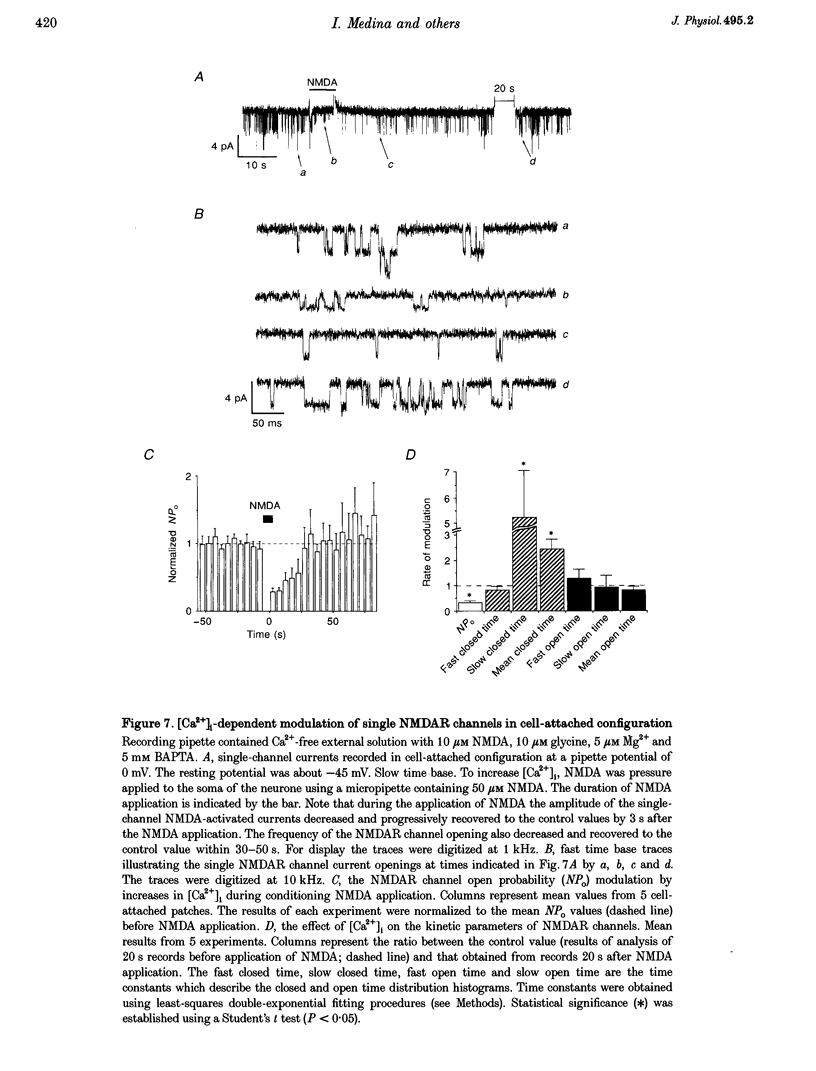
1. Calcium-induced transient inactivation of NMDA receptor (NMDAR) channels was studied in cultured rat hippocampal and cerebellar granule neurones using patch-clamp techniques and confocal scanning microscopy. 2. During whole-cell recordings, in the presence of 2 mM external Ca2+, conditioning (2-20 s) pulses of NMDA (20-100 microM) caused a transient decrease in NMDA responses. Recovery developed in two phases with time constants of 0.6 and 40 s. The slow phase of the recovery could be prevented either by strong intracellular Ca2+ ([Ca2+]i) buffering with 30 mM BAPTA or by using Ca(2+)-free extracellular solution. 3. Simultaneous measurement of currents and Ca(2+)-dependent fluorescence revealed a close correlation between the time constants of [Ca2+]i decay and the slow component of NMDA-activated test current recovery. 4. During prolonged recordings, the transient inactivation was not related to irreversible NMDA-activated current run-down. After 25 min of recording with ATP-free intracellular solution, NMDA-activated currents in hippocampal neurones irreversibly decreased by 49 +/- 5% while inactivation decreased by 8% (n = 9). Calyculin A and FK-506 (phosphatase inhibitors) significantly delayed run-down but did not modulate the transient inactivation. 5. In cerebellar granule cells that did not show run-down (4 mM MgATP in the pipette) the percentage of transient inactivation strongly decreased during 25 min of recording (from 28 +/- 6 to 7 +/- 5%, n = 15). 6. In cell-attached recordings (5 microM NMDA in the pipette), elevation of [Ca2+]i (application of 100 microM NMDA to the soma) caused a reversible reduction of single NMDAR channel open probability (NPo) due to a decrease in the frequency of channel opening. 7. In inside-out patches, application of Ca2+ to the cytoplasmic side of the membrane caused a rapid and reversible decrease in NPo (13 out of 29 patches). In the absence of run-down, the ability of Ca2+ to transiently inhibit NMDAR channel activity disappeared after 3-5 min of recording. 8. These results indicate that Ca(2+)-induced transient inactivation of NMDAR currents develops independently from the run-down and suggest that a diffusible Ca2+ -dependent factor mediates NMDAR channel inactivation.
Full text
PDF


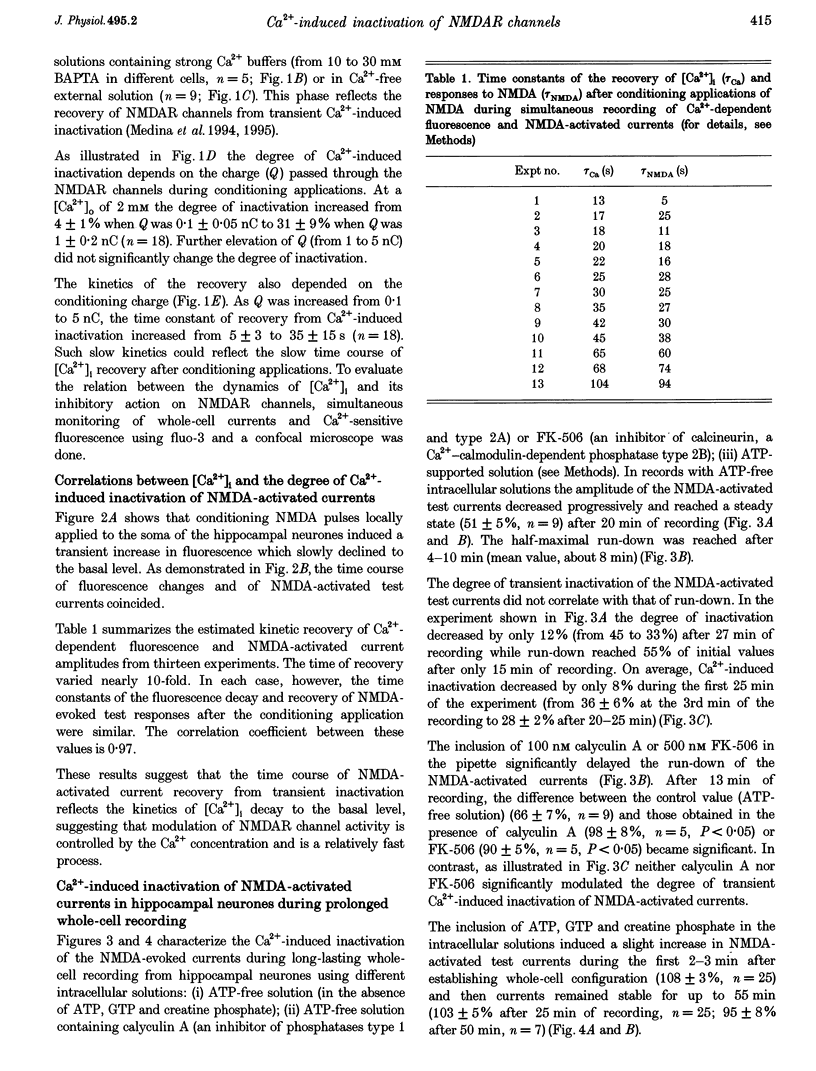
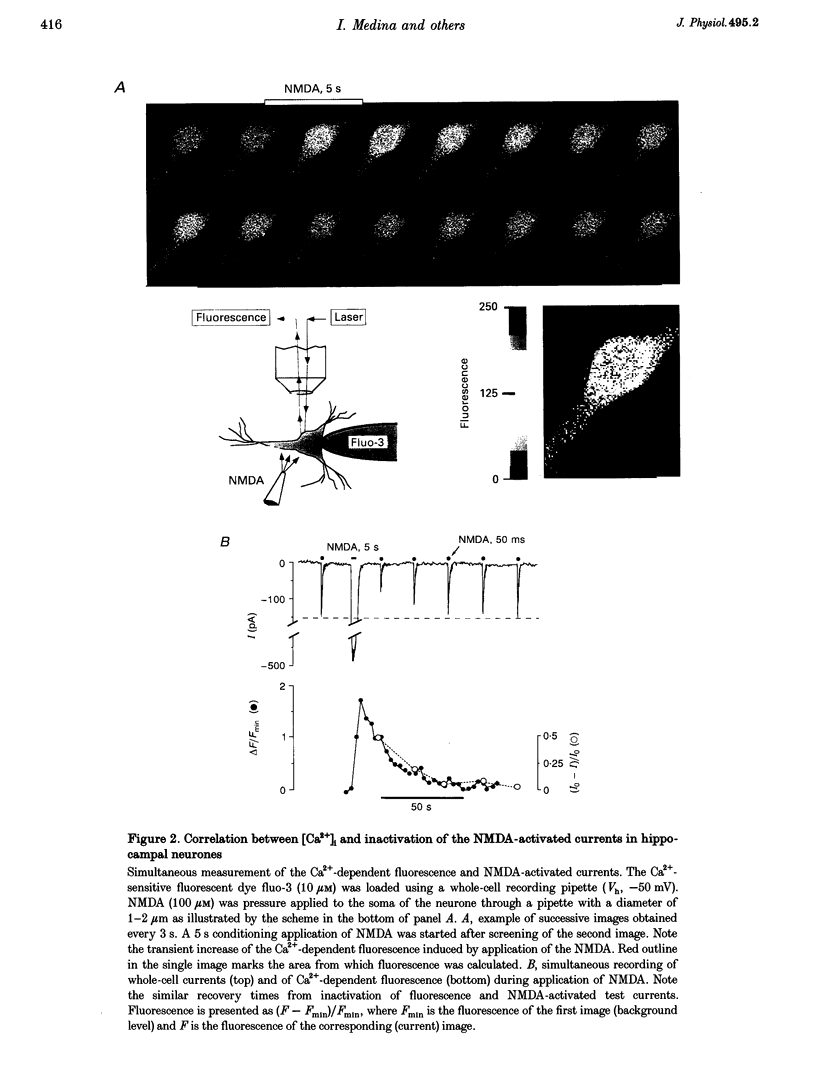
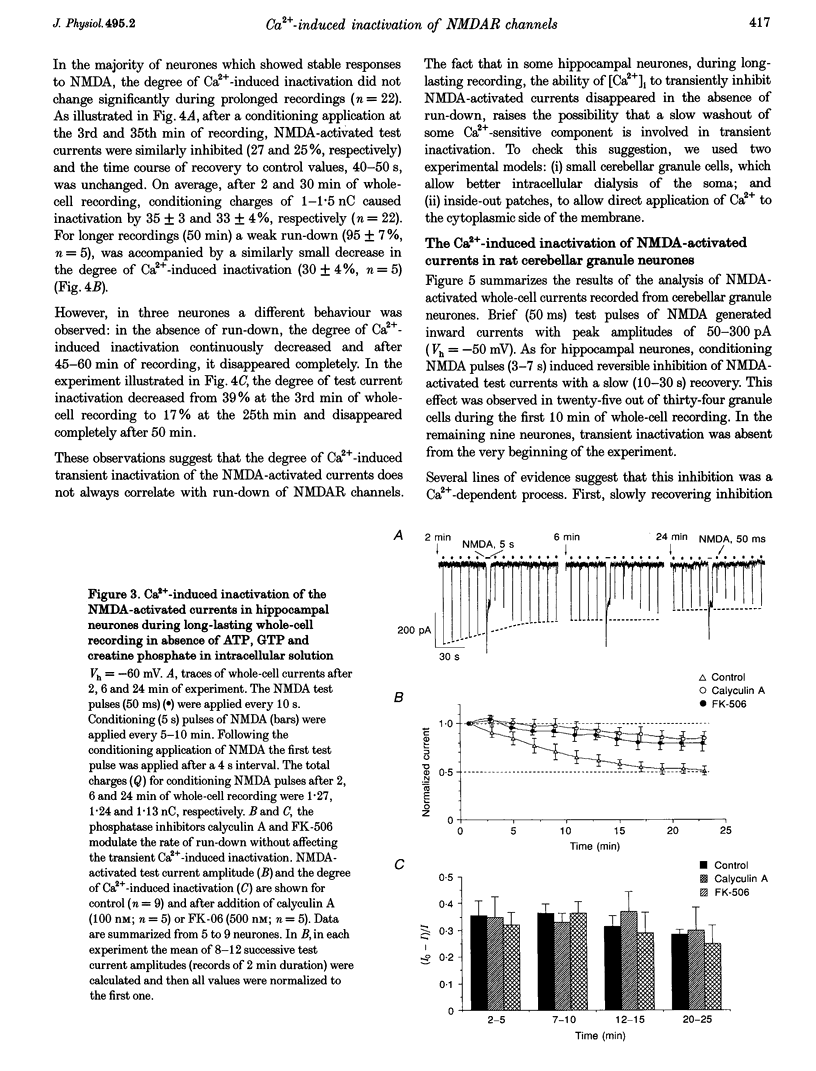
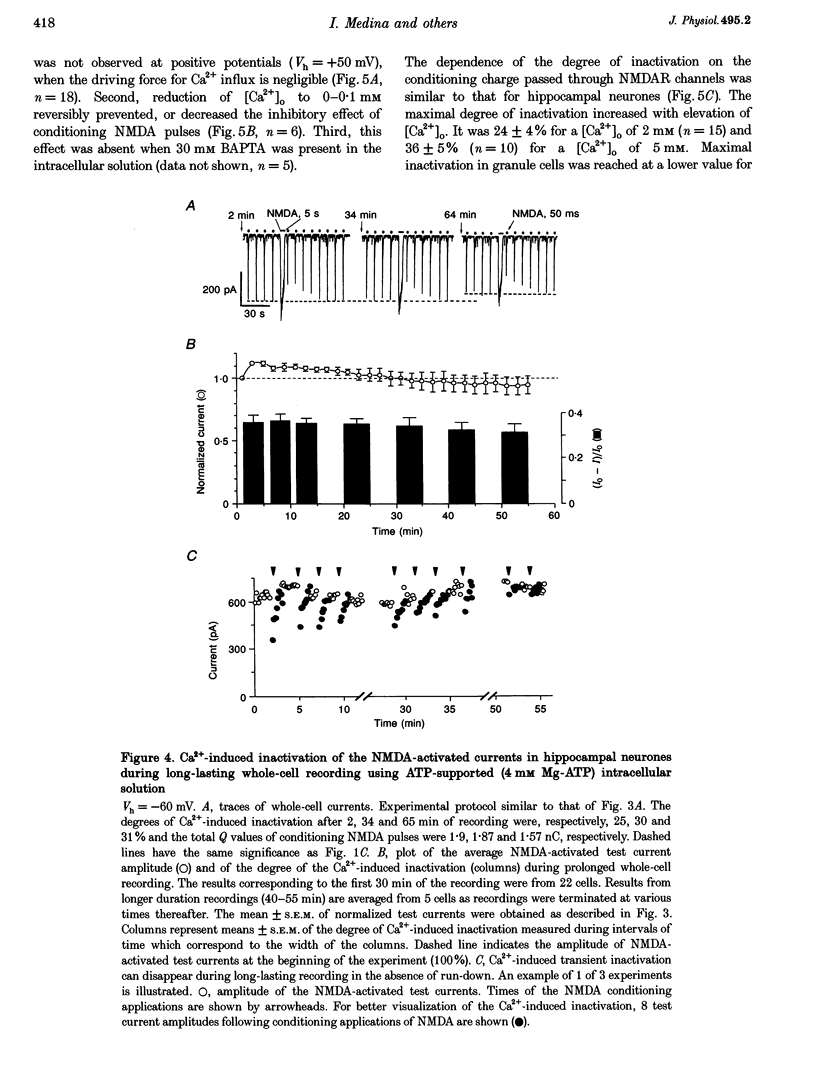
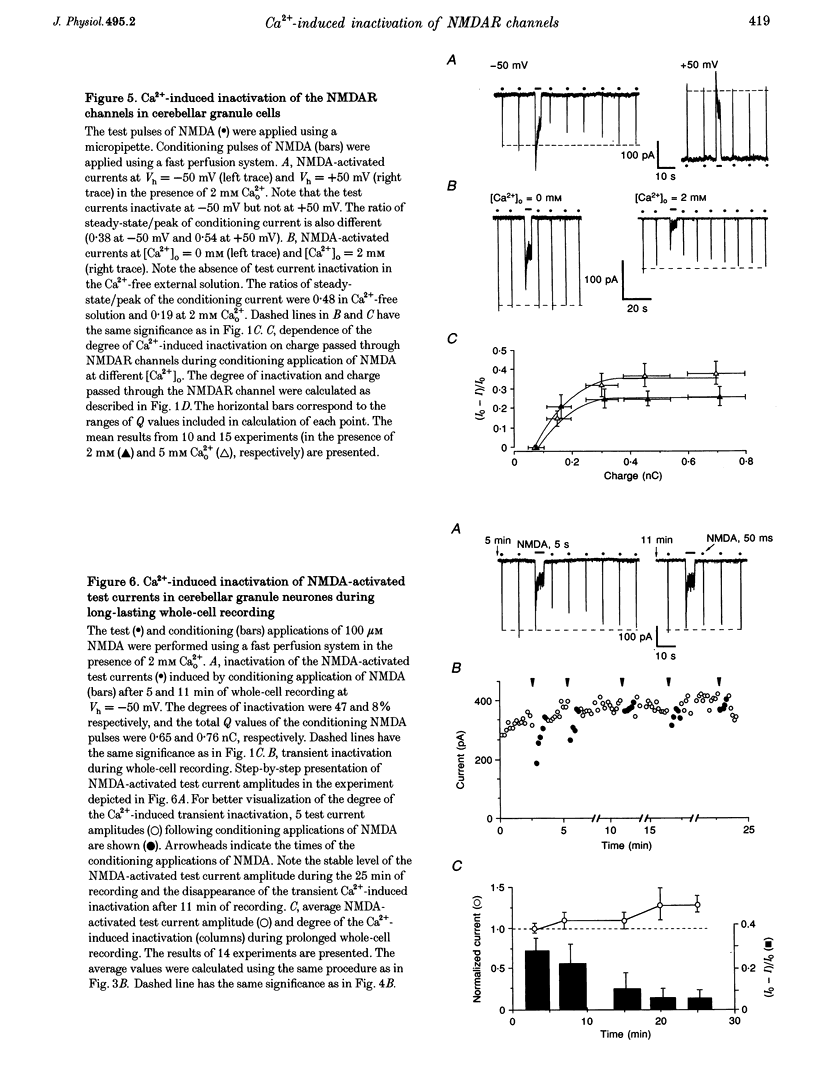





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
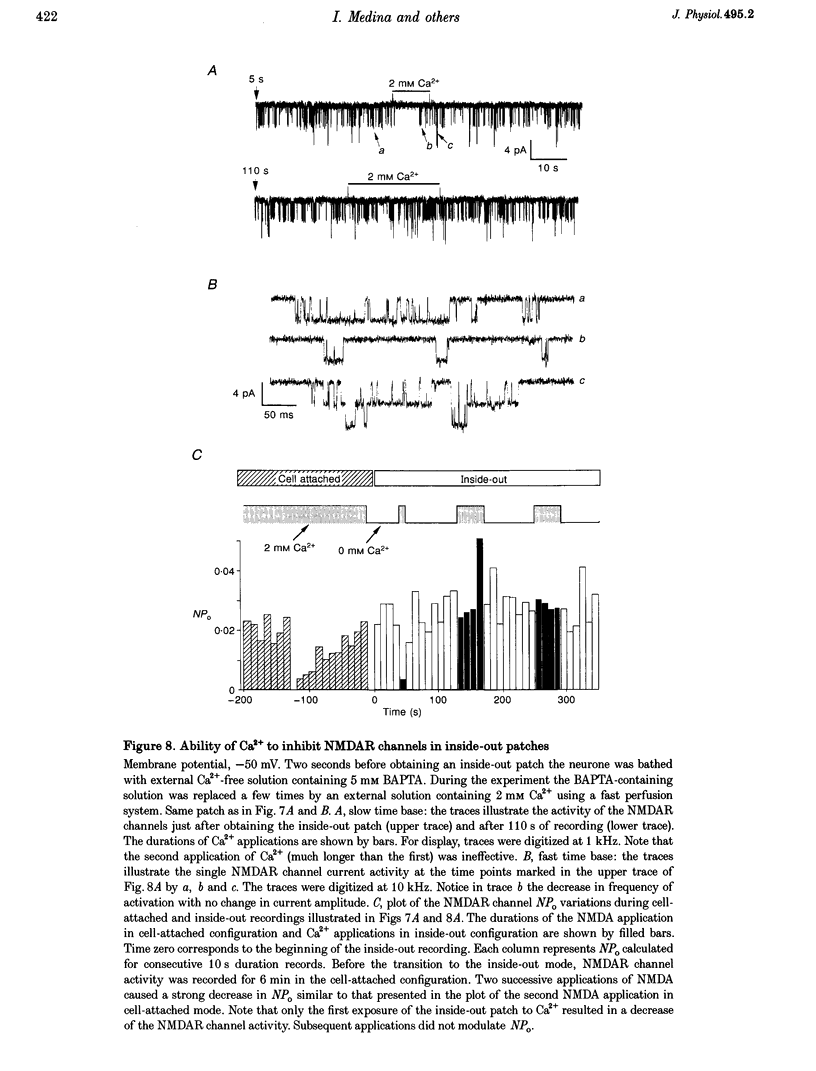
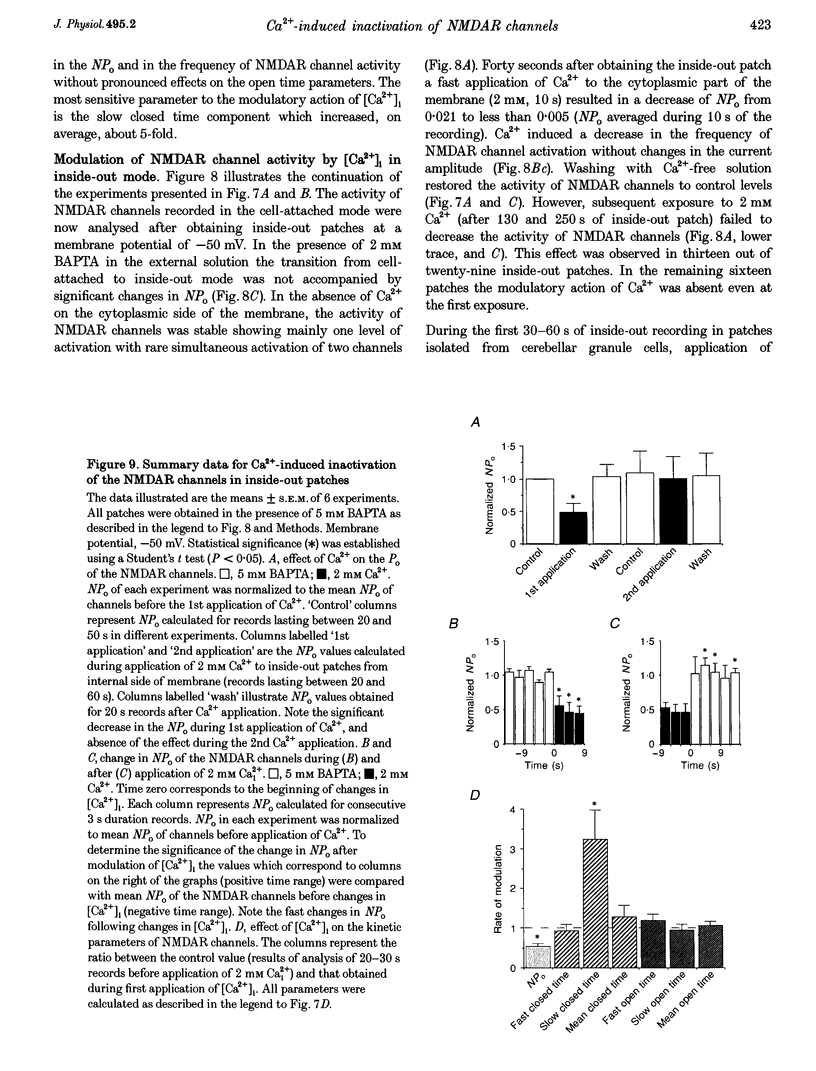
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovski P., Redkozubov A., Alexeev A. Elevation of intracellular calcium reduces voltage-dependent potassium conductance in human T cells. 1986 Feb 27-Mar 5Nature. 319(6056):776–778. doi: 10.1038/319776a0. [DOI] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Chemeris N. K., Kazachenko V. N., Kislov A. N., Kurchikov A. L. Inhibition of acetylcholine responses by intracellular calcium in Lymnaea stagnalis neurones. J Physiol. 1982 Feb;323:1–19. doi: 10.1113/jphysiol.1982.sp014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. X., Wong R. K. Suppression of GABAA receptor responses by NMDA application in hippocampal neurones acutely isolated from the adult guinea-pig. J Physiol. 1995 Jan 15;482(Pt 2):353–362. doi: 10.1113/jphysiol.1995.sp020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. D., Clifford D. B., Zorumski C. F. The effect of agonist concentration, membrane voltage and calcium on N-methyl-D-aspartate receptor desensitization. Neuroscience. 1990;39(3):787–797. doi: 10.1016/0306-4522(90)90261-2. [DOI] [PubMed] [Google Scholar]
- Gibb A. J., Colquhoun D. Activation of N-methyl-D-aspartate receptors by L-glutamate in cells dissociated from adult rat hippocampus. J Physiol. 1992 Oct;456:143–179. doi: 10.1113/jphysiol.1992.sp019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann C. W., Hunziker W. Intracellular calcium-binding proteins: more sites than insights. Trends Biochem Sci. 1991 Mar;16(3):98–103. doi: 10.1016/0968-0004(91)90041-s. [DOI] [PubMed] [Google Scholar]
- Hollmann M., Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Imredy J. P., Yue D. T. Mechanism of Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994 Jun;12(6):1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- Inoue M., Oomura Y., Yakushiji T., Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986 Nov 13;324(6093):156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Jaffe D. B., Johnston D., Lasser-Ross N., Lisman J. E., Miyakawa H., Ross W. N. The spread of Na+ spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature. 1992 May 21;357(6375):244–246. doi: 10.1038/357244a0. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Siegelbaum S. A. Intracellular Ca2+ regulates the sensitivity of cyclic nucleotide-gated channels in olfactory receptor neurons. Neuron. 1992 Nov;9(5):897–906. doi: 10.1016/0896-6273(92)90242-6. [DOI] [PubMed] [Google Scholar]
- Kyrozis A., Goldstein P. A., Heath M. J., MacDermott A. B. Calcium entry through a subpopulation of AMPA receptors desensitized neighbouring NMDA receptors in rat dorsal horn neurons. J Physiol. 1995 Jun 1;485(Pt 2):373–381. doi: 10.1113/jphysiol.1995.sp020736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P., Rosenmund C., Westbrook G. L. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci. 1993 Feb;13(2):674–684. doi: 10.1523/JNEUROSCI.13-02-00674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Mody I., Salter M. W. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989 Jul;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Helm P. J., Sakmann B. Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. J Physiol. 1995 May 15;485(Pt 1):1–20. doi: 10.1113/jphysiol.1995.sp020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Medina I., Filippova N., Barbin G., Ben-Ari Y., Bregestovski P. Kainate-induced inactivation of NMDA currents via an elevation of intracellular Ca2+ in hippocampal neurons. J Neurophysiol. 1994 Jul;72(1):456–465. doi: 10.1152/jn.1994.72.1.456. [DOI] [PubMed] [Google Scholar]
- Medina I., Filippova N., Charton G., Rougeole S., Ben-Ari Y., Khrestchatisky M., Bregestovski P. Calcium-dependent inactivation of heteromeric NMDA receptor-channels expressed in human embryonic kidney cells. J Physiol. 1995 Feb 1;482(Pt 3):567–573. doi: 10.1113/jphysiol.1995.sp020540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen R., Gulyás A. I., Baimbridge K. G., Jacobowitz D. M., Freund T. F. Calretinin is present in non-pyramidal cells of the rat hippocampus--II. Co-existence with other calcium binding proteins and GABA. Neuroscience. 1992;48(1):29–43. doi: 10.1016/0306-4522(92)90335-y. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Koutalos Y., Yau K. W. Ca2+ modulation of the cGMP-gated channel of bullfrog retinal rod photoreceptors. J Physiol. 1995 Apr 1;484(Pt 1):69–76. doi: 10.1113/jphysiol.1995.sp020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Rogers J. H. Immunoreactivity for calretinin and other calcium-binding proteins in cerebellum. Neuroscience. 1989;31(3):711–721. doi: 10.1016/0306-4522(89)90435-1. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Feltz A., Westbrook G. L. Calcium-dependent inactivation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol. 1995 Jan;73(1):427–430. doi: 10.1152/jn.1995.73.1.427. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Westbrook G. L. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993 May;10(5):805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Westbrook G. L. Rundown of N-methyl-D-aspartate channels during whole-cell recording in rat hippocampal neurons: role of Ca2+ and ATP. J Physiol. 1993 Oct;470:705–729. doi: 10.1113/jphysiol.1993.sp019884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W., Dieudonné S., MacDonald J. F., Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol. 1992 May;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko A. A., Brown D. A. Intracellular calcium directly inhibits potassium M channels in excised membrane patches from rat sympathetic neurons. Neuron. 1996 Jan;16(1):151–162. doi: 10.1016/s0896-6273(00)80032-x. [DOI] [PubMed] [Google Scholar]
- Spruston N., Schiller Y., Stuart G., Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995 Apr 14;268(5208):297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- Tong G., Jahr C. E. Regulation of glycine-insensitive desensitization of the NMDA receptor in outside-out patches. J Neurophysiol. 1994 Aug;72(2):754–761. doi: 10.1152/jn.1994.72.2.754. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr Calcium-mediated modulation of N-methyl-D-aspartate (NMDA) responses in cultured rat hippocampal neurones. J Physiol. 1993 Oct;470:575–600. doi: 10.1113/jphysiol.1993.sp019876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C. F., Yang J., Fischbach G. D. Calcium-dependent, slow desensitization distinguishes different types of glutamate receptors. Cell Mol Neurobiol. 1989 Mar;9(1):95–104. doi: 10.1007/BF00711446. [DOI] [PubMed] [Google Scholar]
- Zukin R. S., Bennett M. V. Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci. 1995 Jul;18(7):306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]