Abstract
Purpose
To explore the correlation between fasting C-peptide to diabetes duration ratio (FCP/DD) and diabetic peripheral neuropathy (DPN).
Methods
The study was conducted on 816 patients with type 2 diabetes (T2DM). Subjects were classified into a diabetic peripheral neuropathy group (DPN, n=408) and a non-diabetic peripheral neuropathy group (NDPN, n=408) depending on the presence of DPN. Collected patients’ baseline data, calculated the FCP/DD ratio, and analyzed the correlation between FCP/DD and DPN.
Results
A comparative analysis of general characteristics revealed that the DPN group exhibited higher values for age, DD, proportion of hypertension, proportion of DN, and proportion of DR compared to the NDPN group, Conversely, the DPN group demonstrated lower proportions of eGFR, FCP/DD, FCP, and fatty liver relative to the NDPN group, with all differences achieving statistical significance (P < 0.05). Compared to the high FCP/DD group, the low FCP/DD group exhibited higher values in age, DD, the proportion of DPN, DN, DR, and hypertension, as well as elevated levels of HDL-C and NEUT (P<0.05). Conversely, the low FCP/DD group demonstrated a lower proportion of patients who smoked and those with fatty liver, along with reduced BMI, ALB, FBG, UA, eGFR, TC, TG, and LDL-C levels (P < 0.05). In patients with T2DM, after adjusting for confounding factors, high levels of FCP/DD were found to be a protective factor for DPN (P < 0.05). The area under the curve of the FCP/DD Model predicting DPN (AUC=0.737) was higher than that of single FCP (AUC=0.587), DD (AUC=0.665).
Conclusion
The high FCP/DD ratio was a protective factor for T2DM with DPN. Additionally, the FCP/DD ratio was found to be a better predictor for the occurrence of DPN in T2DM compared to FCP and DD alone.
Keywords: Diabetic peripheral neuropathy, type 2 diabetes, FCP/DD
Introduction
Diabetes is still one of the most significant public health concerns today, with 451 million people globally living with diabetes in 2017. This number is expected to increase to as many as 693 million by 2045.1 The high prevalence of chronic complications of diabetes significantly impacts the quality of life and longevity of patients.2 Diabetic peripheral neuropathy (DPN) is one of the common complications affecting approximately 50% of patients with diabetes (type 1 diabetes and type 2 diabetes).3
Diabetic Peripheral Neuropathy (DPN) can result in numbness and pain in the lower extremities, foot ulcers, and potentially necessitate amputation, thereby markedly diminishing patients’ quality of life. The onset of DPN is often insidious, with up to 50% of affected individuals remaining asymptomatic during the initial stages, which frequently leads to neglect and delayed diagnosis. Therefore, early prevention and detection of diabetic peripheral neuropathy are of paramount importance.4 The pathogenesis of Diabetic Peripheral Neuropathy (DPN) is multifaceted, encompassing oxidative stress, inflammatory responses, neurotrophic dysregulation and various other contributing factors.5–7 Wahren et al8 showed that compared with the placebo group, injected C-peptide analogues can increase the vibration perception thresholds; This indicates that C-peptide replacement therapy may enhance neurological function in patients with diabetic peripheral neuropathy (DPN). C-peptide, a byproduct of glucagon, is more stable than insulin. C-peptide levels and release profiles are commonly used to assess islet function.9 Umaid Potaliya et al10 demonstrated that lower serum C-peptide levels are linked to diabetic peripheral neuropathy (DPN) in people with type 2 diabetes, they also found that the risk of DPN increases with longer duration of diabetes.11 Some studies have shown that C-peptide at physiological concentrations has anti-inflammatory, immunomodulatory, and neurotrophic effects, C-peptide and its analogs have been found to lower blood glucose and alleviate the complications of diabetes.12 At present, no studies have examined the relationship between FCP/DD and type 2 diabetes mellitus in conjunction with DPN. Therefore, this study aims to explore the correlation between the FCP/DD ratio and type 2 diabetes combined with peripheral neuropathy. The objective is to establish a new clinical indicator for the early detection and prediction of DPN.
Methods
Subjects
816 patients with type 2 diabetes who were hospitalized at Hebei Provincial People’s Hospital in 2022 were selected for the study. Inclusion criteria: T2DM was diagnosed according to the diagnostic criteria of 1999 World Health Organization (WHO); Exclusion criteria: (1) type 1 diabetes mellitus, gestational diabetes mellitus, and special types of diabetes mellitus; (2) age <18 years old or age >80 years old; (3) admitted to the hospital with acute diabetes mellitus complications including ketoacidosis, hyperosmolar-hyperglycemic syndrome, and hypoglycemic coma; (4) history of type B history of viral hepatitis, cirrhosis, hepatic encephalopathy, and liver surgery; (5) recent comorbidities of severe renal disease, cardiovascular disease, acute and chronic infections, stress, tumors, or hematological disorders; (6) pregnancy and breastfeeding; (7) other lesions or drug-induced neuropathy; and (8) drug-induced neurotoxicity. Neurological symptoms included burning, numbness, tingling, fatigue, cramping or aching, and neurological signs included vibration sense, pain, temperature sensation and ankle reflex. The symptoms and signs abnormalities assessed for DPN were in a glove/stocking distribution. Evaluating DPN using the Toronto Clinical Scoring System (TCSS) and neurophysiological examination as the gold standard has high diagnostic value for DPN.13 As follows: (1) non-DPN, all neurological symptoms/signs and NCV were normal; (2) clinical DPN, at least two abnormal results among neurological symptoms/signs, or ankle reflex in accordance with a distal symmetrical polyneuropathy and normal NCV; (3) confirmed DPN, at least one abnormal nerve parameter (of NCV, amplitude, latency, and F-wave) in two or more nerves among the median, peroneal, and sural nerves, regardless of neurological signs and symptoms. This study received approval from the Ethics Committee of Hebei Provincial People’s Hospital, and the patient’s information was anonymous and confidential, so the signed informed consent was exempted.
Data Collection and Laboratory Analysis
General and biochemical data were collected: gender, age, diabetes duration, smoking history, drinking history, medication history, hypertension history, fatty liver history, diabetic nephropathy history, diabetic retinopathy history, etc; height and weight were measured, and body mass index(BMI) was calculated; blood samples were collected early in the morning of the following day after 8–10 hours of fasting after admission to the hospital, and the white blood cell count (WBC), percentage of neutrophils (NEUT), albumin (ALB), fasting blood glucose (FBG), glycated hemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), blood creatinine (Cr), blood uric acid (UA), glomerular filtration rate (eGFR), erythrocyte sedimentation rate (ESR), fasting C-peptide (FCP) and other indexes were measured; using flow cytometry to detect routine blood indexes, using automatic biochemical analyzer to detect biochemical indexes, using high-performance liquid-contrast cation-exchange chromatography to determine the glycated hemoglobin, and electrochemiluminescence to determine fasting C-peptide, and so on. Our laboratory doctors perform all of these laboratory tests.
Calculation of Ratio and Grouping
Fasting C-peptide/diabetes duration (FCP/DD) was calculated by dividing fasting C-peptide by diabetes duration; its median was calculated statistically, and the median of this ratio was 0.25, according to which the patients were divided into two groups: the Low FCP/DD group (FCP/DD≤0.25, n=408) and the High FCP/DD group (FCP/DD >0.25, n=408). Patients with type 2 diabetes mellitus (T2DM) were divided into two groups: the DPN group (n=455) and the NDPN group (n=361), based on the presence of diabetic peripheral neuropathy (DPN).
Statistical Methods
Statistical SPSS 25.0 statistical software was used to analyze the collected data. Measurement data that conformed to a normal distribution were expressed as mean ± standard deviation, with group comparisons performed using two independent samples t-tests. Non-normally distributed measurement data were expressed as the median and quantile spacing [M(P25%, P75%)], with group comparisons performed using the Mann–Whitney U-test. Categorical variables were expressed as percentages, and the chi-square test was used for between-group comparisons. Pearson’s correlation analysis was used when continuous variables in the two groups conformed to normal distribution, and Spearman’s rank correlation analysis was used to explore the correlation between DPN and the general information of the study population if any of the continuous variables in the two groups did not obey normal distribution. A binary logistic regression analysis was conducted to investigate the determinants of diabetic peripheral neuropathy (DPN). The predictive value of fasting C-peptide (FCP), diabetes duration (DD) and fasting C-peptide/diabetes duration (FCP/DD) in patients with DPN was assessed by Receiver Operating Characteristic (ROC) curve analysis, including the calculation of the area under the curve (AUC). The DeLong test was used for AUC comparison. Statistical significance was established at P<0.05 for all analyses.
Results
Comparison of General Data and Laboratory-Related Indicators in All Patients
A total of 816 patients with T2DM were included in this study, including 455 patients (55.76%) with DPN and 361 patients (44.24%) with NDPN. The differences in age, diabetes duration, the proportion of history of combined fatty liver, FCP/DD, GFR, and fasting C-peptide were statistically significant between the two groups of study subjects (P < 0.05):the age, diabetes duration, the proportion of history of combined hypertension, the proportion of history of combined diabetic nephropathy, and the proportion of history of combined diabetic retinopathy were higher in the DPN group than those in the NDPN group; and the proportion of history of combined fatty liver, GFR, FCP/DD, and fasting C-peptide were lower than those in the NDPN group. But the differences in the smoking history, drinking history, BMI, ALB, FBG, Cr, UA, TC, TG, HDL-C, LDL-C, HbA1c, ESR, were not statistically significant (P>0.05) (Table 1).
Table 1.
Comparison of General Data and Laboratory-Related Indicators in All Patients
| Variable | DPN | NDPN | Z (χ2) | P |
|---|---|---|---|---|
| n | 455 | 361 | – | – |
| Age (years) | 59 (52,68) | 56 (43,66) | −4.271 | <0.001 |
| Sex (male/female) | 290/164 | 224/137 | 0.246a | 0.620 |
| DD (years) | 11 (5,20) | 4 (1,12) | −8.129 | <0.001 |
| BMI (kg/m2) | 25.92 (23.80,28.18) | 26.18 (23.78,28.87) | −1.239 | 0.215 |
| Smoking (n%) | 122 (26.81%) | 96 (26.59%) | 0.005a | 0.994 |
| Drinking (n%) | 104 (22.86%) | 85 (23.55%) | 0.054a | 0.817 |
| Hypertension (n%) | 253 (55.60%) | 174 (48.20%) | 4.425a | 0.035 |
| Fatty liver (n%) | 233 (51.21%) | 214 (59.28%) | 5.293a | 0.021 |
| DN (n%) | 115 (25.27%) | 32 (8.86%) | 36.703a | <0.001 |
| DR (n%) | 204 (44.84%) | 43 (11.91%) | 103.376a | <0.001 |
| WBC (×109 /L) | 6.37 (5.37,8.07) | 6.27 (5.18, 7.62) | −1.445 | 0.148 |
| NEUT (%) | 63.80(57.10,70.50) | 62.70(56.30,70.85) | −1.357 | 0.175 |
| ALB (g/l) | 39.75(36.93,42.78) | 40.40(37.40,43.30) | −1.939 | 0.053 |
| FBG (mmol/l) | 8.03 (5.97,11.00) | 7.96 (6.08,10.67) | −0.019 | 0.985 |
| Cr (umol/L) | 66.50 (55.93,79.83) | 65.25 (55.40,76.32) | −1.333 | 0.182 |
| UA (umol/L) | 317.65 (258.15,388.68) | 331.15 (270.50,399.40) | −1.396 | 0.163 |
| eGFR (mL/min) | 96.73(82.49,106.25) | 99.95 (87.32,111.43) | −3.38 | 0.001 |
| TC (mmol/l) | 4.71 (3.90,5.57) | 4.70 (3.97,5.65) | −0.792 | 0.429 |
| TG (mmol/l) | 1.37 (0.90,2.09) | 1.50 (0.99,2,21) | −1.752 | 0.08 |
| HDL-C (mmol/l) | 1.09 (0.90,1.30) | 1.05 (0.92,1.22) | −1.147 | 0.251 |
| LDL-C (mmol/l) | 2.94 (2.37,3.57) | 2.96 (2.43,3.64) | −0.865 | 0.387 |
| HBA1c (%) | 8.70 (7.38,10.50) | 8.40 (6.90,10.30) | −1.552 | 0.121 |
| ESR (mm/h) | 10.50 (5.00.21.00) | 10.00 (5.00,20.75) | −0.313 | 0.754 |
| FCP (nmol/l) | 1.83 (1.19,2.71) | 2.28 (1.43,3.21) | −3.882 | <0.001 |
| FCP/DD | 0.18 (0.08,0.45) | 0.45 (0.16,2.23) | −8.316 | <0.001 |
Note: aindicates χ2 value. Significance at a P value of <0.05.
Abbreviations: DPN, diabetic peripheral neuropathy; NDPN,non-diabetic peripheral neuropathy; DN, diabetic nephropathy; DR, diabetic retinopathy; DD, diabetes duration; BMI, body mass index; WBC, white blood cell count; NEUT, percentage of neutrophils; HbA1c, glycated hemoglobin; FBG, fasting blood glucose; ALB, albumin; Cr, blood creatinine; UA, blood uric acid; eGFR, Glomerular filtration rate; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ESR, erythrocyte sedimentation rate; FCP, fasting C-peptide; FCP/DD, fasting C-peptide/ diabetes duration.
Comparison of General Data and Laboratory-Related Indicators on Study Participants from Different FCP/DD Subgroups
The age, diabetes duration, the proportion of history of combined hypertension, proportion of combined diabetic peripheral neuropathy, proportion of combined diabetic nephropathy, and proportion of combined diabetic retinopathy, HDL-C, and NEUT in the High FCP/DD group were lower than those in the Low FCP/DD group (P < 0.05), and the proportion of history of combined fatty liver, proportion of smoking history, BMI, ALB, FBG, UA, eGFR, TC, TG, and LDL-C were higher than those in the Low FCP/DD group (P < 0.05). Comparison of gender, drinking history, HBA1C, ESR, Cr, and WBC between the two study groups was not statistically significant (Table 2).
Table 2.
Comparison of General Data and Laboratory-Related Indicators on Study Participants from Different FCP/DD Subgroups
| Variable | Low FCP/DD | High FCP/DD | Z (χ2) | P |
|---|---|---|---|---|
| n | 408 | 408 | – | – |
| Age (years) | 63 (56,70) | 53 (42,62) | −11.416 | <0.001 |
| Sex (male/female) | 246/162 | 269/139 | 2.544a | 0.111 |
| DD (years) | 17.00 (11.00,20.00) | 3.00 (1.00,6.00) | −22.139 | <0.001 |
| BMI (kg/m2) | 25.60 (23.41,27.71) | 26.39 (24.29,29.55) | −4.402 | <0.001 |
| Smoking (n %) | 94 (23.04%) | 124 (30.39%) | 5.644a | 0.018 |
| Drinking (n %) | 86 (21.08%) | 103 (25.25%) | 1.990a | 0.158 |
| Hypertension (n%) | 237 (58.23%) | 190 (46.57%) | 10.825a | 0.001 |
| Fatty liver (n%) | 180 (44.12%) | 267 (65.44%) | 37.445a | <0.001 |
| DN (n%) | 97 (23.77%) | 50 (12.25%) | 18.329a | <0.001 |
| DR (n%) | 179 (43.87%) | 68 (16.67%) | 71.536a | <0.001 |
| DPN (n %) | 275 (67.40%) | 180 (44.12%) | 44.835a | <0.001 |
| ESR (mm/h) | 10.00 (5.00,23.00) | 10.00 (5.00,18.00) | −1.263 | 0.206 |
| ALB (g/l) | 38.90 (35.95,41.55) | 41.30 (38.20, 44.40) | −7.629 | <0.001 |
| FBG (mmol/l) | 7.67 (5.71,10.50) | 8.17 (6.39,11.17) | −2.69 | 0.007 |
| Cr (μmol/L) | 66.25 (56.10,78.50) | 65.40 (55.08,77.30) | −0.746 | 0.456 |
| UA (μmol/L) | 309.10 (250.00,381.10) | 338.30 (277.00,404.20) | −3.529 | <0.001 |
| eGFR (mL/min) | 93.90 (80.77, 103.26) | 101.95 (89.64, 112.44) | −6.64 | <0.001 |
| TC (mmol/l) | 4.57 (3.75, 5.49) | 4.86 (4.01, 5.66) | −2.827 | 0.005 |
| TG (mmol/l) | 1.21 (0.84,1.82) | 1.62 (1.12,2.56) | −6.956 | <0.001 |
| HDL-C (mmol/l) | 1.09 (0.93,1.30) | 1.05 (0.90,3.66) | −2.208 | 0.027 |
| LDL-C (mmol/l) | 2.89 (2.27,3.55) | 3.08 (2.46,3.66) | −2.923 | 0.003 |
| HBA1C (%) | 8.70 (7.50,10.40) | 8.40 (6.90,10.40) | −1.696 | 0.090 |
| WBC (×109 /L) | 6.37 (5.25,7.98) | 6.28 (5.29,7.73) | −0.687 | 0.492 |
| NEUT (%) | 65.40 (57.60,72.80) | 61.30 (55.80,68.70) | −4.334 | <0.001 |
Note: aindicates χ2 value. Significance at a P value of <0.05.
Abbreviations: DPN, diabetic peripheral neuropathy; DN, diabetic nephropathy;DR, diabetic retinopathy; DD, diabetes duration; BMI, body mass index; HbA1c, glycated hemoglobin; FBG, fasting blood glucose; ALB, albumin;Cr, blood creatinine; UA, blood uric acid; eGFR, Glomerular filtration rate; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ESR, erythrocyte sedimentation rate;WBC, white blood cell count;NEUT, percentage of neutrophils.
Correlation Analysis Between FCP/DD and Indicators
The indicators of general data were included in Spearman’s rank correlation analysis, which showed that FCP/DD was negatively correlated with age, NEUT, HDL-C (P<0.05), positively correlated with BMI, ALB, TC, TG, LDL-C, GFR, UA, FBG (P<0.05), and WBC, Cr, HBA1C, ESR levels were not significantly correlated (Table 3).
Table 3.
Correlation Analysis Between FCP/DD and Indicators (Rs Value)
| Variable | rs | P | Variable | rs | P |
|---|---|---|---|---|---|
| Age | −0.434 | <0.001 | TC | 0.138 | <0.001 |
| BMI | 0.225 | <0.001 | TG | 0.315 | <0.001 |
| ALB | 0.288 | <0.001 | HDL-C | −0.104 | 0.004 |
| NEUT | −0.177 | <0.001 | LDL-C | 0.146 | <0.001 |
| WBC | −0.018 | 0.613 | FBG | 0.162 | <0.001 |
| UA | 0.145 | <0.001 | Cr | −0.035 | 0.325 |
| ESR | −0.07 | 0.091 | eGFR | 0.269 | <0.001 |
| HBA1C | −0.02 | 0.596 |
Note: Significance at a P value of <0.05.
Abbreviations: BMI, body mass index; WBC, white blood cell count;NEUT, percentage of neutrophils;HbA1c, glycated hemoglobin; FBG, fasting blood glucose; ALB, albumin; Cr, blood creatinine; UA, blood uric acid; eGFR, Glomerular filtration rate; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ESR, erythrocyte sedimentation rate.
Multifactor Logistic Regression Analysis of FCP/DD on DPN
Whether T2DM was combined with DPN as the dependent variable, clinical data and biochemical indicators were screened for independent variables, FCP and DD covariates were excluded, and the independent indicators were included in binary Logistics regression analysis. As shown in Table 4, after adjusting for potential confounders, a high ratio of FCP/DD was found to be a protective factor for DPN (Table 4).
Table 4.
Multifactor Logistic Regression Analysis of FCP/DD on DPN
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| FCP/DD | ||||||
| Low | 2.619 (1.970, 3.482) | <0.001 | 1.470 (1.045, 2.068) | 0.027 | 1.554 (1.073, 2.251) | 0.02 |
| High | Ref | Ref | Ref | |||
Notes: Significance at a P value of <0.05. Model 1: no adjustment for confounders; Model 2: adjusted for age, sex, smoking history, drinking history, hypertension history, fatty liver history, diabetic nephropathy history, diabetic retinopathy history based on Model 1; Model 3: further adjusted for BMI, ALB, NEUT, UA, eGFR, TC, TG, FBG, LDL-C, HDL-C based on Model 2.
Abbreviation: FCP/DD, fasting C-peptide/ diabetes duration.
Evaluate the Predictive Value of FCP, DD, and the FCP/DD Model for T2DM with DPN and Compare Using the DeLong Test
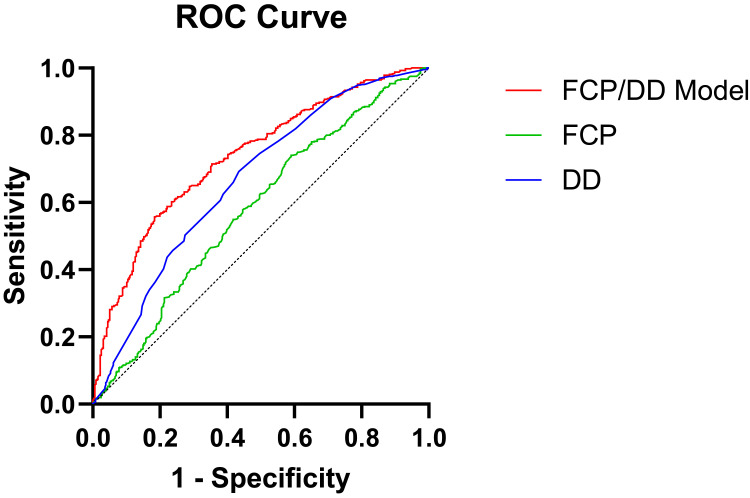
The regression equation for the FCP/DD Model: logit(P)=1.148+0.081×sex-0.097×smoking+0.013×drinking+0.052×Hypertension+0.127×fattyliver-0.844×DN-1.458×DR+0.017×Age-0.018×BMI+0.02×FBG+0.028×ALB-0.01×NEUT-0.001×UA+0.002×eGFR+0.375×TC-0.111×TG-0.464×LDL-C-0.554×HDL-C+0.441×FCP/DD, and compared with FCP and DD, which showed that the AUC of the FCP/DD Model was 0.737, which was greater than that of the FCP (Z=6.000, P<0.01) and DD (Z=4.099, P<0.01) (Table 5 and Figure 1).
Table 5.
ROC Curves of FCP, DD, and FCP/DD Model
| Variable | Cut-off | AUC (95% CI) | Sensitivity | Specificity |
|---|---|---|---|---|
| FCP | 2.667 | 0.587 (0.546, 0.629) | 0.733 | 0.427 |
| DD | 6.500 | 0.665 (0.624, 0.704) | 0.700 | 0.554 |
| FCP/DD Model | 0.609 | 0.737 (0.701, 0.773) | 0.557 | 0.816 |
Note:Significance at a P value of <0.05.
Abbreviations: FCP, fasting C-peptide; DD, diabetes duration; FCP/DD Model, fasting C-peptide/ diabetes duration Model; AUC, the area under the subject’s operating characteristic curve.
Figure 1.
ROC curves for FCP, DD, and FCP/DD Model suggest the risk of type 2 diabetes combined with DPN.
Abbreviation: FCP, fasting C-peptide; DD, diabetes duration; FCP/DD Model, fasting C-peptide/ diabetes duration Model.
Discussion
Diabetic peripheral neuropathy (DPN) is a common complication associated with diabetes mellitus. Early detection of DPN is often challenging due to the difficulty in identifying small fibrotic lesions, which may result in a delayed diagnosis until the condition becomes symptomatic. This delay can lead to severe complications, including the development of ulcers and an increased risk of foot amputation, ultimately resulting in irreversible neuropathy.14 Therefore, early detection or prediction of DPN is of paramount importance, as the current treatment options are frequently inadequate, significantly impairing the patient’s quality of life.
In our study, patients with DPN had higher age, longer diabetes duration, a higher proportion of hypertension, a higher proportion of fatty liver and diabetic complications, and lower blood FCP and eGFR levels, which is consistent with Wang et al.4 And some studies have also shown a correlation between the development of DPN and both a history of diabetic nephropathy and diabetic retinopathy.15 While fasting blood glucose and glycated hemoglobin did not show statistically significant differences, this was observed in the subgroup of patients with type 2 diabetes mellitus with or without comorbid DPN. This finding may be attributable to the critical role of blood glucose management in the selected population, all of whom were administered standardized glucose-lowering medications. Regarding gender, one study claimed that there was no statistically significant association between gender and DPN (p = 0.966), which is consistent with our findings.16
C-peptide, a cleavage product of proinsulin, exhibits greater stability than insulin and remains unaffected by exogenous insulin administration. Consequently, it is frequently utilized in clinical settings to evaluate pancreatic islet function.9 In a study involving Chinese patients with type 2 diabetes, C-peptide demonstrated a potential protective effect against the progression of type 2 diabetes when administered in conjunction with diabetic peripheral neuropathy (DPN);17 a retrospective cohort study showed that higher C-peptide levels (OR: 0.39; 95% CI:0.25–0.61) were negatively associated with the risk of developing neuropathy.18 Zhao et al19 found that the C-peptide area under the curve [AUC (C-pep)] was negatively associated with the prevalence of diabetic peripheral neuropathy. In addition to this, exogenous C-peptide replacement therapy was able to delay and improve diabetic peripheral neuropathy.20 Research has confirmed that the duration of diabetes mellitus is a risk factor for DPN. As the duration of the disease increases, the islet function of diabetic patients gradually decreases, leading to a decrease in serum C-peptide and insulin secretion, and an increase in the prevalence of DPN; In their study, Lian et al14 established a risk factor and risk model for DPN, they found a high correlation between age and duration of diabetes mellitus and DPN, indicating that age and duration of diabetes mellitus are risk factors; Wang et al4 in studying the prevalence and risk factors of DPN showed that DPN was negatively correlated with FCP and positively correlated with diabetes duration. Our findings indicated that FCP levels were lower in DPN patients compared to NDPN patients, and there was a negative correlation. Additionally, the duration of diabetes in DPN patients was longer than in NDPN patients, and there was a positive correlation. These results align with the previous findings. Elafros et al21 conducted a study on DPN prevention and found that diabetes duration strongly influences DPN.
For the first time in this study, FCP (fasting C-peptide) and DD (diabetes duration) are combined to analyze the relationship between the FCP/DD ratio and DPN (diabetic peripheral neuropathy). In this study, there were 816 patients with T2DM with 455 patients (about 55.76%) with combined DPN and 361 patients (about 44.24%) without combined DPN. The FCP levels of patients with DPN were lower than those without DPN, while the DD levels were higher in patients with DPN, consistent with previous studies.4 Upon further analysis of the study, it was found that as the FCP/DD decreased, the incidence of DPN increased. There is a negative correlation between FCP/DD and DPN. Even after adjusting for confounding factors, a high level of FCP/DD was still found to be a protective factor for DPN. This suggests that FCP/DD has the potential to be a predictor of DPN risk.
We analyzed whether the ratio of FCP/DD could be a better predictor of DPN. We plotted the ROC curve and found that FCP/DD had a greater predictive value for the development of DPN in patients with type 2 diabetes than FCP and DD alone, with an AUC of 0.737 (0.701, 0.773). This was superior to FCP and DD alone, suggesting that the FCP/DD ratio is a better clinical predictor of DPN risk.
Chronic inflammation is thought to be closely associated with the onset and progression of DPN, and some studies have shown that neutrophil counts are higher in patients with DPN;22 In our study, we found that there were no statistically significant differences in erythrocyte sedimentation rate, white blood cell count, and percentage of neutrophils between the subgroup of patients with DPN and those without DPN. However, in the subgroup of FCP/DD ratios by the median, we observed that the percentage of neutrophils was higher in patients with a low FCP/DD ratio. This finding aligns with previous research on the topic. Ban et al23 showed that the albumin ratio was lower in patients with DPN than in patients with NDPN, and our study showed that albumin was lower in patients in the low-ratio FCP/DD group, which is consistent with the Ban et al.23 Therefore, patients with type 2 diabetes need to maintain adequate nutritional levels to reduce the risk of DPN.
This study has some limitations that should be taken into account. Firstly, because it’s a retrospective study, it cannot establish a cause-and-effect relationship. Secondly, the study has a small sample size from a single center, which could lead to inaccurate results. Therefore, further validation of the results would require a larger sample size and a more diverse study population. Additionally, conducting a long-term follow-up or a case-control study would help to validate the reliability of the results.
Conclusion
In conclusion, a high FCP/DD ratio is a protective factor for type 2 diabetes combined with DPN. The FCP/DD model has good predictive value for determining whether type 2 diabetes is combined with DPN, and its predictive ability is better than that of FCP and DD alone.
Funding Statement
This study was not supported by any financial sources.
Ethics Statement
The study followed the principles in the Declaration of Helsinki and was approved by the Ethical Committees of Hebei General Hospital (No.2024-LW-145). In addition, this study was a retrospective non-interventional study, and the patient’s information was anonymous and confidential, so the signed informed consent was exempted.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lin X, Xu Y, Pan X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10(1):14790. doi: 10.1038/s41598-020-71908-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Li Y, Deng N, Shi H, Caika S, Sen G. Training and external validation of a predict nomogram for type 2 diabetic peripheral neuropathy. Diagnostics. 2023;13(7):1265. doi: 10.3390/diagnostics13071265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan P, Wu Y, Dan X, et al. Aspartate aminotransferase/alanine aminotransferase ratio was associated with type 2 diabetic peripheral neuropathy in a Chinese population: a cross-sectional study. Front Endocrinol (Lausanne). 2023;14:1064125. doi: 10.3389/fendo.2023.1064125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Ji Q, Ran X, et al. Prevalence and risk factors of diabetic peripheral neuropathy: a population-based cross-sectional study in China. Diabetes Metab Res Rev. 2023;39(8):e3702. doi: 10.1002/dmrr.3702 [DOI] [PubMed] [Google Scholar]
- 5.Azoulay D, Abed S, Sfadi A, et al. Low brain-derived neurotrophic factor protein levels and single-nucleotide polymorphism Val66Met are associated with peripheral neuropathy in type II diabetic patients. Acta Diabetol. 2020;57(7):891–898. doi: 10.1007/s00592-020-01508-6 [DOI] [PubMed] [Google Scholar]
- 6.Mallet ML, Hadjivassiliou M, Sarrigiannis PG, Zis P. The role of oxidative stress in peripheral neuropathy. J Mol Neurosci. 2020;70(7):1009–1017. doi: 10.1007/s12031-020-01495-x [DOI] [PubMed] [Google Scholar]
- 7.Ristikj-Stomnaroska D, Risteska-Nejashmikj V, Papazova M. Role of inflammation in the pathogenesis of diabetic peripheral neuropathy. Open Access Maced J Med Sci. 2019;7(14):2267–2270. doi: 10.3889/oamjms.2019.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahren J, Foyt H, Daniels M, Arezzo JC. Long-acting c-peptide and neuropathy in type 1 diabetes: a 12-month clinical trial. Diabetes Care. 2016;39(4):596–602. doi: 10.2337/dc15-2068 [DOI] [PubMed] [Google Scholar]
- 9.Qin J, Sun R, Ding D. Effects of serum C-peptide level on blood lipid and cardiovascular and cerebrovascular injury in patients with type 2 diabetes mellitus: a meta-analysis. Contrast Media Mol Imaging. 2022;2022(1):6314435. doi: 10.1155/2022/6314435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potaliya U, Tak S, Goyal M. Association of C-peptide level with peripheral neuropathy in type 2 diabetes: an observational cross-sectional preliminary study. Diabetes Metab Syndr. 2023;17(2):102725. doi: 10.1016/j.dsx.2023.102725 [DOI] [PubMed] [Google Scholar]
- 11.Li C, Wang W, Ji Q, et al. Prevalence of painful diabetic peripheral neuropathy in type 2 diabetes mellitus and diabetic peripheral neuropathy: a nationwide cross-sectional study in mainland China. Diabet Res Clin Pract. 2023;198:110602. doi: 10.1016/j.diabres.2023.110602 [DOI] [PubMed] [Google Scholar]
- 12.Pathan R, Purohit N, Choudhary P. Study of serum C-peptide levels in newly diagnosed diabetic mellitus subjects of North Gujarat region of India. Int J Adv Med. 2022;9(2):142. doi: 10.18203/2349-3933.ijam20220124 [DOI] [Google Scholar]
- 13.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments [published correction appears in Diabetes Care. 2010 Dec; 33(12):2725]. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lian X, Qi J, Yuan M, et al. Study on risk factors of diabetic peripheral neuropathy and establishment of a prediction model by machine learning. BMC Med Inform Decis Mak. 2023;23(1):146. doi: 10.1186/s12911-023-02232-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Jiang S. Development and validation of a model that predicts the risk of diabetic nephropathy in type 2 diabetes mellitus patients: a cross-sectional study. Int J Gen Med. 2022;15:5089–5101. doi: 10.2147/IJGM.S363474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinh Le T, Phi Thi Nguyen N, Thanh Thi Tran H, et al. Diabetic peripheral neuropathy associated with cardiovascular risk factors and glucagon-like peptide-1 concentrations among newly diagnosed patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2022;15:35–44. doi: 10.2147/DMSO.S344532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao X, Zheng H, Zhang S, et al. C-peptide is independent associated with diabetic peripheral neuropathy: a community-based study. Diabetol Metab Syndr. 2017;9(1):12. doi: 10.1186/s13098-017-0208-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bo S, Gentile L, Castiglione A, et al. C-peptide and the risk for incident complications and mortality in type 2 diabetic patients: a retrospective cohort study after a 14-year follow-up. Eur J Endocrinol. 2012;167(2):173–180. doi: 10.1530/EJE-12-0085 [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Ma J, Wang S, et al. Relationship between β-cell function, metabolic control, and microvascular complications in type 2 diabetes mellitus. Diabetes Technol The. 2015;17(1):29–34. doi: 10.1089/dia.2014.0214 [DOI] [PubMed] [Google Scholar]
- 20.Ekberg K, Johansson BL. Effect of C-peptide on diabetic neuropathy in patients with type 1 diabetes. Exp Diabetes Res. 2008;2008(1):457912. doi: 10.1155/2008/457912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elafros MA, Andersen H, Bennett DL, et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022;21(10):922–936. doi: 10.1016/S1474-4422(22)00188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Zhang X, Zhang Y, et al. Increased systemic immune-inflammation index was associated with type 2 diabetic peripheral neuropathy: a cross-sectional study in the Chinese population. J Inflamm Res. 2023;16:6039–6053. doi: 10.2147/JIR.S433843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ban J, Pan X, Yang L, et al. Correlation between fibrinogen/albumin and diabetic peripheral neuropathy. Diabetes Metab Syndr Obes. 2023;16:2991–3005. doi: 10.2147/DMSO.S427510 [DOI] [PMC free article] [PubMed] [Google Scholar]