Abstract
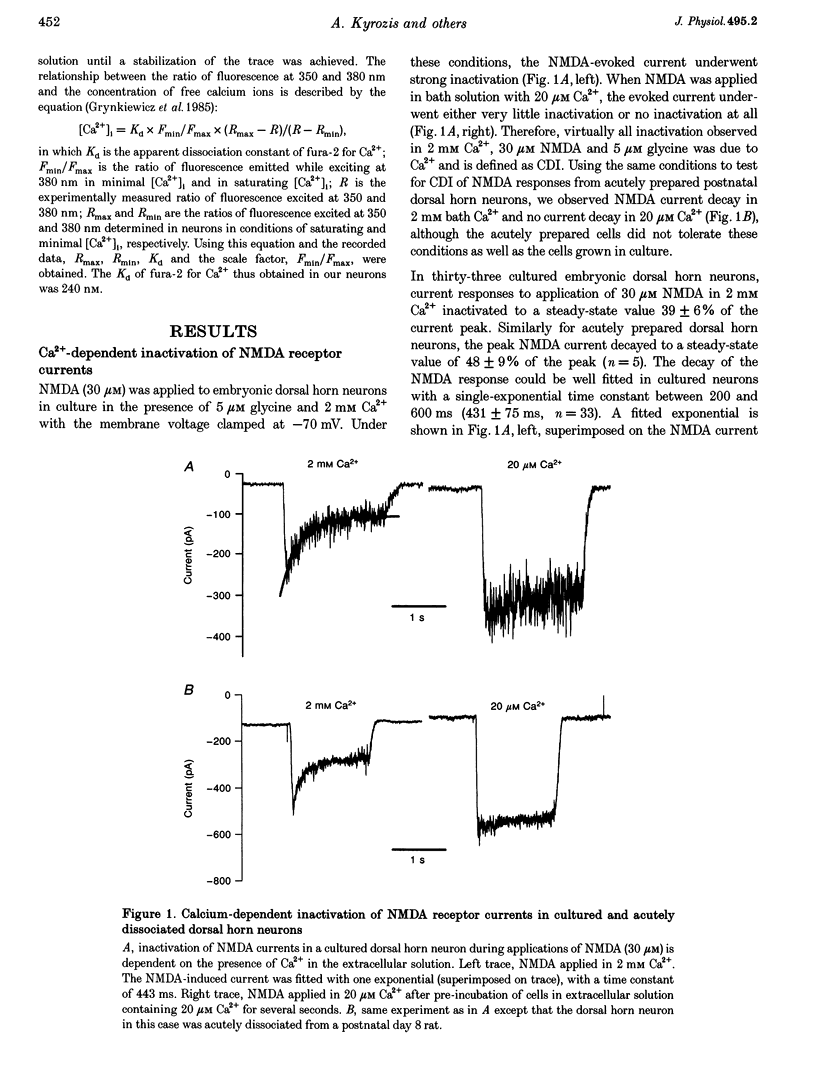
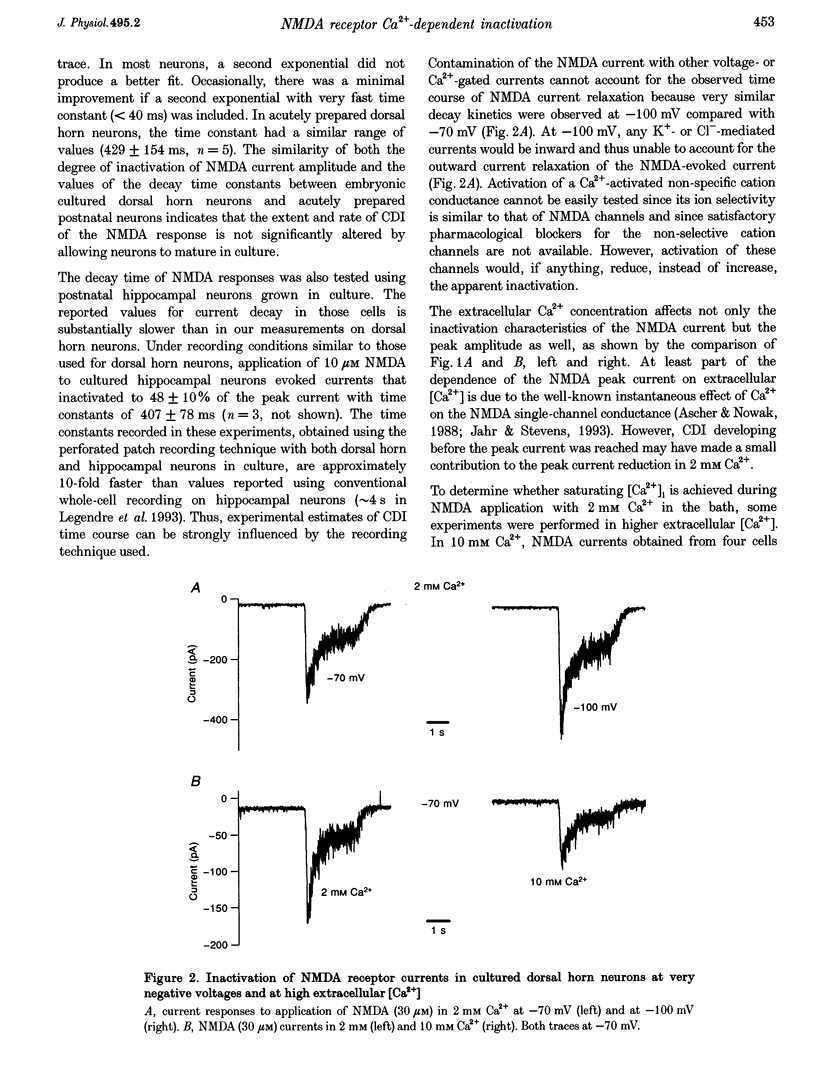
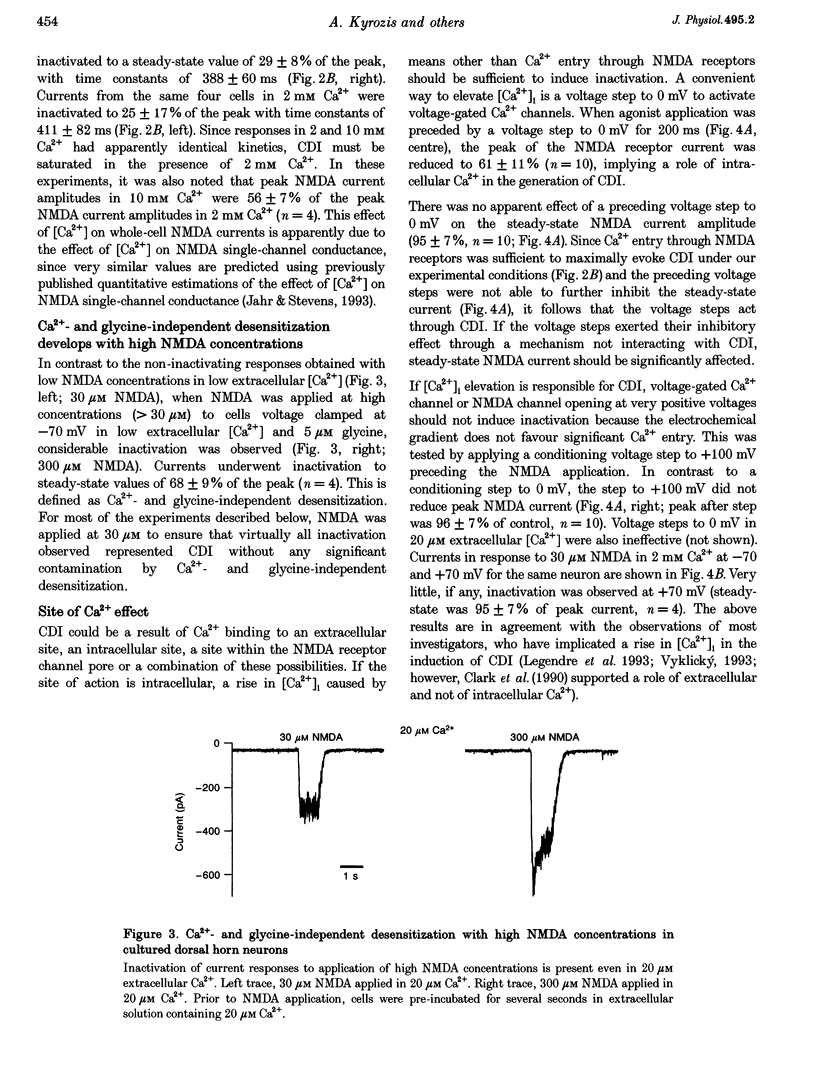
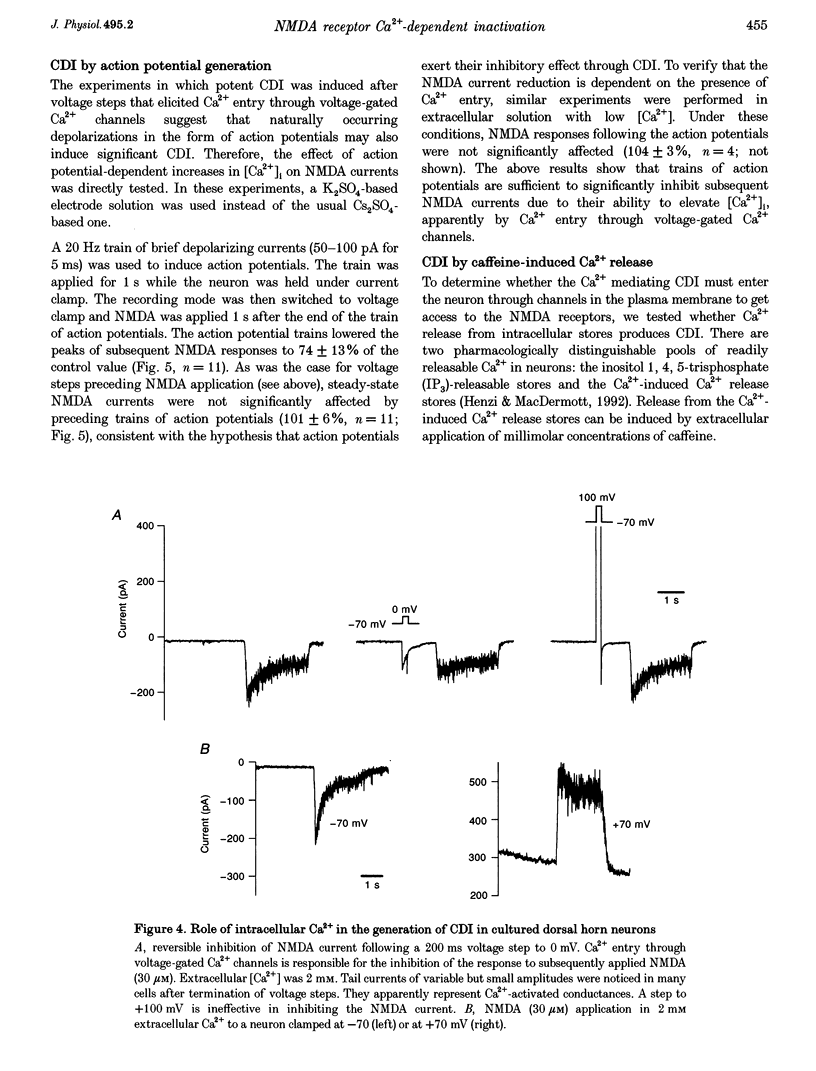
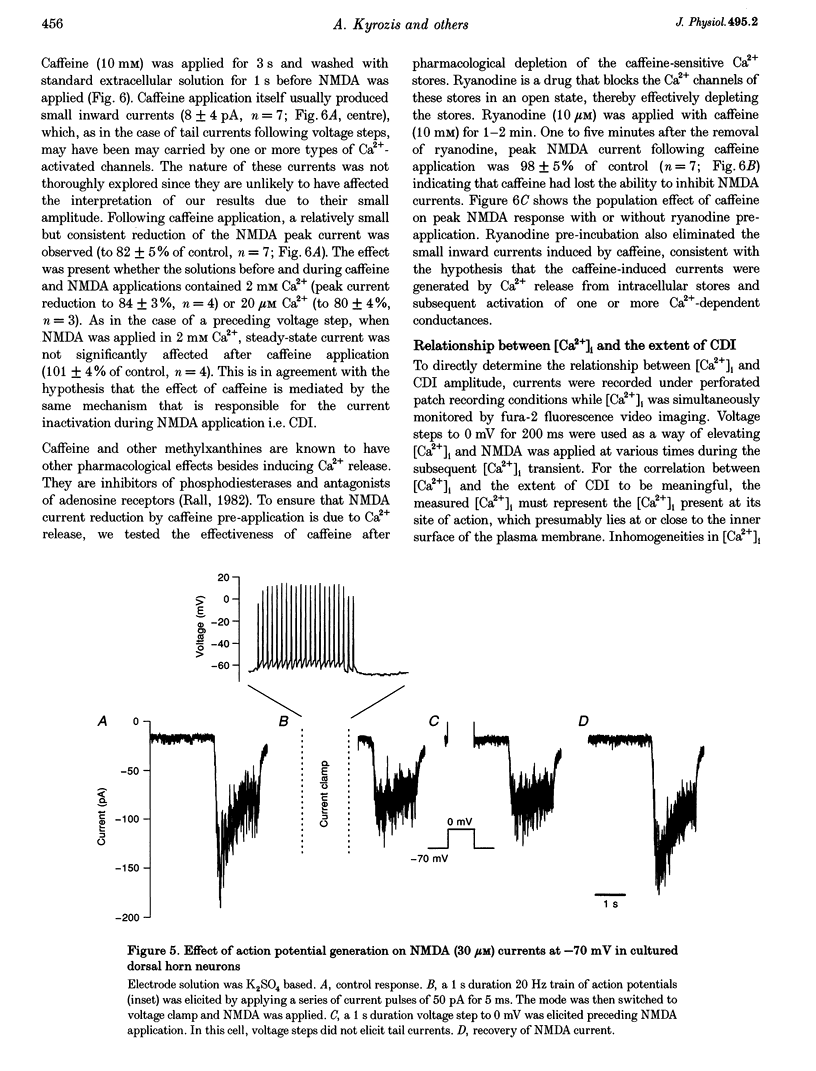
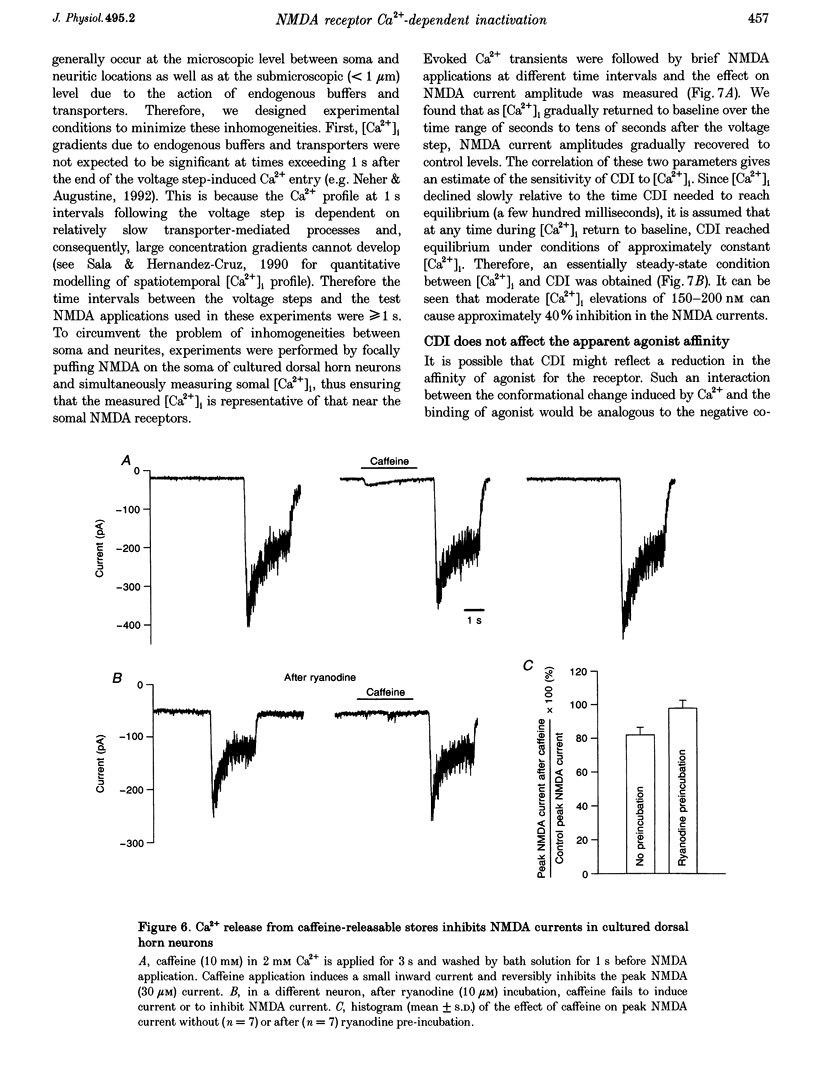
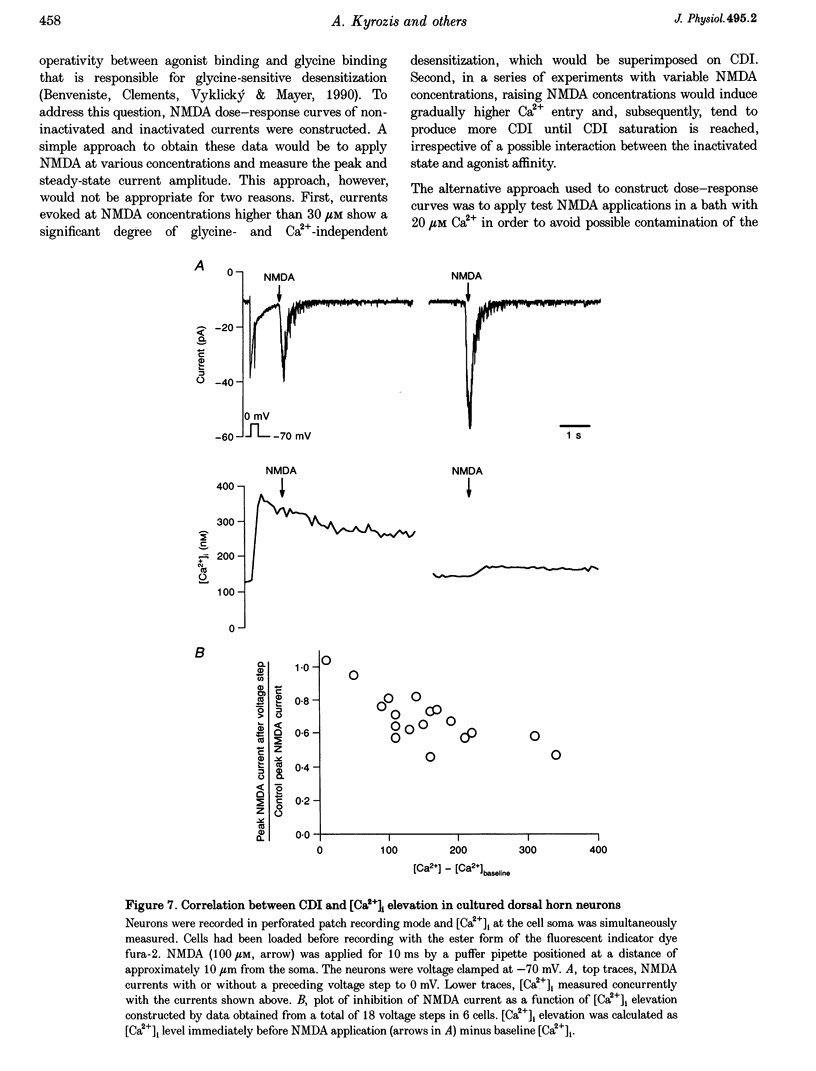
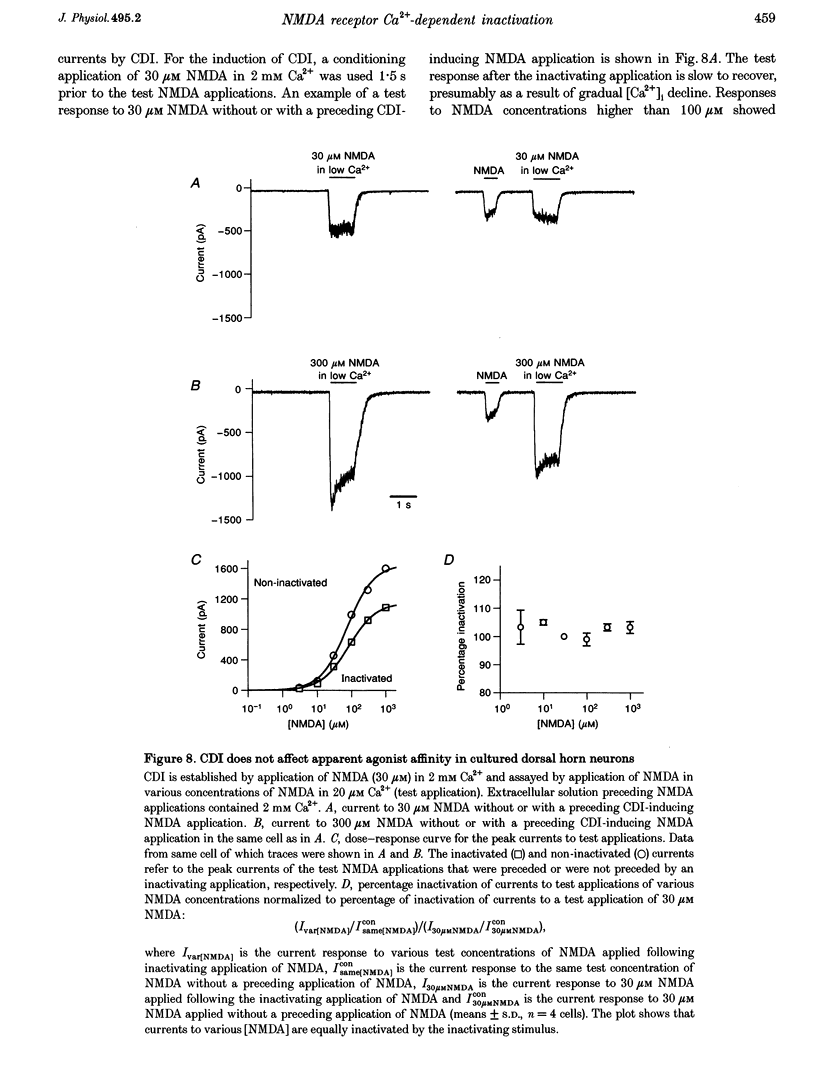
1. Ca(2+)-dependent inactivation (CDI) of NMDA receptors was studied using both cultured embryonic rat dorsal horn neurons and acutely dissociated postnatal rat dorsal horn neurons. The perforated patch recording method was employed in order to preserve intracellular Ca2+ buffers and other cellular constituents. In this way, the kinetics of intracellular Ca2+ concentration ([Ca2+]i) transients and other second messenger signalling systems were maintained in a relatively normal condition. 2. Continuous application of 30 microM NMDA to cultured dorsal horn neurons voltage clamped at -70 mV evoked currents that inactivated to about 40% of the peak value with time constants between 200 and 600 ms. CDI with similar kinetics was also observed in acutely dissociated postnatal rat dorsal horn neurons. 3. When NMDA was applied in a low (20 microM) Ca2+ bath or when dorsal horn neurons were held at +70 mV, inactivation was either very weak or absent. The peaks of NMDA currents were significantly suppressed when preceded by voltage steps to 0 mV or by evoked action potentials. The suppression was dependent on the presence of Ca2+ in the extracellular solution. Voltage steps to +100 mV were ineffective in suppressing NMDA responses. Therefore, the observed inactivation was caused by an increase in [Ca2+]i following Ca2+ entry through NMDA channels or through voltage gated Ca2+ channels. 4. Caffeine application reduced currents evoked by subsequent NMDA applications. This reduction was not dependent on the presence of extracellular Ca2+ but was abolished after incubation of the cells with ryanodine, suggesting that Ca2+ release from intracellular stores also induced CDI. 5. Simultaneous measurements of somal [Ca2+]i and of currents evoked by somal NMDA applications showed that the magnitude of CDI was correlated with [Ca2+]i levels and that [Ca2+]i elevations of 100-300 nM were usually sufficient to inactivate NMDA currents by more than 30%. 6. Dose-response curves of non inactivated and inactivated NMDA responses showed that the apparent receptor affinity for NMDA is not different under the two conditions. CDI is caused instead by non-competitive inhibition of NMDA receptors. CDI was not overcome by increasing glycine concentration, suggesting that it is not mediated by glycine dissociation from the receptor. 7. These results show that, with an intact intracellular environment, CDI in dorsal horn neurons constitutes a potent, inhibitory control of NMDA currents with a faster onset than previously demonstrated. CDI is induced by a variety of [Ca2+]i-elevating stimuli of physiological relevance including Ca2+ entry through ligand- and voltage-gated channels and Ca2+ release from intracellular stores. Our demonstration that CDI is strongly expressed in neurons maturing in vivo supports the hypothesis that CDI may regulate, in part, the postsynaptic integration of excitatory input in the mature or maturing nervous system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artola A., Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993 Nov;16(11):480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Clements J., Vyklický L., Jr, Mayer M. L. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Carmignoto G., Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992 Nov 6;258(5084):1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Clark G. D., Clifford D. B., Zorumski C. F. The effect of agonist concentration, membrane voltage and calcium on N-methyl-D-aspartate receptor desensitization. Neuroscience. 1990;39(3):787–797. doi: 10.1016/0306-4522(90)90261-2. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Lester R. A., Tong G., Jahr C. E., Westbrook G. L. The time course of glutamate in the synaptic cleft. Science. 1992 Nov 27;258(5087):1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Coan E. J., Irving A. J., Collingridge G. L. Low-frequency activation of the NMDA receptor system can prevent the induction of LTP. Neurosci Lett. 1989 Oct 23;105(1-2):205–210. doi: 10.1016/0304-3940(89)90038-4. [DOI] [PubMed] [Google Scholar]
- Enokido Y., Araki T., Aizawa S., Hatanaka H. p53 involves cytosine arabinoside-induced apoptosis in cultured cerebellar granule neurons. Neurosci Lett. 1996 Jan 12;203(1):1–4. doi: 10.1016/0304-3940(95)12247-8. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gu J. G., Albuquerque C., Lee C. J., MacDermott A. B. Synaptic strengthening through activation of Ca2+-permeable AMPA receptors. Nature. 1996 Jun 27;381(6585):793–796. doi: 10.1038/381793a0. [DOI] [PubMed] [Google Scholar]
- Henzi V., MacDermott A. B. Characteristics and function of Ca(2+)- and inositol 1,4,5-trisphosphate-releasable stores of Ca2+ in neurons. Neuroscience. 1992;46(2):251–273. doi: 10.1016/0306-4522(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992 Jun 25;357(6380):686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Hestrin S., Sah P., Nicoll R. A. Mechanisms generating the time course of dual component excitatory synaptic currents recorded in hippocampal slices. Neuron. 1990 Sep;5(3):247–253. doi: 10.1016/0896-6273(90)90162-9. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Calcium permeability of the N-methyl-D-aspartate receptor channel in hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11573–11577. doi: 10.1073/pnas.90.24.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrozis A., Goldstein P. A., Heath M. J., MacDermott A. B. Calcium entry through a subpopulation of AMPA receptors desensitized neighbouring NMDA receptors in rat dorsal horn neurons. J Physiol. 1995 Jun 1;485(Pt 2):373–381. doi: 10.1113/jphysiol.1995.sp020736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrozis A., Reichling D. B. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995 Mar;57(1):27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- Legendre P., Rosenmund C., Westbrook G. L. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci. 1993 Feb;13(2):674–684. doi: 10.1523/JNEUROSCI.13-02-00674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Segal M. Calcimycin potentiates responses of rat hippocampal neurons to N-methyl-D-aspartate. Brain Res. 1991 Feb 1;540(1-2):322–324. doi: 10.1016/0006-8993(91)90529-5. [DOI] [PubMed] [Google Scholar]
- Markram H., Segal M. The inositol 1,4,5-trisphosphate pathway mediates cholinergic potentiation of rat hippocampal neuronal responses to NMDA. J Physiol. 1992 Feb;447:513–533. doi: 10.1113/jphysiol.1992.sp019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The action of N-methyl-D-aspartic acid on mouse spinal neurones in culture. J Physiol. 1985 Apr;361:65–90. doi: 10.1113/jphysiol.1985.sp015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon S. B., Lewin G. R., Wall P. D. Central hyperexcitability triggered by noxious inputs. Curr Opin Neurobiol. 1993 Aug;3(4):602–610. doi: 10.1016/0959-4388(93)90062-4. [DOI] [PubMed] [Google Scholar]
- Medina I., Filippova N., Barbin G., Ben-Ari Y., Bregestovski P. Kainate-induced inactivation of NMDA currents via an elevation of intracellular Ca2+ in hippocampal neurons. J Neurophysiol. 1994 Jul;72(1):456–465. doi: 10.1152/jn.1994.72.1.456. [DOI] [PubMed] [Google Scholar]
- Murase K., Ryu P. D., Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989 Aug 14;103(1):56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell T. J., Hawkins R. D., Kandel E. R., Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Tank D. W. Calcium concentration dynamics produced by synaptic activation of CA1 hippocampal pyramidal cells. J Neurosci. 1992 Nov;12(11):4202–4223. doi: 10.1523/JNEUROSCI.12-11-04202.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C., Feltz A., Westbrook G. L. Calcium-dependent inactivation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol. 1995 Jan;73(1):427–430. doi: 10.1152/jn.1995.73.1.427. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Westbrook G. L. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993 May;10(5):805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W., Johnson J. W., Henderson G., Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990 May;4(5):725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklický L., Jr Calcium-mediated modulation of N-methyl-D-aspartate (NMDA) responses in cultured rat hippocampal neurones. J Physiol. 1993 Oct;470:575–600. doi: 10.1113/jphysiol.1993.sp019876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y., Uteshev V., Sokolova S., Khodorov B. Desensitization of N-methyl-D-aspartate receptors in neurons dissociated from adult rat hippocampus. Mol Pharmacol. 1991 Sep;40(3):337–341. [PubMed] [Google Scholar]


