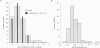Abstract
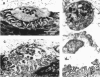
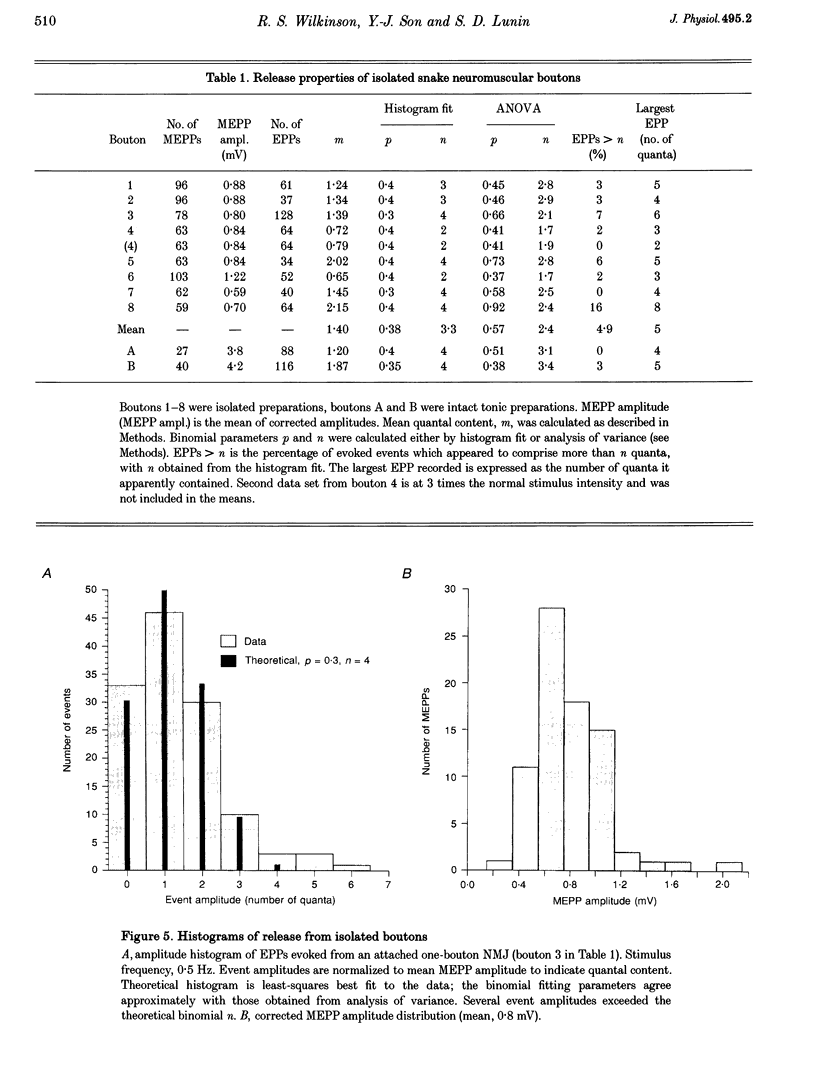
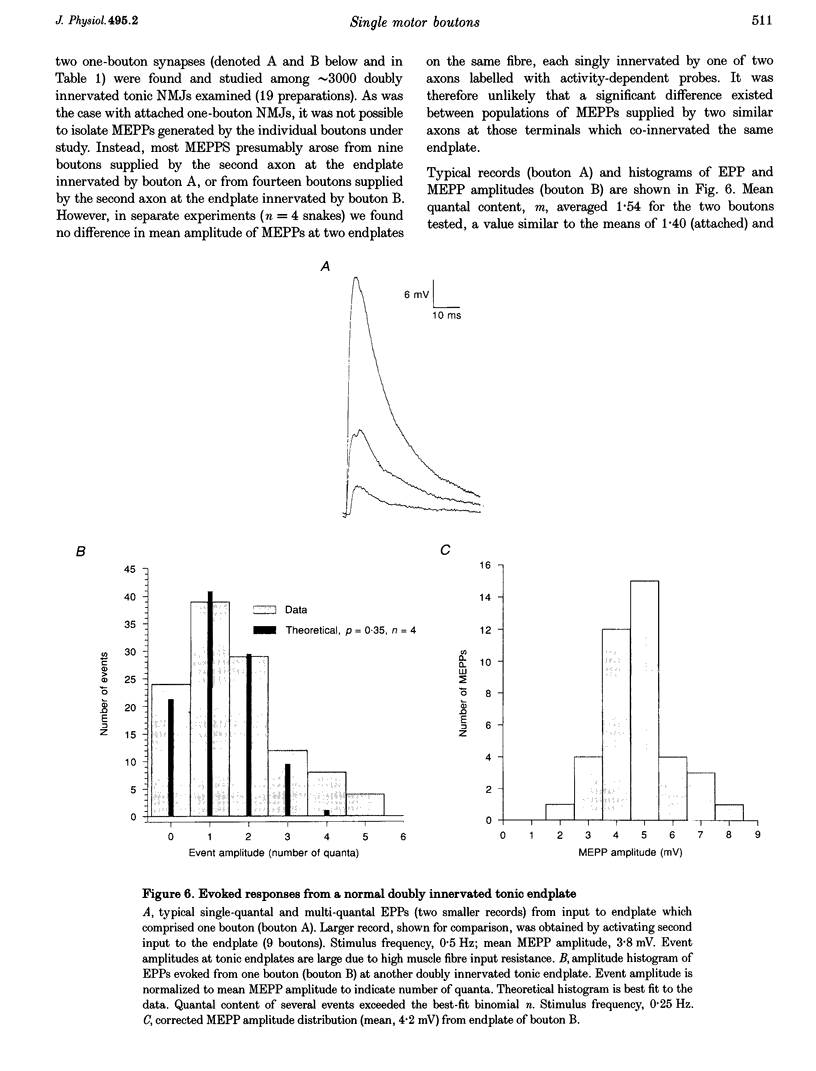
1. Motor nerve terminals innervating fibres in the transversus abdominis muscle of the garter snake comprise discrete boutons. Using a combination of enzymatic digestion and mechanical manipulation, individual boutons were removed from living terminals for study in isolation. 2. Boutons freed from terminals were usually allowed to remain in their original location on the endplate ('attached' one-bouton synapse). Alternatively, they were removed from the endplate, and then placed on the same or another vacant endplate site to form a 'reconstructed' one-bouton synapse. When removed from the endplate, boutons were 2-4 microns in diameter and nearly spherical in shape, in contrast to the variety of complex shapes seen among boutons still in contact with muscle fibre endplates. 3. Transmitter release was assessed by intracellular recording from the postsynaptic fibre. Boutons produced spontaneous miniature endplate potentials (MEPPs) of nearly normal amplitude; extracellular stimulation elicited endplate potentials (EPPs) which resembled MEPPs. Typical EPP amplitudes fluctuated between zero and five quanta per stimulus. For low-frequency stimulation under normal physiological conditions, mean quantal content, m, averaged 1.4; the binomial number of release sites, n, averaged 2.4; and the binomial probability of release, p, averaged 0.57. Statistics of the quantal fluctuations recorded from single boutons agreed only approximately with predictions of simple binomial theory, the discrepancy being that the apparent number of quanta released exceeded n in 5% of the events. 4. In separate experiments, activity-dependent probes were used to locate rare naturally occurring nerve terminals comprising a single bouton. Activation of these small synapses evoked quantal responses similar to those of attached and reconstructed one-bouton synapses described above.
Full text
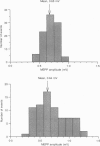
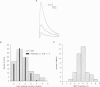
PDF


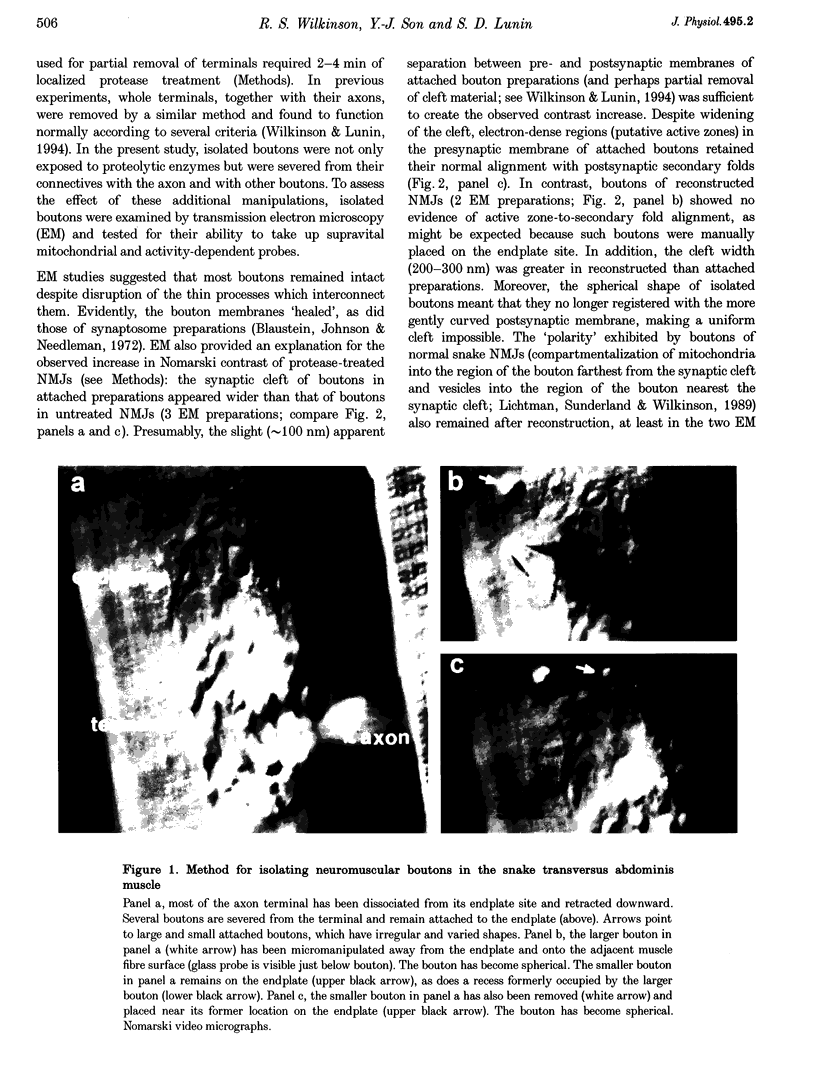
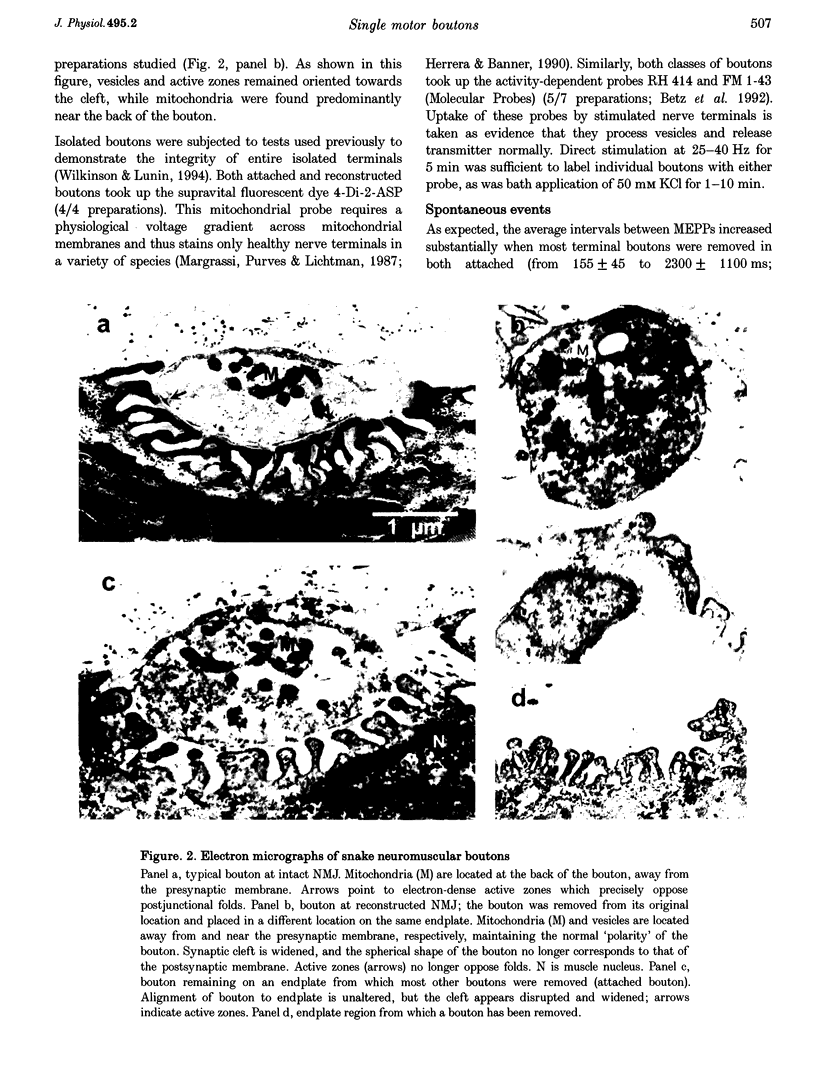
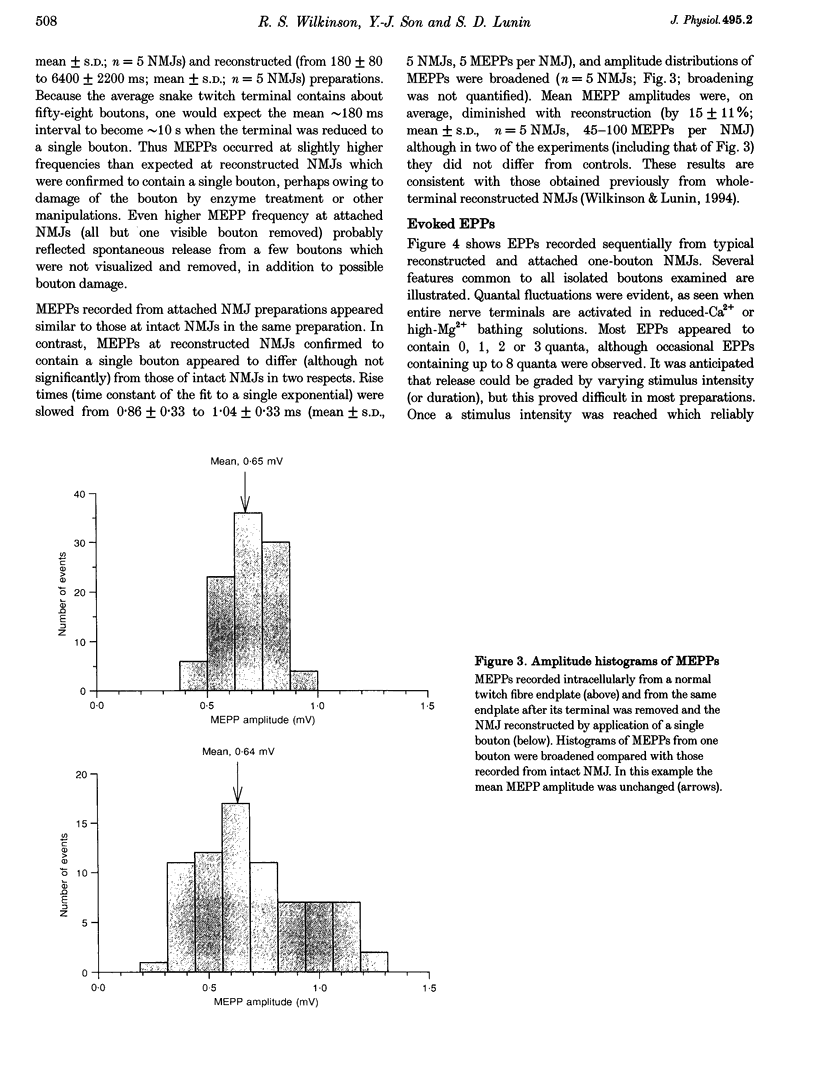
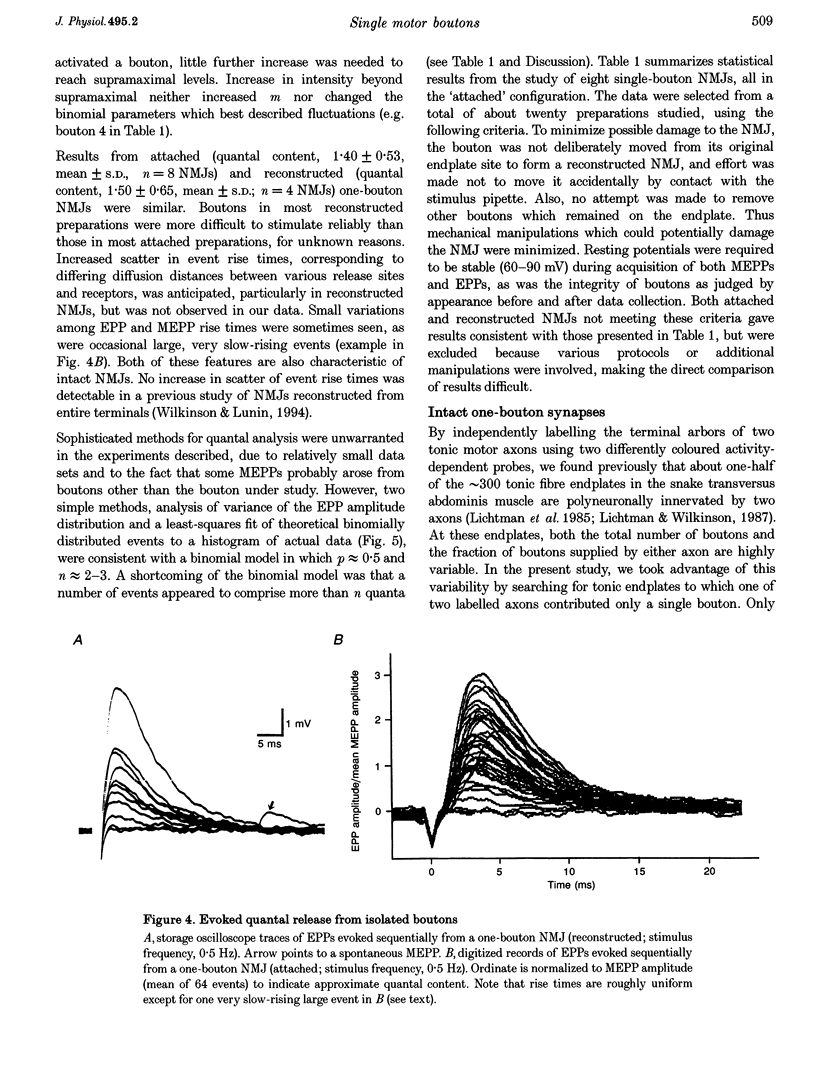



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz W. J., Mao F., Bewick G. S. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. J Neurosci. 1992 Feb;12(2):363–375. doi: 10.1523/JNEUROSCI.12-02-00363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Johnson E. M., Jr, Needleman P. Calcium-dependent norepinephrine release from presynaptic nerve endings in vitro. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2237–2240. doi: 10.1073/pnas.69.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff G. S., Ridge R. M. Innervation of extrafusal and intrafusal fibres in snake muscle. J Physiol. 1973 Aug;233(1):1–18. doi: 10.1113/jphysiol.1973.sp010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S., Tank D. W. Calcium in motor nerve terminals associated with posttetanic potentiation. J Neurosci. 1989 Oct;9(10):3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantyn R., Shapovalov A. I., Shiriaev B. I. Relation between structural and release parameters at the frog sensory-motor synapse. J Physiol. 1984 Apr;349:459–474. doi: 10.1113/jphysiol.1984.sp015167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A. A., Banner L. R. The use and effects of vital fluorescent dyes: observation of motor nerve terminals and satellite cells in living frog muscles. J Neurocytol. 1990 Feb;19(1):67–83. doi: 10.1007/BF01188440. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. Modifications to synaptic transmission at group Ia synapses on cat spinal motoneurones by 4-aminopyridine. J Physiol. 1981 Dec;321:111–126. doi: 10.1113/jphysiol.1981.sp013974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H., Faber D. S. Quantal analysis and synaptic efficacy in the CNS. Trends Neurosci. 1991 Oct;14(10):439–445. doi: 10.1016/0166-2236(91)90042-s. [DOI] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Magrassi L., Purves D. Visualization of neuromuscular junctions over periods of several months in living mice. J Neurosci. 1987 Apr;7(4):1215–1222. doi: 10.1523/JNEUROSCI.07-04-01215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Sunderland W. J., Wilkinson R. S. High-resolution imaging of synaptic structure with a simple confocal microscope. New Biol. 1989 Oct;1(1):75–82. [PubMed] [Google Scholar]
- Lichtman J. W., Wilkinson R. S. Properties of motor units in the transversus abdominis muscle of the garter snake. J Physiol. 1987 Dec;393:355–374. doi: 10.1113/jphysiol.1987.sp016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Wilkinson R. S., Rich M. M. Multiple innervation of tonic endplates revealed by activity-dependent uptake of fluorescent probes. 1985 Mar 28-Apr 3Nature. 314(6009):357–359. doi: 10.1038/314357a0. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. The statistics of transmitter release at chemical synapses. Int Rev Physiol. 1978;17:49–117. [PubMed] [Google Scholar]
- Miyamoto M. D. Probability of quantal transmitter release from nerve terminals: theoretical considerations in the determination of spatial variation. J Theor Biol. 1986 Dec 7;123(3):289–304. doi: 10.1016/s0022-5193(86)80244-2. [DOI] [PubMed] [Google Scholar]
- Nystrom R. R., Ko C. P. Disruption of active zones in frog neuromuscular junctions following treatment with proteolytic enzymes. J Neurocytol. 1988 Feb;17(1):63–71. doi: 10.1007/BF01735378. [DOI] [PubMed] [Google Scholar]
- Provan S. D., Miyamoto M. D. Unbiased estimates of quantal release parameters and spatial variation in the probability of neurosecretion. Am J Physiol. 1993 Apr;264(4 Pt 1):C1051–C1060. doi: 10.1152/ajpcell.1993.264.4.C1051. [DOI] [PubMed] [Google Scholar]
- Redman S., Walmsley B. Amplitude fluctuations in synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983 Oct;343:135–145. doi: 10.1113/jphysiol.1983.sp014885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. Post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1969 Jul;203(1):121–133. doi: 10.1113/jphysiol.1969.sp008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triller A., Korn H. Transmission at a central inhibitory synapse. III. Ultrastructure of physiologically identified and stained terminals. J Neurophysiol. 1982 Sep;48(3):708–736. doi: 10.1152/jn.1982.48.3.708. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W., Molgó J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol Rev. 1994 Oct;74(4):899–991. doi: 10.1152/physrev.1994.74.4.899. [DOI] [PubMed] [Google Scholar]
- Walrond J. P., Reese T. S. Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. J Neurosci. 1985 May;5(5):1118–1131. doi: 10.1523/JNEUROSCI.05-05-01118.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A. Estimates of statistical release parameters from crayfish and frog neuromuscular junctions. J Physiol. 1975 Jan;244(1):207–221. doi: 10.1113/jphysiol.1975.sp010792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. S., Lichtman J. W. Regular alternation of fiber types in the transversus abdominis muscle of the garter snake. J Neurosci. 1985 Nov;5(11):2979–2988. doi: 10.1523/JNEUROSCI.05-11-02979.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. S., Lunin S. D. Properties of "reconstructed" motor synapses of the garter snake. J Neurosci. 1994 May;14(5 Pt 2):3319–3332. doi: 10.1523/JNEUROSCI.14-05-03319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. S., Lunin S. D., Stevermer J. J. Regulation of single quantal efficacy at the snake neuromuscular junction. J Physiol. 1992 Mar;448:413–436. doi: 10.1113/jphysiol.1992.sp019049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. S., Nemeth P. M. Metabolic fiber types of snake transversus abdominis muscle. Am J Physiol. 1989 Jun;256(6 Pt 1):C1176–C1183. doi: 10.1152/ajpcell.1989.256.6.C1176. [DOI] [PubMed] [Google Scholar]
- Zefirov A., Benish T., Fatkullin N., Cheranov S., Khazipov R. Localization of active zones. Nature. 1995 Aug 3;376(6539):393–394. doi: 10.1038/376393b0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H., Matthews G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 1994 Feb 24;367(6465):735–739. doi: 10.1038/367735a0. [DOI] [PubMed] [Google Scholar]