Abstract
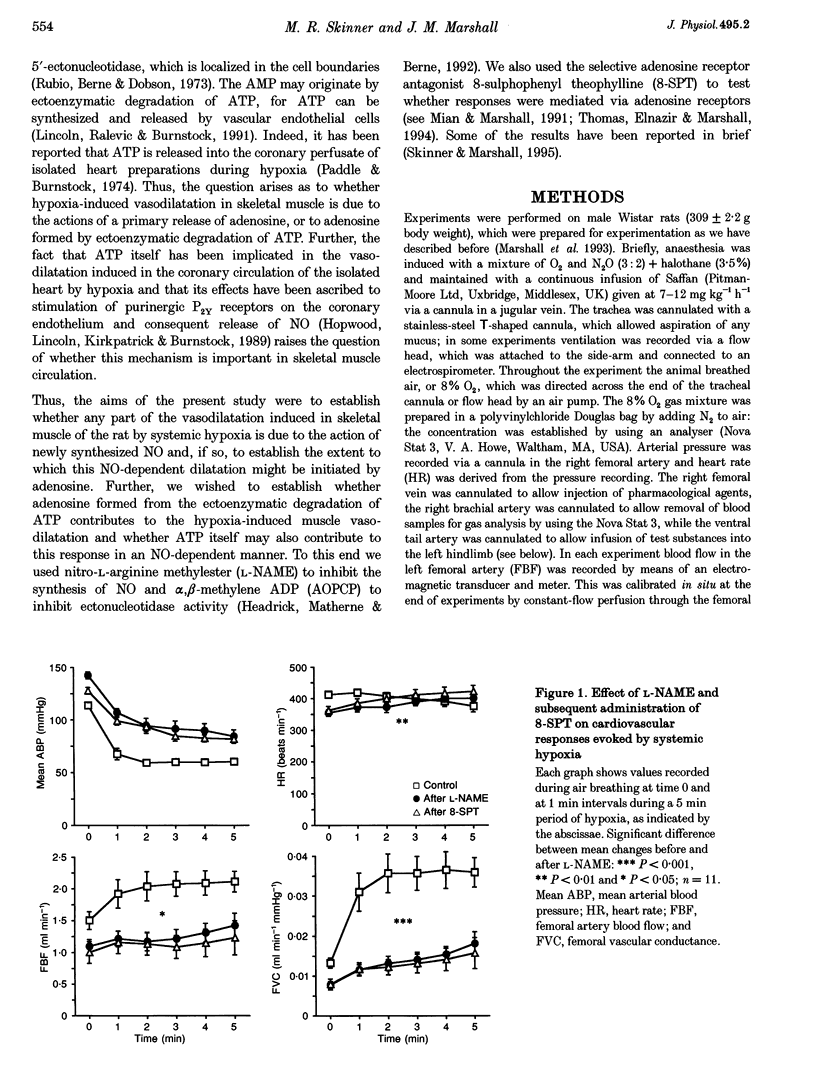
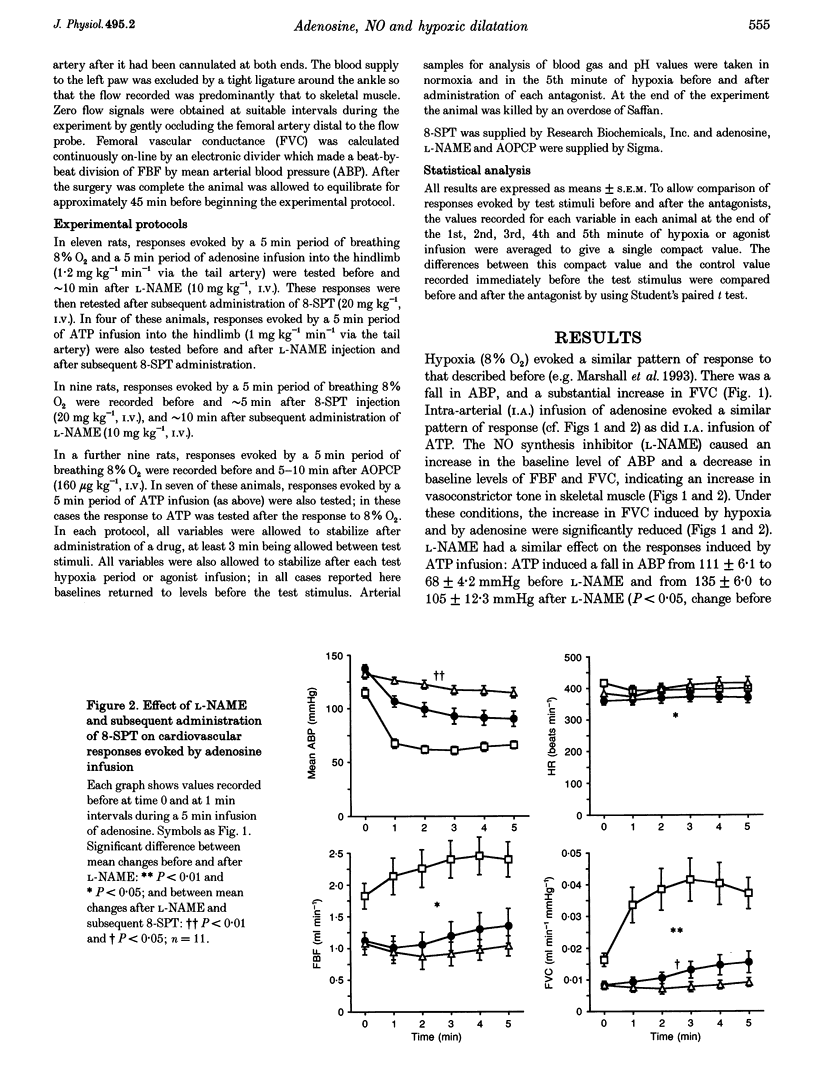
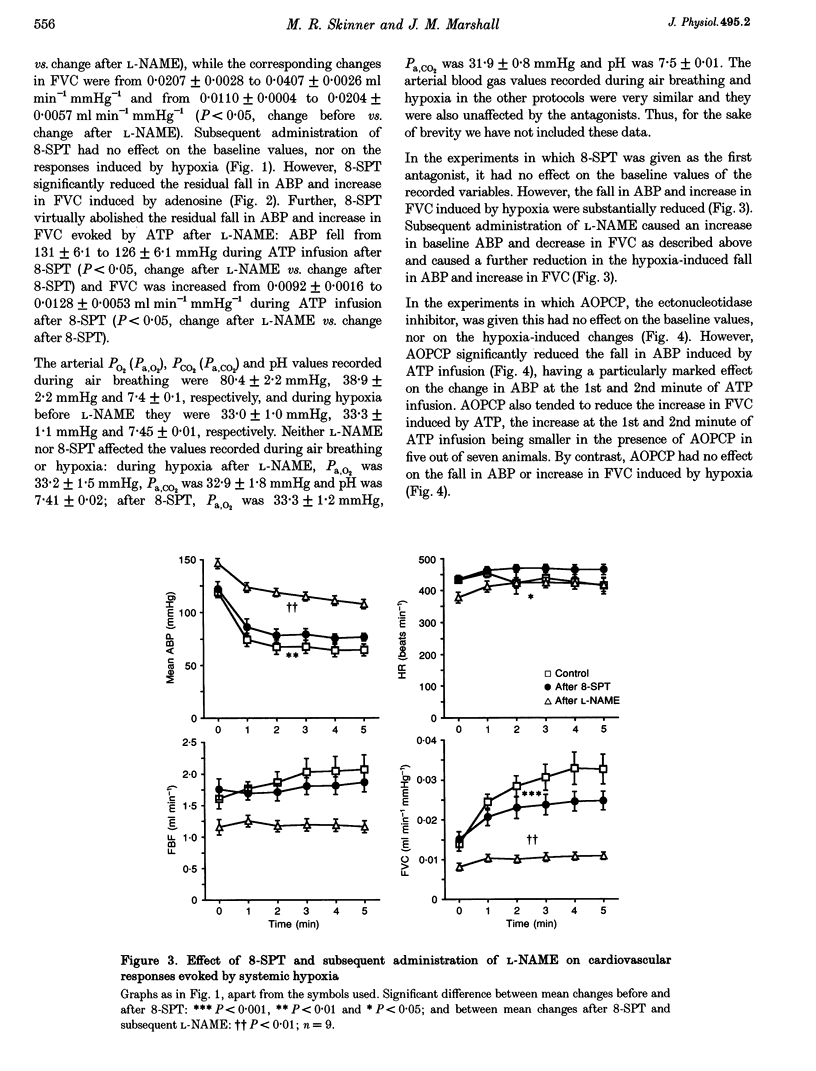
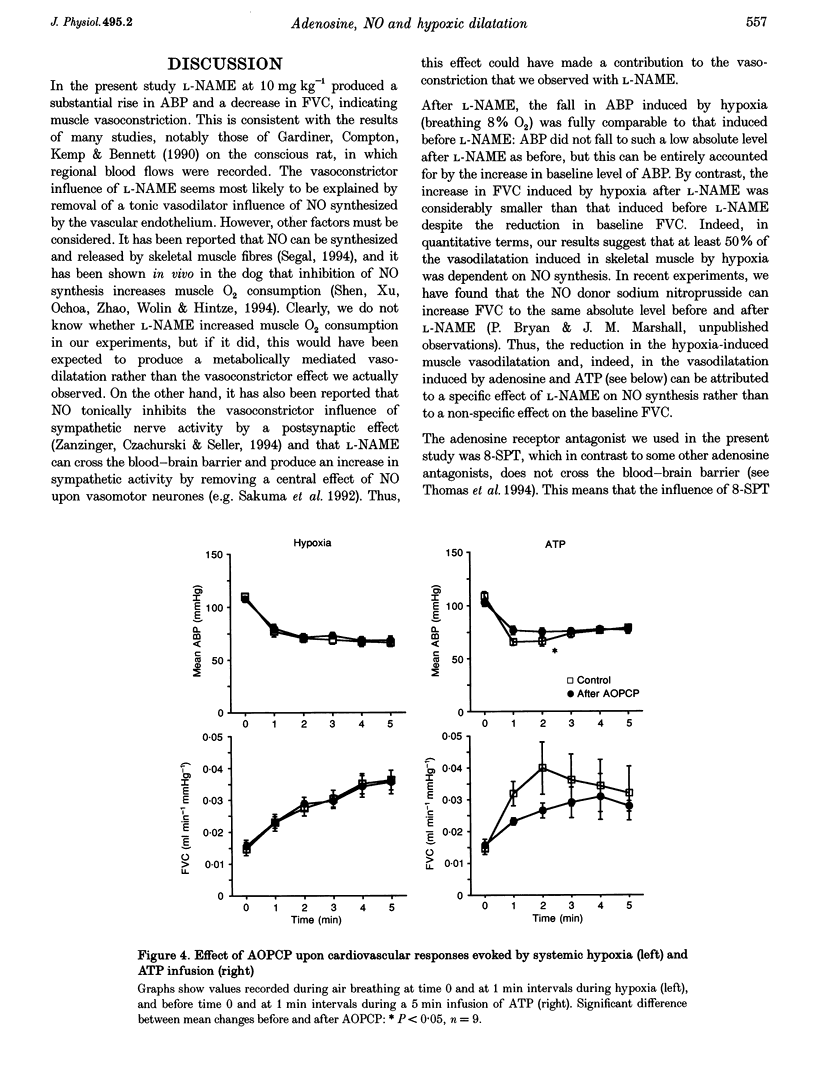
1. In Saffan-anaesthetized rats, we have further investigated the mechanisms underlying the vasodilatation induced by adenosine in skeletal muscle by acute systemic hypoxia (breathing 8% O2 for 5 min). 2. In eleven rats the nitric oxide (NO) synthesis inhibitor nitro-L-arginine methyl ester (L-NAME, 10 mg kg-1, i.v.) reduced the increase in femoral vascular conductance (FVC) induced by hypoxia by approximately 50%. L-NAME had similar effects on the increase in FVC induced by intra-arterial (I.A.) infusion of adenosine (at 1.2 mg kg-1 min-1 for 5 min via the tail artery) and by ATP (I.A., 1 mg kg-1 min-1 for 5 min). Subsequent administration of the adenosine receptor antagonist 8-sulphophenyl theophylline (8-SPT, 20 mg kg-1, i.v.) virtually abolished the adenosine- and ATP-induced increase in FVC. 3. In a further nine rats, 8-SPT reduced the increase in FVC induced by hypoxia by approximately 50%. This remaining increase in FVC was substantially reduced by L-NAME. 4. In an additional nine rats, alpha,beta-methyleneADP (160 micrograms kg-1, i.v.) which inhibits the 5'-ectonucleotidase that degrades AMP to adenosine, reduced the peripheral vasodilatation (fall in arterial blood pressure, ABP) induced by ATP infusion, but had no effect on the increase in FVC or decrease in ABP evoked by systemic hypoxia. 5. These results provide the first evidence that the muscle vasodilatation induced by adenosine during systemic hypoxia is mainly dependent on NO synthesis. They also suggest that adenosine is released as such rather than being formed extracellularly from AMP. Given evidence that extraluminal adenosine acts in an NO-independent fashion we propose that hypoxia releases adenosine from the endothelium. Our results also indicate that hypoxia induces muscle vasodilatation that is adenosine independent but NO dependent: they allow the possibility that this is partly mediated by ATP released from the endothelium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. H., Sutton E. T. Antagonism of acetylcholine and adenosine rat cremaster arteriolar vasodilation by combination of NO antagonists. Int J Microcirc Clin Exp. 1993 Jun;12(3):275–286. [PubMed] [Google Scholar]
- Deussen A., Möser G., Schrader J. Contribution of coronary endothelial cells to cardiac adenosine production. Pflugers Arch. 1986 Jun;406(6):608–614. doi: 10.1007/BF00584028. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br J Pharmacol. 1990 Nov;101(3):625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headrick J. P., Matherne G. P., Berne R. M. Myocardial adenosine formation during hypoxia: effects of ecto-5'-nucleotidase inhibition. J Mol Cell Cardiol. 1992 Mar;24(3):295–303. doi: 10.1016/0022-2828(92)93166-h. [DOI] [PubMed] [Google Scholar]
- Hopwood A. M., Lincoln J., Kirkpatrick K. A., Burnstock G. Adenosine 5'-triphosphate, adenosine and endothelium-derived relaxing factor in hypoxic vasodilatation of the heart. Eur J Pharmacol. 1989 Jun 20;165(2-3):323–326. doi: 10.1016/0014-2999(89)90730-9. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Delbro D., Burnstock G. P2-purinoceptors mediate both vasodilation (via the endothelium) and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol. 1985 Jan 2;107(2):161–168. doi: 10.1016/0014-2999(85)90055-x. [DOI] [PubMed] [Google Scholar]
- Marshall J. M., Thomas T., Turner L. A link between adenosine, ATP-sensitive K+ channels, potassium and muscle vasodilatation in the rat in systemic hypoxia. J Physiol. 1993 Dec;472:1–9. doi: 10.1113/jphysiol.1993.sp019931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian R., Marshall J. M. The role of adenosine in dilator responses induced in arterioles and venules of rat skeletal muscle by systemic hypoxia. J Physiol. 1991 Nov;443:499–511. doi: 10.1113/jphysiol.1991.sp018847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian R., Marshall J. M. The role of adenosine in mediating vasodilatation in mesenteric circulation of the rat in acute and chronic hypoxia. J Physiol. 1995 Nov 15;489(Pt 1):225–234. doi: 10.1113/jphysiol.1995.sp021044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody C. J., Meghji P., Burnstock G. Stimulation of P1-purinoceptors by ATP depends partly on its conversion to AMP and adenosine and partly on direct action. Eur J Pharmacol. 1984 Jan 13;97(1-2):47–54. doi: 10.1016/0014-2999(84)90511-9. [DOI] [PubMed] [Google Scholar]
- Paddle B. M., Burnstock G. Release of ATP from perfused heart during coronary vasodilatation. Blood Vessels. 1974;11(3):110–119. doi: 10.1159/000158005. [DOI] [PubMed] [Google Scholar]
- Pohl U., Dézsi L., Simon B., Busse R. Selective inhibition of endothelium-dependent dilation in resistance-sized vessels in vivo. Am J Physiol. 1987 Aug;253(2 Pt 2):H234–H239. doi: 10.1152/ajpheart.1987.253.2.H234. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., Standen N. B. KATP channels in vascular smooth muscle. Cardiovasc Res. 1994 Jun;28(6):797–804. doi: 10.1093/cvr/28.6.797. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Togashi H., Yoshioka M., Saito H., Yanagida M., Tamura M., Kobayashi T., Yasuda H., Gross S. S., Levi R. NG-methyl-L-arginine, an inhibitor of L-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ Res. 1992 Mar;70(3):607–611. doi: 10.1161/01.res.70.3.607. [DOI] [PubMed] [Google Scholar]
- Segal S. S. "Nitric oxide release is present from incubated skeletal muscle preparations". J Appl Physiol (1985) 1994 Dec;77(6):2517–2518. doi: 10.1152/jappl.1994.77.6.2517. [DOI] [PubMed] [Google Scholar]
- Shen W., Xu X., Ochoa M., Zhao G., Wolin M. S., Hintze T. H. Role of nitric oxide in the regulation of oxygen consumption in conscious dogs. Circ Res. 1994 Dec;75(6):1086–1095. doi: 10.1161/01.res.75.6.1086. [DOI] [PubMed] [Google Scholar]
- Taylor E. M., Parsons M. E., Wright P. W., Pipkin M. A., Howson W. The effects of adenosine triphosphate and related purines on arterial resistance vessels in vitro and in vivo. Eur J Pharmacol. 1989 Feb 28;161(2-3):121–133. doi: 10.1016/0014-2999(89)90834-0. [DOI] [PubMed] [Google Scholar]
- Thomas T., Elnazir B. K., Marshall J. M. Differentiation of the peripherally mediated from the centrally mediated influences of adenosine in the rat during systemic hypoxia. Exp Physiol. 1994 Sep;79(5):809–822. doi: 10.1113/expphysiol.1994.sp003809. [DOI] [PubMed] [Google Scholar]
- Zanzinger J., Czachurski J., Seller H. Inhibition of sympathetic vasoconstriction is a major principle of vasodilation by nitric oxide in vivo. Circ Res. 1994 Dec;75(6):1073–1077. doi: 10.1161/01.res.75.6.1073. [DOI] [PubMed] [Google Scholar]


