Abstract
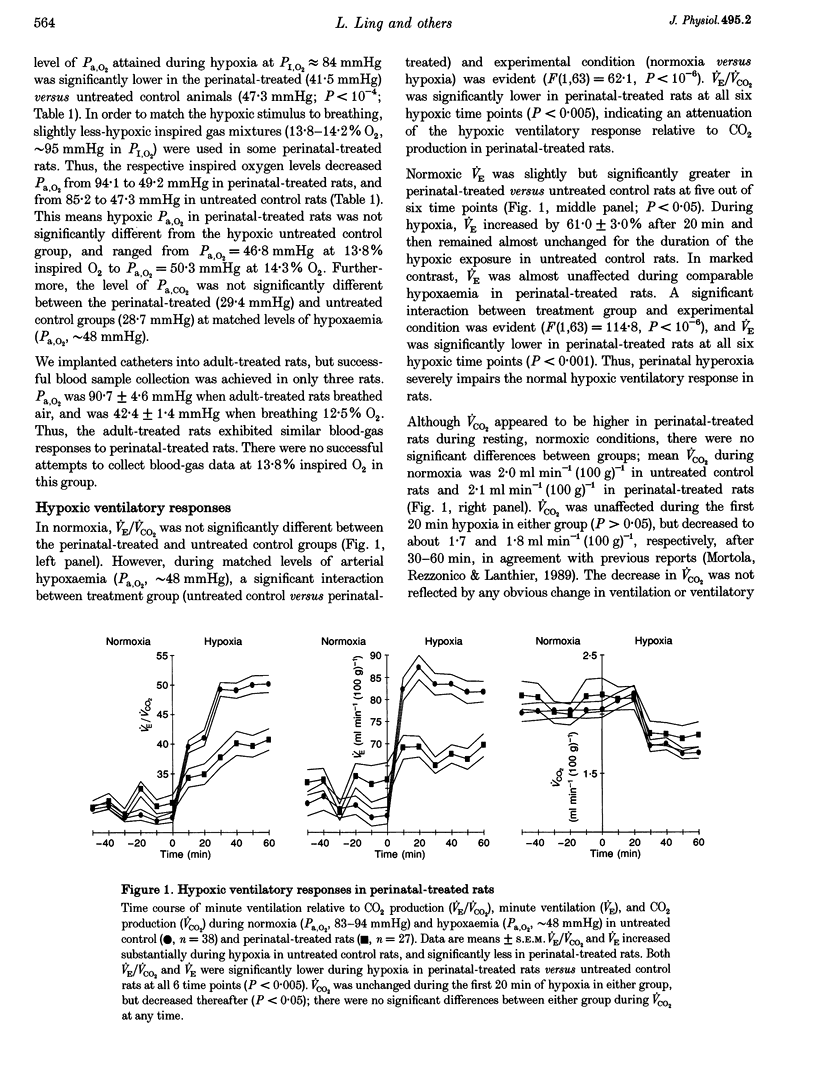
1. This study was designed to test the hypothesis that perinatal suppression of peripheral arterial chemoreceptor inputs attenuates the hypoxic ventilatory response in adult rats. Perinatal suppression of peripheral chemoreceptor activity was achieved by exposing rats to hyperoxia throughout the first month of life. 2. Late-gestation pregnant rats were housed in a 60% O2 environment, exposing the pups to hyperoxia from several days prior to birth until they were returned to normoxia on postnatal day 28. These perinatally treated rats were then reared to adulthood (3-5 months old) in normoxia. In addition to the mother rats, adult male rats were also exposed to hyperoxia, creating an adult-treated control group. Two to four months after the hyperoxic exposure, treated rats were compared with untreated male rats of similar age. 3. A whole-body, flow-through plethysmograph was used to measure hypoxic and hypercapnic ventilatory responses of the unanaesthetized adult rats. In moderate hypoxia (arterial oxygen partial pressure, Pa,O2 approximately 48 mmHg). VE (minute ventilation) and the ratio VE/VCO2 (ventilation relative to CO2 production) increased by 16.7 +/- 4.0 and 35.4 +/- 3.4%, respectively, in perinatal-treated rats (means +/- S.E.M.), but increased more in untreated control rats (51.4 +/- 2.8 and 83.1 +/- 4.3%; both P < 10(-6)). 4. In contrast to the impaired hypoxic ventilatory response, ventilatory responses to hypercapnia (5% CO2) were similar between untreated control and perinatal-treated rats. 5. Impaired hypoxic responsiveness was unique to the perinatal-treated rats since hypoxic ventilatory responses were not attenuated in adult-treated rats. 6. The results indicate that ventilatory responses to hypoxaemia are greatly attenuated in adult rats that had experienced hyperoxia during their first month of life, and suggest that normal hypoxic ventilatory control mechanisms are susceptible to developmental plasticity.
Full text
PDF








Images in this article
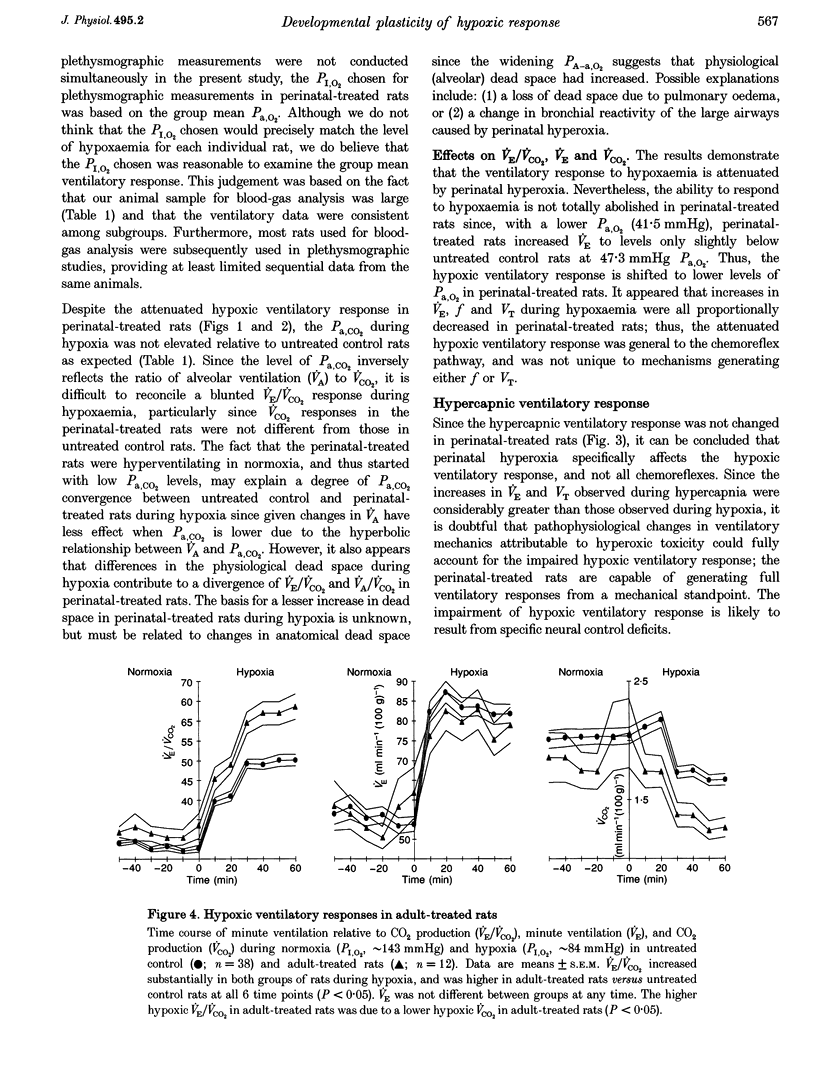
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanco C. E., Hanson M. A., McCooke H. B. Effects on carotid chemoreceptor resetting of pulmonary ventilation in the fetal lamb in utero. J Dev Physiol. 1988 Apr;10(2):167–174. [PubMed] [Google Scholar]
- Bureau M. A., Lamarche J., Foulon P., Dalle D. Postnatal maturation of respiration in intact and carotid body-chemodenervated lambs. J Appl Physiol (1985) 1985 Sep;59(3):869–874. doi: 10.1152/jappl.1985.59.3.869. [DOI] [PubMed] [Google Scholar]
- Calder N. A., Williams B. A., Smyth J., Boon A. W., Kumar P., Hanson M. A. Absence of ventilatory responses to alternating breaths of mild hypoxia and air in infants who have had bronchopulmonary dysplasia: implications for the risk of sudden infant death. Pediatr Res. 1994 Jun;35(6):677–681. doi: 10.1203/00006450-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Charbonneau P., Brun M., Azoulay E., Bernaudin J. F., Blayo M. C., Pocidalo J. J. Respiratory and non-respiratory causes of death in the rat exposed to normobaric oxygen. Bull Eur Physiopathol Respir. 1982 Jul-Aug;18(4):633–642. [PubMed] [Google Scholar]
- DRORBAUGH J. E., FENN W. O. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955 Jul;16(1):81–87. [PubMed] [Google Scholar]
- Donnelly D. F., Haddad G. G. Prolonged apnea and impaired survival in piglets after sinus and aortic nerve section. J Appl Physiol (1985) 1990 Mar;68(3):1048–1052. doi: 10.1152/jappl.1990.68.3.1048. [DOI] [PubMed] [Google Scholar]
- Dotta A., Mortola J. P. Postnatal development of the denervated lung in normoxia, hypoxia, or hyperoxia. J Appl Physiol (1985) 1992 Oct;73(4):1461–1466. doi: 10.1152/jappl.1992.73.4.1461. [DOI] [PubMed] [Google Scholar]
- Frank L. Developmental aspects of experimental pulmonary oxygen toxicity. Free Radic Biol Med. 1991;11(5):463–494. doi: 10.1016/0891-5849(91)90062-8. [DOI] [PubMed] [Google Scholar]
- Hofer M. A. Lethal respiratory disturbance in neonatal rats after arterial chemoreceptor denervation. Life Sci. 1984 Jan 30;34(5):489–496. doi: 10.1016/0024-3205(84)90505-8. [DOI] [PubMed] [Google Scholar]
- Hofer M. A. Role of carotid sinus and aortic nerves in respiratory control of infant rats. Am J Physiol. 1986 Oct;251(4 Pt 2):R811–R817. doi: 10.1152/ajpregu.1986.251.4.R811. [DOI] [PubMed] [Google Scholar]
- Hofer M. A. Sleep-wake state organization in infant rats with episodic respiratory disturbance following sinoaortic denervation. Sleep. 1985;8(1):40–48. doi: 10.1093/sleep/8.1.40. [DOI] [PubMed] [Google Scholar]
- King A. J., Moore D. R. Plasticity of auditory maps in the brain. Trends Neurosci. 1991 Jan;14(1):31–37. doi: 10.1016/0166-2236(91)90181-s. [DOI] [PubMed] [Google Scholar]
- Lahiri S., Mokashi A., Shirahata M., Andronikou S. Chemical respiratory control in chronically hyperoxic cats. Respir Physiol. 1990 Nov;82(2):201–215. doi: 10.1016/0034-5687(90)90035-w. [DOI] [PubMed] [Google Scholar]
- Lahiri S., Mulligan E., Andronikou S., Shirahata M., Mokashi A. Carotid body chemosensory function in prolonged normobaric hyperoxia in the cat. J Appl Physiol (1985) 1987 May;62(5):1924–1931. doi: 10.1152/jappl.1987.62.5.1924. [DOI] [PubMed] [Google Scholar]
- Liberzon I., Arieli R., Kerem D. Attenuation of hypoxic ventilation by hyperbaric O2: effects of pressure and exposure time. J Appl Physiol (1985) 1989 Feb;66(2):851–856. doi: 10.1152/jappl.1989.66.2.851. [DOI] [PubMed] [Google Scholar]
- Mokashi A., Lahiri S. Aortic and carotid body chemoreception in prolonged hyperoxia in the cat. Respir Physiol. 1991 Nov;86(2):233–243. doi: 10.1016/0034-5687(91)90083-u. [DOI] [PubMed] [Google Scholar]
- Mortola J. P., Rezzonico R., Lanthier C. Ventilation and oxygen consumption during acute hypoxia in newborn mammals: a comparative analysis. Respir Physiol. 1989 Oct;78(1):31–43. doi: 10.1016/0034-5687(89)90140-0. [DOI] [PubMed] [Google Scholar]
- Okubo S., Mortola J. P. Long-term respiratory effects of neonatal hypoxia in the rat. J Appl Physiol (1985) 1988 Mar;64(3):952–958. doi: 10.1152/jappl.1988.64.3.952. [DOI] [PubMed] [Google Scholar]
- Olson E. B., Jr, Dempsey J. A., McCrimmon D. R. Serotonin and the control of ventilation in awake rats. J Clin Invest. 1979 Aug;64(2):689–693. doi: 10.1172/JCI109510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. B., Jr, Dempsey J. A. Rat as a model for humanlike ventilatory adaptation to chronic hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1978 May;44(5):763–769. doi: 10.1152/jappl.1978.44.5.763. [DOI] [PubMed] [Google Scholar]
- Olson E. B., Jr Physiological dead space increases during initial hours of chronic hypoxemia with or without hypocapnia. J Appl Physiol (1985) 1994 Sep;77(3):1526–1531. doi: 10.1152/jappl.1994.77.3.1526. [DOI] [PubMed] [Google Scholar]
- Reinstorff D., Fenner A. Ventilatory response to hyperoxia in premature and newborn infants during the first three days of life. Respir Physiol. 1972 Jun;15(2):159–165. doi: 10.1016/0034-5687(72)90095-3. [DOI] [PubMed] [Google Scholar]
- Thomas A. J., Austin W., Friedman L., Strohl K. P. A model of ventilatory instability induced in the unrestrained rat. J Appl Physiol (1985) 1992 Oct;73(4):1530–1536. doi: 10.1152/jappl.1992.73.4.1530. [DOI] [PubMed] [Google Scholar]
- Torbati D., Wafapoor H., Peyman G. A. Hyperbaric oxygen tolerance in newborn mammals--hypothesis on mechanisms and outcome. Free Radic Biol Med. 1993 Jun;14(6):695–703. doi: 10.1016/0891-5849(93)90152-k. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N. Postnatal development of the visual cortex and the influence of environment. Nature. 1982 Oct 14;299(5884):583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]




