Abstract
Acute kidney injury (AKI) secondary to severe falciparum malaria possesses a high mortality rate; however, a prognostic marker of renal dysfunction has not yet been identified. Thus, we reported a case of a patient with AKI secondary to falciparum malaria who underwent hemodialysis and a renal biopsy due to prolonged renal dysfunction. The male patient, in his 50 s, presented to our hospital with vomiting, diarrhea, fever, and decreased level of consciousness. The Giemsa-stained peripheral blood film revealed approximately 5% parasitemia, and a rapid diagnostic test was positive for Plasmodium falciparum. He was diagnosed with severe falciparum malaria and was started on quinine hydrochloride. Hemodialysis was initiated due to the decreased urine output and fluid retention. Subsequently, he was weaned off hemodialysis. The histopathological analysis of a renal biopsy revealed interstitial fibrosis, tubular atrophy, and chronic inflammatory cell infiltration; thus, malarial nephropathy was diagnosed. Thereafter, his renal function stabilized, and he was discharged from the hospital. The urinary liver-type fatty acid-binding protein (L-FABP) level decreased before renal function improved. Our report highlighted that long-term follow-up is essential for severe AKI secondary to malaria. The urinary L-FABP level may be a useful prognostic indicator of AKI secondary to severe falciparum malaria.
Keywords: Falciparum malaria, Hemodialysis, Urinary liver-type fatty acid-binding protein, Plasmodium falciparum, Malarial nephropathy
Introduction
Malaria is endemic in > 100 countries, and the World Health Organization (WHO) estimates that > 240 million cases and > 600,000 mortalities occur annually [1]. Most tropical and subtropical regions of the world are malaria-endemic areas; and the disease is transmitted through the bite of an infective female Anopheles mosquito, which carries these malarial protozoan parasites. In Japan, before the COVID-19 pandemic, approximately 50–100 cases were reported annually [1] as imported infections.
Falciparum malaria is a serious public health problem responsible for approximately 200,000 mortalities worldwide [2]. Prophylactic medications, including mefloquine hydrochloride or atovaquone-proguanil, are recommended for travel to malaria-endemic areas. If not treated within 24 h of onset, the outcomes of this disease are severe, causing acute kidney injury (AKI) with secondary hyperkalemia and hyponatremia, metabolic acidosis, encephalopathy, pulmonary edema, acute respiratory distress syndrome, disseminated intravascular coagulation, anemia, thrombocytopenia, and hypoglycemia. AKI is a known complication, particularly of severe falciparum malaria, in which 58% of patients develop AKI [3]. AKI secondary to malaria is associated with a high mortality rate; however, a prognostic marker of renal dysfunction has not yet been identified. Moreover, few reports exist regarding dialysis and renal biopsies for patients with severe malaria. Consequently, in this study, we report a case of severe AKI, secondary to falciparum malaria who underwent hemodialysis and a renal biopsy due to prolonged renal dysfunction.
Case report
A male patient in his 50 s presented to the initial hospital with vomiting and diarrhea. He had been working as a resident representative in Africa for a year. There was no remarkable history until he boarded the plane to return home, during which he vomited three times. The vomiting continued after his return to Japan. At dawn on the day after his return, he had repeated episodes of diarrhea and incontinence and was unable to move; hence, an ambulance was called. He presented with a fever of 40.4 °C, a decreased level of consciousness, and a reduced peripheral blood oxygen saturation of 93% on room air. A Giemsa-stained peripheral blood film revealed approximately 5% of ring-form trophozoites and a rapid diagnostic test was positive for Plasmodium falciparum (P. falciparum); therefore, a working diagnosis of severe falciparum malaria was made.
The patient was subsequently transferred to our hospital for treatment. On admission, the body temperature; pulse; blood pressure; and respiratory rate were E3V5M6, 40.4 °C, 140 beats/min and regular, 136/80 mmHg, and 24 breaths/min, respectively. The patient was responsive with coherent speech; however, he showed a delayed response, was drowsy, and closed his eyes when not being spoken to. The palpebral conjunctivae were yellowish-in-color and nonanemic. Xerostomia of the oral cavity was noted; however, hemorrhage and a lichenoid reaction were absent. Bilateral submandibular and subclavian lymphadenopathy were palpated. Auscultation of the respiratory system revealed normal pulmonary and cardiac sounds. Furthermore, on palpation, the abdomen was soft and tender, with the absence of hepatosplenomegaly. Edema, skin rashes, and purpura of the lower extremities were not observed. Yellow skin discoloration was absent.
The results of the urinary and blood tests are presented in Table 1. Proteinuria and occult hematuria were observed. Granular casts were present, however, erythrocyte casts were not. Thrombocytopenia as well as hepatic and renal dysfunction with secondary hyponatremia were observed. An arterial blood gas test revealed the presence of acidosis. Severe malaria is defined as a parasitemia of 10% or more[4]. Although this case had a parasitemia of 5%, it was considered severe because it was complicated by hyperbilirubinemia and renal impairment. Culture test results were negative. Simple computed tomography revealed enlargement of the spleen and bilateral kidneys.
Table 1.
Laboratory test results from the day of admission
| On admission | Normal range | |
|---|---|---|
| White blood cell (/μL) | 11 200 | 3300–8600 |
| Erythrocyte (× 106/μL) | 4.9 | 4.3–5.5 |
| Hemoglobin (g/dL) | 14.6 | 13.7–16.8 |
| Platelet (× 103/μL) | 14 | 155–350 |
| Total protein (g/dL) | 5.2 | 6.9–8.4 |
| Albumin (g/dL) | 2.9 | 4.1–5.1 |
| Urea (mg/dL) | 29 | 8–20 |
| Creatinine (mg/dL) | 1.74 | 0.6–1.0 |
| C-reactive protein (mg/dL) | 14.4 | < 0.3 |
| Aspartate aminotransferase (IU/L) | 228 | 11–38 |
| Alanine aminotransferase (IU/L) | 145 | 10–42 |
| Sodium (mEq/L) | 122 | 138–145 |
| Potassium (mEq/L) | 3.4 | 3.6–4.8 |
| Chloride (mEq/L) | 87 | 101–108 |
| Calcium (mg/dL) | 6.6 | 8.8–10.1 |
| Inorganic phosphorus (mg/dL) | 4.1 | 2.7–4.6 |
| Lactate dehydrogenase (IU/L) | 1078 | 103–190 |
| Alkaline phosphatase (U/L) | 83 | 38–113 |
| Total bilirubin (mg/dL) | 4.5 | 0.4–1.5 |
| Prothrombin time (%) | 74 | > 75 |
| Activated partial thromboplastin time (s) | 47 | 25–35 |
| D dimers (μg/mL) | 119.5 | < 0.99 |
| Hematuria | 3 + | Negative |
| Proteinuria | 2 + | Negative |
| Urinary leukocytes (/HPF) | 1–4 | < 10 |
| Urinary erythrocytes (/HPF) | 1–4 | < 5 |
| Urinary protein (g/gCr) | 1.05 | < 0.15 |
| Arterial blood gas: pH | 7.43 | 7.35–7.45 |
| Arterial blood gas: PaCO2 (mm Hg) | 28.5 | 35–45 |
| Arterial blood gas: PaO2 (mm Hg) | 64.3 | 80–100 |
| Arterial blood gas: HCO3 (mmol/L) | 19 | 22.5–26.9 |
| Arterial blood gas: lactate dehydrogenase (mmol/L) | 4.1 | 0.4–1.8 |
| Anion gap (mmol/L) | 10.3 | 8–16 |
HCO3 bicarbonate, HPF high power field, PaCO2 partial pressure of carbon dioxide, PaO2 partial pressure of oxygen
The patient was diagnosed with AKI secondary to severe malaria evidenced by a high parasitemia of P. falciparum. On the first day of admission, treatment was initiated with intravenous quinine hydrochloride after obtaining informed consent to participate in the study “Efficacy and safety of injectable quinine in malaria patients (injectable quinine for malaria)” by the Development of Optimal Medical Care Network for the Diagnosis and Treatment of Tropical and Parasitic Diseases in Japan. The first dose was 1000 mg intravenously, followed by 500 mg, every 8 h. On the third day, the parasitemia rate decreased to 0.2%. The patient’s nausea had improved considerably, and he could tolerate oral artemisinin-combination therapy; thus, quinine hydrochloride was discontinued, as it was theorized that it had resulted in renal dysfunction. The patient was treated with an artemisinin-based combination therapy comprising a 160 mg twice daily oral dosage of artemisinin–lumefantrine combination tablets for 3 days as per the WHO’s guidelines [4].
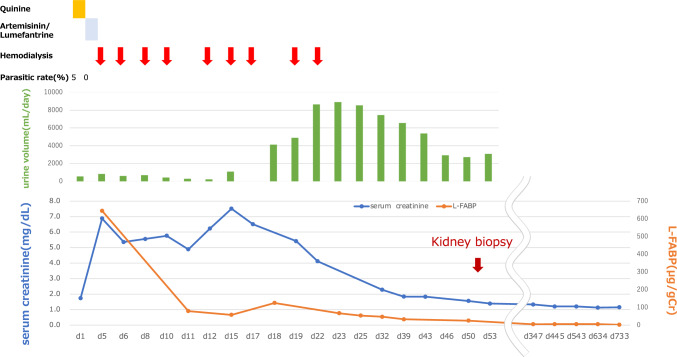
Antimalarial therapy was terminated when the infected erythrocytes were confirmed to be negative on the fifth day of treatment. The anorexia and hyponatremia gradually improved. From the time of admission to the fourth day, the patient’s serum creatinine worsened from 1.74 mg/dL to 6.43 mg/dL, his urine output decreased, and he became fluid-retentive (Fig. 1). Urinary liver-type fatty acid-binding protein (L-FABP) measured 645 μg/gCr. A temporary vascular catheter was inserted through the right internal jugular vein. From day four onwards, weekly hemodialysis (HD) was started as well as continuous infusion management. A total of ten sessions were conducted. The patient’s urine output gradually recovered, and the urine toxins began to decrease spontaneously; on the 22nd day of hospitalization, he was weaned off HD. At this point, the urinary L-FABP level measured 67.1 μg/gCr.
Fig. 1.
Clinical course of AKI secondary to malaria is depicted. Quinine hydrochloride has been administered on the day of admission. The parasitemia rate has decreased to 0.2%, on the third day. Serum creatinine has worsened from 1.74 mg/dL to 6.88 mg/dL, and urine output has decreased. Hemodialysis has been initiated, on the fourth day. The patient’s renal function has slowly improved, and he has been weaned off hemodialysis, on the 22nd day. The urinary L-FABP levels have decreased from day 5, which has preceded the recovery of renal function. AKI acute kidney injury, L-FABP liver-type fatty acid-binding protein
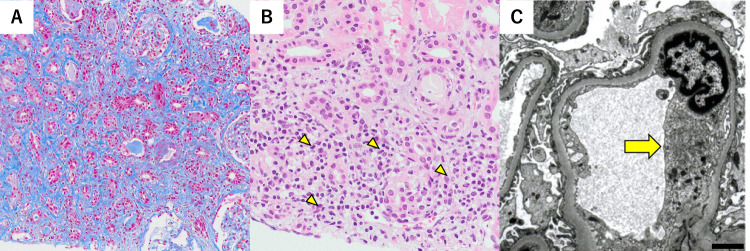
The patient’s baseline serum creatinine was 1.08 mg/dL; however, post-weaning subsequent to HD, this level did not steadily decline. Therefore, on the 51st day, a renal biopsy was performed for histopathological analysis (Fig. 2). Light microscopy revealed that three of the 36 glomeruli were diffusely sclerotic. The glomeruli appeared unremarkable; however, interstitial fibrosis, tubular atrophy, and mild, chronic, inflammatory cell infiltration were observed in 70% of the biopsied renal tissue. In addition, focal tubulointerstitial inflammation was observed. Intense tubulitis with neutrophilia and lymphocytosis was observed in some of the non-atrophic tubular areas. Immunofluorescence stainings for immunoglobulin (Ig) A, IgM, and IgG; complement components C3 and C4; and the kappa and lambda chains were negative. Electron microscopy revealed mild swelling of the glomerular endothelial cells; nevertheless, foot-process effacement of the podocytes was preserved. Electron-dense deposits were not observed in the glomeruli. Histopathological findings revealed a focal acute tubulointerstitial nephritis, which was confirmed to be part of the healing process of malarial nephropathy. On the 56th day, the patient was discharged from the hospital.
Fig. 2.
Renal biopsy findings are depicted. A Light microscopy revealing interstitial fibrosis and tubular atrophy, using the Masson trichrome staining, (magnification × 200; scale bar = 50 µm). B Intense tubulitis with neutrophilia and lymphocytosis is observed in a few non-atrophic tubular areas, using the periodic acid–Schiff staining (as indicated by the yellow arrowheads; magnification × 400; scale bar = 100 µm). C Electron microscopy revealing mild swelling of the glomerular endothelial cells (as indicated by the yellow arrow; scale bar = 2 µm)
He continued to attend the outpatient clinic, and his urinary L-FABP level improved from 24.8 μg/gCr to 1.9 μg/gCr. His serum creatinine level recovered to 1.39 mg/dL with an estimated glomerular filtration rate of 43.5 ml/min/1.73 m2 (Fig. 1). He has currently returned to work and is leading a healthy life.
Discussion
We reported a case in which HD and renal biopsy were performed subsequent to the diagnosis of AKI secondary to severe falciparum malaria. A renal biopsy was performed to investigate the prolonged renal dysfunction secondary to malaria. A progressive form of nephropathy with persistent tubulointerstitial inflammation was revealed, resulting in interstitial fibrosis and tubular atrophy, particularly after the resolution of the malarial infection was confirmed by smear.
The initial treatment of severe falciparum malaria is recommended to be parenteral artesunate or quinine. Parenteral artesunate is preferred as the first-line treatment because it is more effective and safer than parenteral quinine [5]. However, parenteral artesunate is difficult to obtain in Japan; therefore, parenteral quinine, the second-choice medication, is used instead. Since injectable quinine is not approved for malaria treatment in Japan, it was administered on the first day of admission after obtaining informed consent from the patient to participate in the study “Efficacy and safety of injectable quinine in malaria patients (injectable quinine for malaria)” (jRCTs071180063) by the Development of Optimal Medical Care Network for the Diagnosis and Treatment of Tropical and Parasitic Diseases in Japan. Following AKI practice guidelines [6], the decision to initiate RRT was based not only on blood urea nitrogen and serum creatinine thresholds but also on broad clinical presentation and trends in disease status and laboratory changes that RRT improves. Likewise, RRT was discontinued when renal function improved to a satisfactory level.
Several mechanisms underlie renal involvement in malaria [7]. The mechanisms of renal dysfunction in this case were as follows: 1) acute tubular necrosis and thrombotic microangiopathy (TMA), due to microvascular sequestration, induced by infected erythrocyte rosetting; 2) acute interstitial nephritis, due to the activation of mononuclear cells, by the malaria antigen; 3) tubular damage due to free iron produced by hemolysis; and 4) quinine-induced TMA and tubulointerstitial nephritis.
In this case, regarding acute tubular necrosis, due to microvascular occlusion, induced by infected erythrocyte rosetting, the infected erythrocytes were not identified in the renal biopsy specimens. Nguansangiam et al. revealed a greater number of infected erythrocytes and monocytes in the glomerular basement membranes (GBMs) and peritubular capillaries (PTCs) in the AKI-infected cohort than that in the AKI-uninfected cohort of patients infected with malaria [8]. This suggests that monocytosis in the GBMs and PTCs may contribute to malarial renal damage, which is consistent with the histopathological findings of the present study.
Iron staining was not performed in this case, and no hemoglobin casts were observed in the biopsied renal specimens to indicate that tubular damage had been caused by free iron. However, the long interval between malaria infection and renal biopsy and the presence of tubulointerstitial damage suggests that free iron may have caused tubular damage. The importance of hemozoin in the growth cycle of P. falciparum malaria has been noted [9]. It is undeniable that such iron-induced tubular cell damage may also contribute to elevated urinary L-FABP expression after malaria infection[10]. There are no reports of quinine or artemisinin–lumefantrine directly affecting L-FABP levels. Contrarily, a known mechanism of quinine-induced renal injury is TMA, which can occur even after a single dose and sometimes several hours later [11]. However, in the present case, the patient had AKI with decreased urine output at the time of admission, i.e., before quinine administration, and the subsequent course of the disease made this unlikely.
In vivo, mice infected with malaria developed interstitial nephritis and were subsequently cured of the infection. Moreover, murine renal dysfunction persisted for at least 14 days, possibly due to monocyte activation and the production of inflammatory cytokines [12]. In the present case, renal dysfunction persisted particularly after the malaria was cured, and renal histopathology revealed tubulointerstitial damage. Therefore, the most likely etiology of renal dysfunction was acute interstitial nephritis, caused by the activation of mononuclear cells, due to the malaria antigen. The presence of endothelial cell swelling, elevated lactate dehydrogenase, anemia, and thrombocytopenia suggests that TMA may have occurred and free iron may have caused tubular cell damage.
Cystatin C, kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, and nuclear factor kappa B have been studied as prognostic indicators of malaria; nonetheless, no consistent conclusions regarding the reliability thereof have been deduced [13]. The urinary L-FABP, found in the proximal renal tubules and detected early in urine, is characterized as an ischemic and oxidative stress marker, reflecting renal microcirculatory impairment, rather than an inflammatory marker. In 2012, in the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guidelines for Acute Kidney Injury [6], the urinary L-FABP was introduced as one of the biomarkers for AKI. In patients with AKI after cardiovascular surgery, the urinary L-FABP levels can predict the onset of AKI earlier than seen with the serum creatinine levels [14, 15]. In principle, urine L-FABP is calculated by insurance only once every 3 months; however, if the benefit is high, it can be calculated more than once every 3 months. In falciparum malaria, infected erythrocytes form a rosette and adhere to the vascular endothelium, causing microvascular sequestration and resulting in circulatory disturbances. In our in vivo experiments investigating the relationship between malaria infection and urinary human L-FABP (hL-FABP) in transgenic mice carrying the hL-FABP gene, we found a parallel increase or decrease in the peripheral blood-infected erythrocyte rate and urinary hL-FABP levels, coinciding with a peak on day 10 of Plasmodium inoculation [16]. In the present case, the urinary L-FABP and serum creatinine levels were elevated, which was theorized to reflect a microcirculatory disturbance; however, the urinary L-FABP level improved prior to the serum creatinine level. These findings suggest that the urinary L-FABP level may be a useful prognostic indicator of malarial nephropathy and malaria infection.
Prompt diagnosis and treatment of severe malaria are necessary because, without early and appropriate intervention, mortality rates are reportedly high. In this case, the severity of the malaria infection and the course of the disease after treatment could be assessed not only by measuring the rate of infected erythrocytes in the peripheral blood and clinical symptoms but also by measuring the urinary L-FABP and performing a renal biopsy. The urinary L-FABP was measured twice a week and a marked improvement was observed at the time of the second measurement, suggesting that frequent measurements early in the course of the disease may be an early marker for determining treatment efficacy. In this case, the renal function had been stabilizing; nonetheless, the patient transitioned from AKI to chronic kidney disease. This study had some limitations. First, this paper is a case report. There are inherent limitations in describing the efficacy of L-FABP for renal impairment due to severe fatigue from a single case. The possibility of confounding factors for the observed results cannot be ruled out. Second, several mechanisms of AKI in malarial nephropathy have been reported; however, in this case, renal biopsy could not provide a clear diagnosis because of the time that had elapsed since malaria infection. If systemic conditions permit, a renal biopsy should be performed as early as possible to predict renal prognosis and provide appropriate treatment.
In conclusion, this study highlighted that long-term, continuous follow-up may be necessary for AKI secondary to malaria. Regarding the high incidence and mortality rates of AKI secondary to severe falciparum malaria, urinary L-FABP level may be a useful prognostic indicator.
Funding
Japan Society for the Promotion of Science,23K07687,Daisuke Katagiri,Japan Agency for Medical Research and Development,JP23fk0108639h0002,Yutaro Akiyama.
Declarations
Conflict of interest
This study was supported by a grant from the Japan Society for the Promotion of Science (grant number 23K07687), and the Emerging/Re-emerging Infectious Diseases Project of Japan from the Japan Agency for Medical Research and Development, AMED (JP23fk0108639h0002).
Research involving humans and/or animals
All procedures performed herein were in accordance with the Institutional Review Board (IRB) of the National Center for Global Health and Medicine on 10/31/2022 (IRB approval number NCGM-S-004548–00) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Institute of Infectious Diseases. Characteristics of reported cases of malaria in the Surveillance of Infectious Disease Outbreak Trends 2006—first semester of 2014 https://www.niid.go.jp/niid/ja/malaria-m/malaria-iasrd.html. accessed on Dec, 1, 2023. 224–226. 2014;35.
- 2.WHO (2023) Malaria. https://www.who.int/news-room/fact-sheets/detail/malaria. accessed on Nov, 3, 2023
- 3.Plewes K, Turner GDH, Dondorp AM. Pathophysiology, clinical presentation, and treatment of coma and acute kidney injury complicating falciparum malaria. Curr Opin Infect Dis. 2018;31(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. WHO guidelines for malaria https://www.mmv.org/sites/default/files/content/document/WHO-UCN-GMP-2023.01-eng.pdf. Dec, 1, 2023. 2023.
- 5.Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376(9753):1647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KDIGO. Clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–124. [Google Scholar]
- 7.Barsoum RS. Malarial acute renal failure. J Am Soc Nephrol. 2000;11(11):2147–54. [DOI] [PubMed] [Google Scholar]
- 8.Nguansangiam S, Day NP, Hien TT, Mai NT, Chaisri U, Riganti M, et al. A quantitative ultrastructural study of renal pathology in fatal Plasmodiumfalciparum malaria. Trop Med Int Health. 2007;12(9):1037–50. [DOI] [PubMed] [Google Scholar]
- 9.Rathi A, Chowdhry Z, Patel A, Zuo S, Veettil TCP, Adegoke JA, et al. Hemozoin in malaria eradication—from material science, technology to field test. NPG Asia Mater. 2023;15(1):70. [Google Scholar]
- 10.Toyokuni S, Uchida K, Okamoto K, Hattori-Nakakuki Y, Hiai H, Stadtman ER. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc Natl Acad Sci USA. 1994;91(7):2616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liles NW, Page EE, Liles AL, Vesely SK, Raskob GE, George JN. Diversity and severity of adverse reactions to quinine: a systematic review. Am J Hematol. 2016;91(5):461–6. [DOI] [PubMed] [Google Scholar]
- 12.Abreu TP, Silva LS, Takiya CM, Souza MC, Henriques MG, Pinheiro AA, et al. Mice rescued from severe malaria are protected against renal injury during a second kidney insult. PLoS ONE. 2014;9(4):e93634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte DB, Lacerda M, Ribeiro YJP, Ribeiro MZD, Frederico MA, Oliveira MJC. Kidney biomarkers in tropical infections: an update. Pathog Glob Health. 2020;114(6):302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noiri E, Doi K, Negishi K, Tanaka T, Hamasaki Y, Fujita T, et al. Urinary fatty acid-binding protein 1: an early predictive biomarker of kidney injury. Am J Physiol Renal Physiol. 2009;296(4):F669-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katagiri D, Doi K, Honda K, Negishi K, Fujita T, Hisagi M, et al. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann Thorac Surg. 2012;93(2):577–83. [DOI] [PubMed] [Google Scholar]
- 16.Sanjoba C, Osada Y, Asada M, Gantuya S, Negishi K, Sugaya T, et al. Liver type fatty acid-binding protein (L-FABP) as a novel biomarker for malaria infection. Jpn US Coop Med Sci Progr Forty-third Joint Conf Parasit Dis. 2009;1:7–8. [Google Scholar]